Abstract
In vivo selection may provide a means to increase the relative number of cells of donor origin in recipients with hemopoietic chimerism. We have tested whether in vivo selection using chemical inducers of dimerization (CIDs) can direct the expansion of transduced normal donor erythrocytes in recipients with chimerism using a mouse model of pyruvate kinase deficiency. Marrow cells from normal CBA/N mice were transduced with a vector (F36VmplGFP) that promotes cell growth in the presence of CIDs. Transduced cells were then transplanted into minimally conditioned, pyruvate kinase-deficient recipients (CBA-Pk-1slc/Pk-1slc) to establish stable chimerism. CID administration resulted in expansion of normal donor erythrocytes and improvement of the anemia. The preferential expansion of normal erythrocytes also resulted in a decrease in erythropoietin levels, reducing the drive for production of pyruvate kinase-deficient red blood cells. CID-mediated expansion of genetically modified erythrocytes could prove a useful adjunct to transplantation methods that achieve erythroid chimerism. (Blood. 2004;103:4432-4439)
Introduction
Although many inherited blood disorders are curable through stem cell transplantation, the applicability of the procedure is limited in part by its toxicity. One way to reduce the toxicity associated with stem cell transplantation is through the use of attenuated conditioning regimens that fall short of producing complete myeloablation and lead to the establishment of donor/host chimerism. Studies in inherited red blood cell (RBC) disorders have shown that relatively small percentages of normal donor stem cells can substantially ameliorate the severity of these diseases. Patients in whom chimerism has developed after stem cell transplantation for β-thalassemia or sickle cell disease demonstrate that as few as 10% of normal donor progenitors can functionally compensate for the anemia that characterizes these disorders.1-3 Similar conclusions pertain to the relevant mouse models of these disorders.4-9
However, chimerism alone does not correct all the manifestations of hemolysis. Transplantation studies in a sickle cell mouse model demonstrated that hematologic and pathologic correction required 100% peripheral RBC chimerism.10 In studies of hematopoietic chimerism in a dog model of pyruvate kinase (PK) deficiency, improvement in the anemia failed to eliminate persistent hemolysis in 1 animal and resulted in severe iron overload and cirrhosis.11 After stem cell transplantation for thalassemia, persistent iron overload is treated with aggressive phlebotomy to forestall progression of liver fibrosis.12 However, patients with chimerism may not be able to tolerate phlebotomy because of low hematocrits, necessitating treatment with chelation therapy.3,13 Methods that would allow for the erythropoietin-independent expansion of donor-origin erythropoiesis may suppress erythropoietin production and, in turn, residual aberrant erythropoiesis. Additionally, expanding donor hematopoiesis in vivo may aid in preventing the graft rejection that plagues nonmyeloablative transplantation for hemoglobinopathies.14
We have previously described a system that uses small-molecule drugs as artificial growth factors for genetically modified cells. These drugs, called chemical inducers of dimerization (CIDs), exert their activity by directing the self-association of hybrid signaling molecules composed of a CID-binding domain and the intracellular portion of a growth factor receptor.15,16 We have shown that CID-mediated activation of the intracellular portion of the thrombopoietin receptor, mpl, can induce growth of genetically modified hemopoietic cells from mice, dogs, and humans.17-19 On in vivo administration, CID-induced activation of mpl induces a dramatic expansion of genetically modified RBCs. In this study, we have used a mouse model of pyruvate kinase (PK) deficiency to test whether CIDs can be used to augment the contribution of normal donor erythrocytes in patients with mixed chimerism. The PK-deficient mouse (CBA-Pk-1slc/Pk-1slc) closely reproduces the disease found in the canine model and in humans,20-22 exhibiting a phenotype that includes anemia, reticulocytosis, and splenomegaly. Marrow cells from the congenic founder strain, CBA/N, can be used in transplantation experiments to completely correct the disorder.21 We show that the selective, CID-mediated expansion of normal donor RBCs can improve inherited anemia and reduce abnormal erythropoiesis.
Materials and methods
Retroviral vector
The retroviral vector F36VmplGFP has been described in a previous publication.18 Briefly, this vector is based on murine stem cell virus (MSCV)23 and expresses a single transcript that encodes the CID-responsive cytoplasmic portion of the thrombopoietin receptor (mpl) and the enhanced green fluorescence protein (GFP) (Figure 2A). A point mutation was introduced into the FKBP domain that changed a phenylalanine to a valine at amino acid position 36, allowing for binding of the CIDs AP1903 and AP20187.19,24 A stable ecotropic producer line (GP+E86) with a titer of 1 × 106 was used for coculture-based gene transfer.
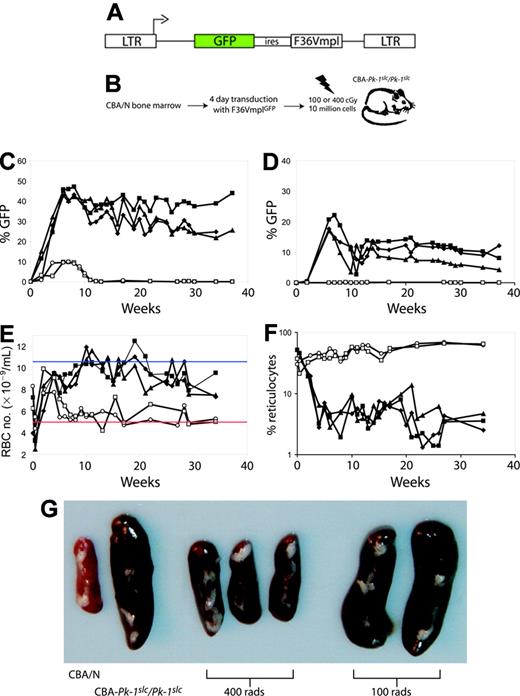
400 cGy gamma irradiation allows long-term stable engraftment of transduced marrow cells. (A) Five CBA-Pk-1slc/Pk-1slc mice underwent transplantation with 10 million 5-FU-treated marrow cells from CBA/N mice transduced with the retroviral vector F36VmplGFP. (B) The schematic shows the experimental plan. (C-F) Two mice received conditioning with 100 cGy (open symbols), and 3 mice received 400 cGy (filled symbols). All 3 mice that were conditioned with 400 cGy before transplantation exhibited stable engraftment of GFP-positive erythrocytes (C) and platelets (D) and correction of their anemia as evidenced by an increase in RBC number (E) and a decrease in the percentage of reticulocytes (F). Mice conditioned with 100 cGy did not have evidence of GFP-positive erythrocytes beyond 10 weeks after transplantation (C; open symbols), did not have GFP-positive platelets (D), remained anemic (E), and had persistently elevated reticulocyte levels (F). Panel G shows the spleens from the 5 mice that received transplants and 2 control mice. The weights are as follows: CBA/N, 60 mg; CBA-Pk-1slc/Pk-1slc, 310 mg; 400 cGy, 120 mg, 100 mg, 100 mg; 100 cGy, 350 mg, 360 mg. In panel E, the blue line represents the average RBC number for CBA/N mice, and the red line represents the average RBC number for CBA-Pk-1slc/Pk-1slc (Figure 1).
400 cGy gamma irradiation allows long-term stable engraftment of transduced marrow cells. (A) Five CBA-Pk-1slc/Pk-1slc mice underwent transplantation with 10 million 5-FU-treated marrow cells from CBA/N mice transduced with the retroviral vector F36VmplGFP. (B) The schematic shows the experimental plan. (C-F) Two mice received conditioning with 100 cGy (open symbols), and 3 mice received 400 cGy (filled symbols). All 3 mice that were conditioned with 400 cGy before transplantation exhibited stable engraftment of GFP-positive erythrocytes (C) and platelets (D) and correction of their anemia as evidenced by an increase in RBC number (E) and a decrease in the percentage of reticulocytes (F). Mice conditioned with 100 cGy did not have evidence of GFP-positive erythrocytes beyond 10 weeks after transplantation (C; open symbols), did not have GFP-positive platelets (D), remained anemic (E), and had persistently elevated reticulocyte levels (F). Panel G shows the spleens from the 5 mice that received transplants and 2 control mice. The weights are as follows: CBA/N, 60 mg; CBA-Pk-1slc/Pk-1slc, 310 mg; 400 cGy, 120 mg, 100 mg, 100 mg; 100 cGy, 350 mg, 360 mg. In panel E, the blue line represents the average RBC number for CBA/N mice, and the red line represents the average RBC number for CBA-Pk-1slc/Pk-1slc (Figure 1).
Mice
Breeding pairs of the wild-type strain CBA/N and the pyruvate kinase mutant strain CBA-Pk-1slc/Pk-1slc were obtained from the SLC facility in Japan.21 They were maintained in a specific pathogen-free facility. In addition, the animals were maintained on a breeder diet to limit the development of folate deficiency.
Gene transfer and transplantation
Twelve- to 16-week-old donor mice were treated with 150 mg/kg 5-fluorouracil (5-FU) by intraperitoneal (IP) injection 2 days before bone marrow mononuclear cells were harvested. Cells were prestimulated for 48 hours in Dulbecco minimal essential medium containing 16% fetal calf serum (FCS), 5% interleukin-3 (IL-3)-conditioned medium, 100 ng/mL recombinant human IL-6, and 50 ng/mL recombinant murine stem cell factor (SCF) ina37°C, 5% CO2 incubator. Cells were then transferred to irradiated (1500 cGy) producer cells and cocultivated using identical growth conditions except for the addition of polybrene (8 μg/mL). Marrow cells were harvested after 48 hours and were immediately infused by tail vein injection. Eight- to 12-week-old recipient mice received variable doses of radiation from a dual cesium Cs 137 gamma source (GammaCell 40; AEC, Kanata, ON, Canada) before infusions ranging from 2 to 10 × 106 transduced bone marrow. Gene transfer was monitored by methylcellulose plating and by scoring progenitor colonies for GFP expression. All mice that underwent transplantation in this study are listed in Table 1, as are mice that underwent similar transplantations and were followed up long term for adverse events.
Summary of mouse transplantation experiments
Experiment and mouse no. . | cGy . | Cell dose, × 106 . | CFC transduction, % . | % GFP-positive RBCs at 12 wks . | % GFP-positive RBCs at 24 wks . | % GFP-positive platelets at 24 wks . | Treated with CID . | Response to CID . | Comments/adverse events . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | |||||||||
| 2160 | 400 | 10 | 57 | 29.63 | 25.26 | 11.12 | No | — | Killed wk 37 |
| 2161 | 400 | 10 | 57 | 37.73 | 35.08 | 12.15 | No | — | Killed wk 37 |
| 2162 | 400 | 10 | 57 | 35.67 | 27.25 | 6.62 | No | — | Killed wk 37 |
| 2168 | 100 | 10 | 57 | 0.11 | 0.1 | 0.1 | No | — | Killed wk 37 |
| 2172 | 100 | 10 | 57 | 0.12 | 0.1 | 0.09 | No | — | Killed wk 37 |
| 2 | |||||||||
| 2781 | 400 | 2 | 49 | 0.07 | 0.05 | 0.01 | Yes | No | Killed wk 41 |
| 2782 | 400 | 2 | 49 | 4.15 | 0.13 | 0.02 | No | — | Killed wk 41 |
| 2778 | 400 | 2 | 49 | 4.51 | 0.07 | 0.03 | No | — | Killed wk 41 |
| 2779 | 400 | 2 | 49 | 1.47 | 0.04 | 0.03 | No | — | Killed wk 41 |
| 2785 | 400 | 4 | 49 | 8.79 | 48.75* | 1.85 | Yes | Yes | Killed wk 45 |
| 2752 | 400 | 4 | 49 | 10.84 | 12.41 | 2.91 | No | — | Killed wk 45 |
| 2777 | 400 | 4 | 49 | 7.01 | — | — | — | — | Died after single CID dose, wk 17 |
| 2780 | 400 | 4 | 49 | 8.21 | 12.96 | 1.51 | No | — | Died wk 40 |
| 3 | |||||||||
| 2407 | 400 | 5 | 55 | 15.72 | 19.94 | 2.35 | No | — | Killed wk 67 |
| 2408 | 400 | 5 | 55 | 10.29 | 7.52 | 1.09 | Yes | Yes | Died wk 50 |
| 2409 | 400 | 5 | 55 | 8.53 | 8.59 | 1.01 | Yes | Yes | Killed wk 67 |
| 2410 | 400 | 5 | 55 | 9.98 | 4.44 | 0.83 | No | — | Killed wk 67 |
| 2411 | 400 | 5 | 55 | 11.71 | 11.81 | 2.65 | Yes | Yes | Killed wk 67 |
| 2412 | 400 | 5 | 55 | 19.12 | 24.02 | 6.07 | No | — | Killed wk 67 |
| 2413 | 400 | 5 | 55 | 17.13 | 22.17 | 3.97 | Yes† | Yes† | Killed wk 67; CID wk 63 |
| 2414 | 400 | 5 | 55 | 10.21 | 8.97 | 1.88 | Yes | Yes | Died wk 48 |
| 2415 | 400 | 5 | 55 | 14.58 | 7.46 | 2.72 | Yes | Yes | Died wk 62 |
| 2416 | 400 | 5 | 55 | 13.5 | 17.27 | 3.13 | No | — | Died wk 39 |
| 4 | |||||||||
| 1674 | 0 | 5 | 49 | 0 | — | — | No | — | Killed wk 50 |
| 1246 | 0 | 5 | 49 | 0 | — | — | Yes | No | Killed wk 50 |
| 1247 | 0 | 5 | 49 | 0 | — | — | Yes | No | Died |
| 1675 | 0 | 5 | 49 | 0 | — | — | Yes | No | Killed wk 50 |
| 5 | |||||||||
| 2952 | 400 | 10 | 66.7 | 55.42 | 47.84 | 7.46 | Yes | Yes | Died wk 110 |
| 2953 | 400 | 10 | 66.7 | 40.6 | 53.02 | 8.47 | Yes | Yes | Died wk 123 |
| 2954 | 400 | < 2 | 66.7 | 5.76 | 5.25 | 0.13 | No | — | Died wk 124 |
| 6 | |||||||||
| 2891 | 100 | 2 | 66 | 2.89 | 0.96 | 0.08 | Yes | No | Killed wk 48 |
| 2163 | 100 | 5 | 66 | 2.25 | 0.75 | 0.05 | No | — | Died wk 47 |
| 2165 | 100 | 10 | 66 | 5.21 | 5.25 | 0.4 | Yes | Yes | Died wk 33 |
Experiment and mouse no. . | cGy . | Cell dose, × 106 . | CFC transduction, % . | % GFP-positive RBCs at 12 wks . | % GFP-positive RBCs at 24 wks . | % GFP-positive platelets at 24 wks . | Treated with CID . | Response to CID . | Comments/adverse events . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | |||||||||
| 2160 | 400 | 10 | 57 | 29.63 | 25.26 | 11.12 | No | — | Killed wk 37 |
| 2161 | 400 | 10 | 57 | 37.73 | 35.08 | 12.15 | No | — | Killed wk 37 |
| 2162 | 400 | 10 | 57 | 35.67 | 27.25 | 6.62 | No | — | Killed wk 37 |
| 2168 | 100 | 10 | 57 | 0.11 | 0.1 | 0.1 | No | — | Killed wk 37 |
| 2172 | 100 | 10 | 57 | 0.12 | 0.1 | 0.09 | No | — | Killed wk 37 |
| 2 | |||||||||
| 2781 | 400 | 2 | 49 | 0.07 | 0.05 | 0.01 | Yes | No | Killed wk 41 |
| 2782 | 400 | 2 | 49 | 4.15 | 0.13 | 0.02 | No | — | Killed wk 41 |
| 2778 | 400 | 2 | 49 | 4.51 | 0.07 | 0.03 | No | — | Killed wk 41 |
| 2779 | 400 | 2 | 49 | 1.47 | 0.04 | 0.03 | No | — | Killed wk 41 |
| 2785 | 400 | 4 | 49 | 8.79 | 48.75* | 1.85 | Yes | Yes | Killed wk 45 |
| 2752 | 400 | 4 | 49 | 10.84 | 12.41 | 2.91 | No | — | Killed wk 45 |
| 2777 | 400 | 4 | 49 | 7.01 | — | — | — | — | Died after single CID dose, wk 17 |
| 2780 | 400 | 4 | 49 | 8.21 | 12.96 | 1.51 | No | — | Died wk 40 |
| 3 | |||||||||
| 2407 | 400 | 5 | 55 | 15.72 | 19.94 | 2.35 | No | — | Killed wk 67 |
| 2408 | 400 | 5 | 55 | 10.29 | 7.52 | 1.09 | Yes | Yes | Died wk 50 |
| 2409 | 400 | 5 | 55 | 8.53 | 8.59 | 1.01 | Yes | Yes | Killed wk 67 |
| 2410 | 400 | 5 | 55 | 9.98 | 4.44 | 0.83 | No | — | Killed wk 67 |
| 2411 | 400 | 5 | 55 | 11.71 | 11.81 | 2.65 | Yes | Yes | Killed wk 67 |
| 2412 | 400 | 5 | 55 | 19.12 | 24.02 | 6.07 | No | — | Killed wk 67 |
| 2413 | 400 | 5 | 55 | 17.13 | 22.17 | 3.97 | Yes† | Yes† | Killed wk 67; CID wk 63 |
| 2414 | 400 | 5 | 55 | 10.21 | 8.97 | 1.88 | Yes | Yes | Died wk 48 |
| 2415 | 400 | 5 | 55 | 14.58 | 7.46 | 2.72 | Yes | Yes | Died wk 62 |
| 2416 | 400 | 5 | 55 | 13.5 | 17.27 | 3.13 | No | — | Died wk 39 |
| 4 | |||||||||
| 1674 | 0 | 5 | 49 | 0 | — | — | No | — | Killed wk 50 |
| 1246 | 0 | 5 | 49 | 0 | — | — | Yes | No | Killed wk 50 |
| 1247 | 0 | 5 | 49 | 0 | — | — | Yes | No | Died |
| 1675 | 0 | 5 | 49 | 0 | — | — | Yes | No | Killed wk 50 |
| 5 | |||||||||
| 2952 | 400 | 10 | 66.7 | 55.42 | 47.84 | 7.46 | Yes | Yes | Died wk 110 |
| 2953 | 400 | 10 | 66.7 | 40.6 | 53.02 | 8.47 | Yes | Yes | Died wk 123 |
| 2954 | 400 | < 2 | 66.7 | 5.76 | 5.25 | 0.13 | No | — | Died wk 124 |
| 6 | |||||||||
| 2891 | 100 | 2 | 66 | 2.89 | 0.96 | 0.08 | Yes | No | Killed wk 48 |
| 2163 | 100 | 5 | 66 | 2.25 | 0.75 | 0.05 | No | — | Died wk 47 |
| 2165 | 100 | 10 | 66 | 5.21 | 5.25 | 0.4 | Yes | Yes | Died wk 33 |
Experiment 1 is depicted in Figure 2; experiment 2 in Figure 3; and experiment 3 in Figures 4, 5, 6. Experiments 4 to 6 are not depicted in the figures. CFC indicates colony-forming cells.—indicates that testing was not performed.
Mouse 2785 was treated at week 17 with CID, resulting in an elevation in the percentage of GFP-positive RBCs.
AP20187 administration
The drug used to regulate cell expansion was AP20187 (ARIAD Pharmaceuticals, Cambridge, MA). This drug was designed to specifically bind to 2 mutated FK1012 binding proteins (FKBPs). Lyophilized AP20187 was solubilized in 100% ethanol to produce a 5-mg/mL stock that was stored at -20°C. From this stock solution, AP20187 was diluted fresh on the day of injection. The final solution for injection contained 10% polyethylene glycol (PEG) 400 and 1.4% Tween 80. We administered AP20187 by daily intraperitoneal injection in a total volume of 400 μL per injection.
Hematologic parameters
RBC number, reticulocyte percentage, and RBC and platelet GFP expression were determined every 1 to 2 weeks through a 10-μL sample of blood taken from a tail vein. RBC numbers were determined on a Coulter Z series after a 1:50 000 dilution in isotonic saline. Reticulocyte percentage was determined by staining peripheral blood with new methylene blue (Sigma, St Louis, MO). Reticulum-positive cells were counted, and at least 500 cells were scored per sample. Flow cytometry was performed using FACScan (Becton Dickinson, Mountain View, CA). RBCs and platelets were identified based on forward- and side-scatter characteristics. Statistical analysis of groups (with and without CID) used a 2-tailed t test assuming equal variance (Microsoft Excel Statistical analysis package).
Erythropoietin levels
Mice were killed and serum was collected. Levels of serum erythropoietin were measured using a murine erythropoietin-specific enzyme-linked immunosorbent assay (ELISA), as described.25 Briefly, monoclonal rat antimurine erythropoietin antibody (BD BioSciences, Mississauga, ON, Canada) was used as the capturing antibody, and polyclonal rabbit antihuman erythropoietin antibody (R&D Systems, Minneapolis, MN) was used as the detecting antibody. Reactivity was revealed using ABTS substrate (Boehringer Mannheim-Roche, Laval, PQ, Canada), and optical density values were read at a wavelength of 405 nm with 492 nm as the reference. Concentrations of erythropoietin in serum samples were calculated against the standard curve generated using recombinant murine erythropoietin (Boehringer Mannheim-Roche).
PCR for the genomic liver-RBC pyruvate kinase (lr-pk) gene
The following primers were used for polymerase chain reaction (PCR) across the region of the lr-pk gene that contains the single base pair change that causes PK deficiency in this animal model: 5′ primer, ATCTTTTGGATCTGCGCTTC; 3′ primer, GCCAAGAAAACCTTCTCTGCT. The resultant product is 367 bp in length. The mutation results in the loss of a BstEII site. The PCR product from the CBA/N lr-pk gene can be digested with BstEII to result in fragments of 326 and 41 bp. DNA was purified from the peripheral blood of mice that underwent transplantation and was used as the template for the PCR reaction. After digestion with BstEII, the DNA fragments were resolved on 2% agarose. Kodak DS 1D software (Eastman Kodak, Rochester, NY) was used to assign relative fluorescence intensities to the bands and to determine ratios within samples. Values were adjusted to compensate for the differences in band size.
Results
Amelioration of anemia can be achieved with 10% donor chimerism
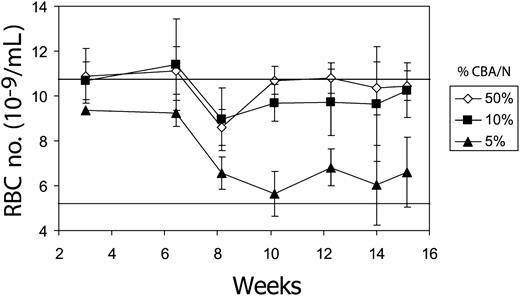
We first determined the relative level of donor chimerism required to improve the anemia associated with pyruvate kinase deficiency. Marrow cells from the CBA/N mouse and the mutant CBA-Pk-1slc/Pk-1slc were subjected to the conditions necessary for gene transfer, mixed in various proportions, and infused into lethally irradiated CBA/N recipients. Although 5% of normal donor marrow cells were insufficient to correct the anemia, 10% resulted in near normal RBC numbers (Figure 1).
Ten percent CBA/N-derived stem cells can correct the anemia of pyruvate kinase-deficient mice. Three million marrow cells from a mixture of CBA/N and CBA-Pk-1slc/Pk-1slc were transplanted into CBA/N mice that had undergone complete myeloablation with 1050 cGy radiation. The percentage of CBA/N cells in the transplanted cell dose is indicated in the inset to the right. Mice were then observed for 15 weeks to monitor the severity of the anemia. The top horizontal line represents the average RBC number for CBA/N mice (10.8 ± 1.7; n = 20), and the lower horizontal line represents the RBC number for CBA-Pk-1slc/Pk-1slc (5.4 ± 0.8; n = 20). Error bars show 1 standard deviation.
Ten percent CBA/N-derived stem cells can correct the anemia of pyruvate kinase-deficient mice. Three million marrow cells from a mixture of CBA/N and CBA-Pk-1slc/Pk-1slc were transplanted into CBA/N mice that had undergone complete myeloablation with 1050 cGy radiation. The percentage of CBA/N cells in the transplanted cell dose is indicated in the inset to the right. Mice were then observed for 15 weeks to monitor the severity of the anemia. The top horizontal line represents the average RBC number for CBA/N mice (10.8 ± 1.7; n = 20), and the lower horizontal line represents the RBC number for CBA-Pk-1slc/Pk-1slc (5.4 ± 0.8; n = 20). Error bars show 1 standard deviation.
400 cGy conditioning results in stable chimeric marrow
We next identified a minimal conditioning regimen for the CBA-Pk-1slc/Pk-1slc mice that would allow for the engraftment of transduced marrow cells of normal donor origin. Radiation doses as low as 100 cGy have been shown to improve engraftment of donor stem cells.26,27 However, studies have demonstrated that retroviral transduction impairs stem cell engraftment.28-30 Therefore, we compared 2 doses of gamma radiation, 100 cGy and 400 cGy, as conditioning regimens for achieving stable engraftment of transduced donor stem cells.
CBA-Pk-1slc/Pk-1slc mice were conditioned with either 100 cGy or 400 cGy gamma irradiation in a single dose on the day of transplantation (schematic; Figure 2B). Marrow cells from the CBA/N donors were transduced with the MSCV-based retroviral vector F36VmplGFP (Figure 2A) using a 4-day coculture method that resulted in gene transfer into 49% to 67% of IL-3-responsive colony-forming cells (data not shown).18 Ten million posttransduction donor cells were injected per recipient. Blood (5-10 μL) was sampled from the tail veins of the mice every 1 to 2 weeks to analyze GFP expression in erythrocytes and platelets, changes in the peripheral blood erythrocyte number, and effects on the percentage of reticulocytes. As shown in Figure 2C, GFP-positive cells disappeared from the blood of mice conditioned with 100 cGy irradiation. By 12 weeks after transplantation, GFP-positive RBCs were no longer evident in the peripheral blood, and GFP-positive platelets were not detected at any time point after transplantation (Figure 2D). These findings suggested that 100 cGy allowed for only brief short-term engraftment resulting in a transient production of GFP-positive donor RBCs. This loss of transduced RBCs might have resulted from a failure to engraft or from an immune-mediated rejection of the transduced GFP-positive cells.31
In contrast, mice conditioned with 400 cGy exhibited long-term engraftment (more than 40 weeks) with transduced CBA/N stem cells, as evidenced by the continued presence of GFP-positive erythrocytes and platelets. Differences in the percentages of GFP-positive erythrocytes compared with platelets are attributable to the prolonged survival of the CBA/N erythrocytes relative to their PK-deficient counterparts. Mice conditioned with 400 cGy had long-term improvements in RBC numbers (Figure 2E), decreased reticulocytes (Figure 2F), and near-complete normalization in spleen size (Figure 2G.) In contrast, mice conditioned with 100 cGy were indistinguishable from CBA-Pk-1slc/Pk-1slc mice.
Determination of the number of transduced donor marrow cells required for stable chimerism
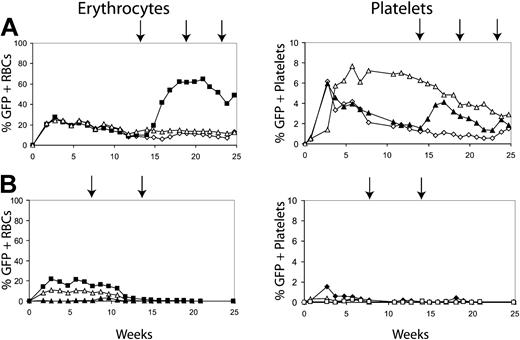
We next determined whether conditioning with 400 cGy could allow a donor graft to be established with fewer transduced marrow cells. Mice underwent transplantation with a range of transduced donor cells. Figure 3 shows the results using either 2 or 4 million transduced donor cells. Figure 3A shows the percentage of GFP-positive erythrocytes and platelets in 3 mice that underwent transplantation with 4 million donor cells. All 3 mice maintained transduced cells past 20 weeks after transplantation. In addition, the transduced cells were responsive to CID, as evidenced by the increase in GFP-positive RBCs in the treated mouse (Figure 3A, solid squares). Three mice that received 2 million cells did not establish donor grafts, as evidenced by the loss of GFP expression in erythrocytes and platelets (Figure 3B), and CID administration failed to induce the reappearance of GFP-positive cells in these mice.
Determining the number of transduced cells required to establish long-term erythrocyte chimerism. Transduced 5-FU-treated marrow cells were injected into mice conditioned with 400 cGy at 2 cell doses, 4 million cells (A) and 2 million cells (B). The percentages of GFP-positive erythrocytes and GFP-positive platelets are displayed. Arrows represent treatment with AP20187 at 10 μg/kg for 3 days by intraperitoneal (IP) injection. Filled symbols represent the GFP-positive erythrocytes and platelets of mice that received AP20187. Open symbols represent mice that did not receive injections of CID. Results indicated that only mice that received 4 million transplanted cells maintained GFP-positive cells and could respond to the CIDs.
Determining the number of transduced cells required to establish long-term erythrocyte chimerism. Transduced 5-FU-treated marrow cells were injected into mice conditioned with 400 cGy at 2 cell doses, 4 million cells (A) and 2 million cells (B). The percentages of GFP-positive erythrocytes and GFP-positive platelets are displayed. Arrows represent treatment with AP20187 at 10 μg/kg for 3 days by intraperitoneal (IP) injection. Filled symbols represent the GFP-positive erythrocytes and platelets of mice that received AP20187. Open symbols represent mice that did not receive injections of CID. Results indicated that only mice that received 4 million transplanted cells maintained GFP-positive cells and could respond to the CIDs.
Improvement of anemia with CID administration
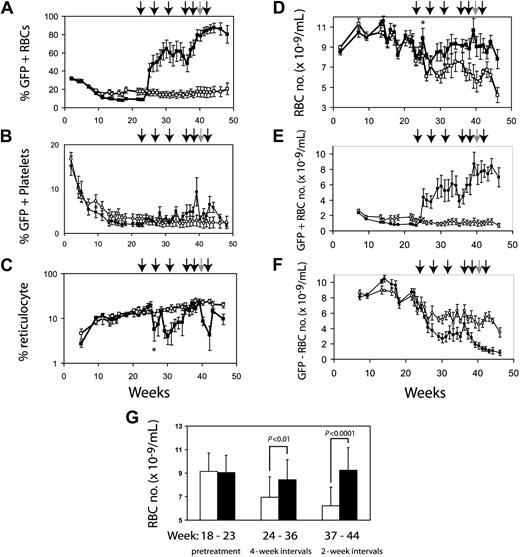
We expected that anemic mice that had undergone reduced-intensity transplantation with F36VmplGFP-transduced normal marrow cells would respond to CID administration with an increase in RBC numbers. Ten CBA-Pk-1slc/Pk-1slc mice irradiated with 400 cGy underwent transplantation with 5 million transduced CBA/N marrow cells. GFP-positive cells from the CBA/N donor persisted long term in all the mice. Initially, all mice showed near normalization of RBC numbers, likely because of the engraftment of short-term repopulating cells from donor CBA/N marrow. By 23 weeks after transplantation, all mice were anemic (RBCs, 7.87 ± 0.69 SEM). At that point the mice were divided into 2 groups, matched for RBC numbers and reticulocyte counts. One group was treated with CIDs, and the control group received injections of the carrier alone without CIDs. The mice that did not receive CIDs had a slow, steady worsening of anemia (Figure 4D), similar to the decline in hematocrit observed in thalassemic mice that underwent transplantation with transduced marrow cells.32 Initial treatment using 5 days of drug every 4 weeks led to clear increases in GFP-positive RBCs with variable responses in individual mice. In general, mice with low percentages of GFP-expressing blood cells were more anemic and exhibited less pronounced responses to CID treatment. At 36 weeks, the frequency of drug administration was increased to 5 days every other week, resulting in a more uniform increase in GFP-positive RBCs (Figure 4A). Platelets showed a modest response to CID treatment (Figure 4B). The reticulocyte percentage decreased in the animals treated with CID (Figure 4C), except for transient increases in reticulocytes immediately after CID administration. There were significant differences in reticulocyte percentages (P < .01) between CID-treated and untreated mice at 30 and 42 weeks. Mice treated with CID had significantly increased RBC number (Figure 4D, G). The average RBC number after week 30 was 6.39 ± 0.88 × 109/mL for control animals and 9.09 ± 0.67 × 109/mL for animals treated with CID (P < .01). Figure 4G demonstrates that with more frequent administration of CIDs, the treated group developed clear improvements in RBC number compared with untreated mice. The sixth CID dose was withheld for 2 mice because of elevated RBC numbers (more than 13 × 109/mL) (Figure 4, gray arrow). After CIDs were withheld, the percentages of GFP-positive RBCs decreased, with a half-life of 56 days (data not shown). The relatively slow decay rate of transduced donor RBCs was likely caused by the relatively long lifespan of this cell population combined with the persistence of CID-induced RBC production for 1 to 2 weeks after CID withdrawal, as we have noted previously.18
CID administration preferentially expands genetically modified erythrocytes. Ten CBA-Pk-1slc/Pk-1slc mice were conditioned with 400 cGy radiation and then infused with 5 million F36VmplGFP-transduced CBA/N marrow cells. The mice were observed for 22 weeks, at which time they were paired based on RBC number and percentage of reticulocytes. The cohort with lower GFP expression (8.6% ± 1.4% vs 17.6% ± 8.1%) was treated with AP20187, 10 μg/kg by IP injection every day for 5 days (▪). Initially, CIDs were administered every 4 weeks. Frequency was increased to every 2 weeks at 34 weeks after transplantation. Each arrow represents a course of CID. Five control mice received injections of the carrier compound at a similar schedule (□). The 40-week injection was given to only 3 of the 5 CID-treated mice because 2 of the mice had more than 13 × 109 RBCs/mL. Panel A demonstrates that the expansion of GFP-positive RBCs is dependent on the administration of CID. GFP-positive platelets are shown in panel B. Effects of CID on reticulocytes are shown in panel C. Panel D demonstrates the differences in the RBC numbers between treated and untreated animals. The absolute numbers of GFP-positive and GFP-negative RBCs are shown in panels E and F, respectively. Panel F shows the decrease in nontransduced RBCs that occurs coincident with CID administration relative to the nontreated control croup. Panel G demonstrates the separation in the 2 groups during the pretreatment phase, the period when the mice were treated every 4 weeks, and the period when the animals were treated every other week. Error bars indicate standard error of the mean, but in panel G they show 1 SD. Significance of the asterisks in panels C and D is discussed in “Results.”
CID administration preferentially expands genetically modified erythrocytes. Ten CBA-Pk-1slc/Pk-1slc mice were conditioned with 400 cGy radiation and then infused with 5 million F36VmplGFP-transduced CBA/N marrow cells. The mice were observed for 22 weeks, at which time they were paired based on RBC number and percentage of reticulocytes. The cohort with lower GFP expression (8.6% ± 1.4% vs 17.6% ± 8.1%) was treated with AP20187, 10 μg/kg by IP injection every day for 5 days (▪). Initially, CIDs were administered every 4 weeks. Frequency was increased to every 2 weeks at 34 weeks after transplantation. Each arrow represents a course of CID. Five control mice received injections of the carrier compound at a similar schedule (□). The 40-week injection was given to only 3 of the 5 CID-treated mice because 2 of the mice had more than 13 × 109 RBCs/mL. Panel A demonstrates that the expansion of GFP-positive RBCs is dependent on the administration of CID. GFP-positive platelets are shown in panel B. Effects of CID on reticulocytes are shown in panel C. Panel D demonstrates the differences in the RBC numbers between treated and untreated animals. The absolute numbers of GFP-positive and GFP-negative RBCs are shown in panels E and F, respectively. Panel F shows the decrease in nontransduced RBCs that occurs coincident with CID administration relative to the nontreated control croup. Panel G demonstrates the separation in the 2 groups during the pretreatment phase, the period when the mice were treated every 4 weeks, and the period when the animals were treated every other week. Error bars indicate standard error of the mean, but in panel G they show 1 SD. Significance of the asterisks in panels C and D is discussed in “Results.”
The increased percentages of GFP-positive RBCs after the initiation of CID treatment preceded a delayed increase in total RBC number. For example, GFP-positive RBCs rose from approximately 5% at baseline to approximately 50% before the second dose of CID (Figure 4A). However, this dramatic rise in genetically modified RBCs was not reflected in an increase in the absolute number of RBCs measured at the same time (Figure 4D). We determined the absolute number of GFP-positive RBCs (Figure 4E) and the absolute number of GFP-negative RBCs (Figure 4F) in the treated and untreated mice. With the initiation of CID treatment, there was a clear increase in GFP-positive RBCs (Figure 4E) and a coincident decrease in GFP-negative RBCs (Figure 4F). This resulted in a large increase in the percentage of GFP-positive RBCs without a proportional increase in total RBC numbers. By increasing the frequency of CID administration, the total number of RBCs in the CID-treated mice rose to levels significantly higher than in the control mice (Figure 4D, G). We conjectured that the suppression of nontransduced RBCs after the initiation of CID treatment might have resulted from an inhibition of erythropoietin-dependent erythropoiesis. This interpretation was supported by an initial increase in total RBC numbers that was detectable at a single time point 3 weeks after the first dose of AP20187 (Figure 4D, asterisk) and was accompanied by a transient decrease in reticulocyte count (Figure 4C, asterisk). These findings suggested that AP20187 induced a surge in genetically modified RBCs and a temporary increase in RBC numbers, resulting in a physiologically relevant decrease in circulating erythropoietin levels, a decrease in reticulocyte count, and a sharp decrease in short-lived PK deficient RBC levels. We proceeded to directly test whether CID administration could suppress circulating levels of erythropoietin.
CID administration decreases erythropoietin levels
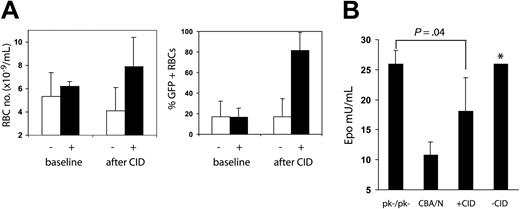
To determine whether CID treatment could reduce erythropoietin levels and, therefore, erythropoietin-dependent erythropoiesis, we measured erythropoietin levels in mice after CID administration. All the surviving mice (2 treated, 4 control) from the group shown in Figure 4 were observed for 12 weeks after the last course of CID, by which time the percentage of GFP-positive RBCs decreased to that at the previous baseline (Figure 5A). Three mice (2 treated, 1 control) were treated with CID for 4 weeks to increase the peripheral blood RBC numbers and the percentage of GFP-positive RBCs (Figure 5A). Mice were then killed, and erythropoietin levels were measured. As shown in Figure 5B, mice that received CID had erythropoietin levels that were intermediate between the levels measured in CBA/N and those measured in CBA-Pk-1slc/Pk-1slc mice, demonstrating that CID administration can suppress erythropoietin levels through the selective expansion of transduced erythrocytes.
Erythropoietin levels decrease with CID administration. Six mice (depicted in Figure 4) were used as samples to measure erythropoietin levels in CID-treated and control mice. RBC numbers were measured 10 weeks after the last CID administration; RBC numbers and the percentage of GFP-positive RBCs had fallen to baseline (A-B, baseline). Three of the 6 mice were treated with 10 μg/kg CID 3 days a week for 2 weeks, then every day for 2 weeks, at which time all mice were killed (week 67 after transplantation). (A) Erythrocyte numbers and the percentage of GFP-positive erythrocytes increased in CID treated mice (+) relative to untreated mice (-). (B) Erythropoietin levels were measured in the 6 mice, 3 CBA/N mice, and 3 CBA-Pk-1slc/Pk-1slc mice (PK-/PK-). Treated mice had erythropoietin levels between those of CBA/N mice and those of CBA-Pk-1slc/Pk-1slc mice. *Data from 1 of the untreated mice with an erythropoietin level of 570 mU/mL was not included in the bar chart.
Erythropoietin levels decrease with CID administration. Six mice (depicted in Figure 4) were used as samples to measure erythropoietin levels in CID-treated and control mice. RBC numbers were measured 10 weeks after the last CID administration; RBC numbers and the percentage of GFP-positive RBCs had fallen to baseline (A-B, baseline). Three of the 6 mice were treated with 10 μg/kg CID 3 days a week for 2 weeks, then every day for 2 weeks, at which time all mice were killed (week 67 after transplantation). (A) Erythrocyte numbers and the percentage of GFP-positive erythrocytes increased in CID treated mice (+) relative to untreated mice (-). (B) Erythropoietin levels were measured in the 6 mice, 3 CBA/N mice, and 3 CBA-Pk-1slc/Pk-1slc mice (PK-/PK-). Treated mice had erythropoietin levels between those of CBA/N mice and those of CBA-Pk-1slc/Pk-1slc mice. *Data from 1 of the untreated mice with an erythropoietin level of 570 mU/mL was not included in the bar chart.
The experiments outlined in Figures 4 and 5 demonstrate that erythropoiesis from normal donor cells can be selectively expanded using CIDs, resulting in CID-dependent increases in RBC number. These findings also indicate that CID treatment can suppress the erythropoietin-dependent generation of PK-deficient RBCs. We cannot exclude the involvement of an erythropoietin-independent process in which CID-responsive increases in GFP-positive RBCs result in the suppression of erythropoiesis arising from the nontransduced RBC compartment.
Further analysis of the 6 surviving animals 67 weeks after transplantation is presented as supplemental data (available at the Blood website; see the Supplemental Materials link at the top of the online article). However, conclusions related to toxicity are limited given the small numbers of experimental animals.
Lack of CID response in leukocytes
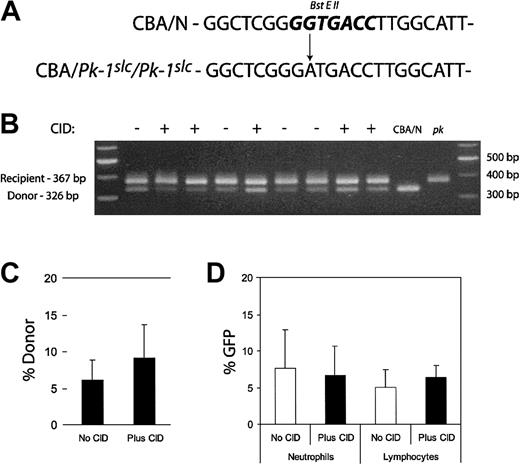
We also examined whether CID administration expanded transduced peripheral blood leukocytes. Because of the relatively large volume of blood required for these studies, leukocytes were evaluated only after a clear effect of CID treatment on RBCs was demonstrated. Retro-orbital blood sampling was performed on the mice presented in Figure 4 at week 44. DNA was purified from the leukocytes. PCR was performed using primers that flanked the mutation responsible for the enzyme deficiency. The mutation resulted in the loss of a BstEII restriction enzyme site, allowing the determination of donor leukocyte contribution (Figure 6A). Resultant bands were separated on an agarose gel, and band intensity was determined (Figure 6B). The average of the donor contribution in CID-treated and untreated mice is shown in the bar graph in Figure 6C. Donor contribution in untreated mice was 6.13%, and in treated mice it was 9.16%. This difference was not significant (P = .28). Leukocytes were also examined for GFP expression by flow cytometry. There was no significant difference in the proportion of neutrophils or lymphocytes that expressed GFP between the treated and untreated groups (Figure 6D). Unlike previous findings in the dog transplantation model,19 lymphocyte expansion was not observed.
Analysis of leukocytes after CID administration. PCR primers were designed to flank the mutation responsible for the PK deficiency. The A>G transition results in the loss of a BstEII site (A, bold). DNA was purified from 50 μL peripheral blood from the experimental mice in Figure 4 at week 44. DNA was also obtained from the peripheral blood of CBA/N and CBA-Pk-1slc/Pk-1slc (pk) mice. This genomic DNA was used as a template for PCR. The resultant product was digested with BstEII and subjected to agarose gel electrophoresis (B). Relative donor contribution was determined based on band intensity (C). Fifty microliters peripheral blood was also used for flow cytometric analysis to determine GFP expression in the leukocytes. Forward and side scatter was used to identify the neutrophil and lymphocyte populations. Percentages of GFP expression in the 2 cell types did not differ significantly between CID-treated and untreated mice (D). Error bars indicate 1 standard deviation.
Analysis of leukocytes after CID administration. PCR primers were designed to flank the mutation responsible for the PK deficiency. The A>G transition results in the loss of a BstEII site (A, bold). DNA was purified from 50 μL peripheral blood from the experimental mice in Figure 4 at week 44. DNA was also obtained from the peripheral blood of CBA/N and CBA-Pk-1slc/Pk-1slc (pk) mice. This genomic DNA was used as a template for PCR. The resultant product was digested with BstEII and subjected to agarose gel electrophoresis (B). Relative donor contribution was determined based on band intensity (C). Fifty microliters peripheral blood was also used for flow cytometric analysis to determine GFP expression in the leukocytes. Forward and side scatter was used to identify the neutrophil and lymphocyte populations. Percentages of GFP expression in the 2 cell types did not differ significantly between CID-treated and untreated mice (D). Error bars indicate 1 standard deviation.
Discussion
A new approach to cell therapy aims to identify methods to specifically control the fate of transplanted cells.33 Hemopoietic growth factors provide a means for controlling cell growth in vivo. However, growth factors lack the ability to selectively expand a subpopulation of genetically modified cells within a particular hemopoietic lineage. Selectivity can be achieved by engineering cells ex vivo so their fate can be controlled in vivo using exogenously supplied small molecules. We have developed a means whereby cell expansion can be directed using CIDs.16 This approach could be advantageous in gene therapy in which only a fraction of cells contain the therapeutic gene or for nonmyeloablative transplantation in which donor and host hemopoietic elements coexist.
In the studies outlined here, selective expansion of normal donor erythrocytes with normal pyruvate kinase activity resulted in improvements in the anemia of the recipient mice. CIDs functioned similarly to erythropoietin, targeting erythroid cells expressing the F36Vmpl fusion protein. Much like the use of growth factors, the dose of CID can be adjusted to achieve the desired level of RBC production (Figure 4E). The combination of reduced intensity conditioning followed by repeated administrations of CID was well tolerated by the CBA-Pk-1slc/Pk-1slc mice. One concern with repeated administrations of CIDs was that transduced progenitors would be deleted. Initial resistance to CID-mediated expansion in some mice was overcome with more frequent administration of CIDs. This resulted in a median RBC number in the normal range for the 5 treated mice. In fact, erythrocytosis developed in 2 of the CID-treated mice with RBC numbers exceeding 13 × 109 RBCs/mL, necessitating a pause in CID treatment. We monitored mice that underwent transplantation for the development of therapy-associated leukemia, as has been reported in other murine gene transfer experiments.34 Marrow from treated and untreated animals appeared morphologically indistinguishable, though one untreated mouse that underwent transplantation did acquire severe anemia 67 weeks after transplantation that was not associated with an increase in GFP-positive cells (Supplemental Materials).
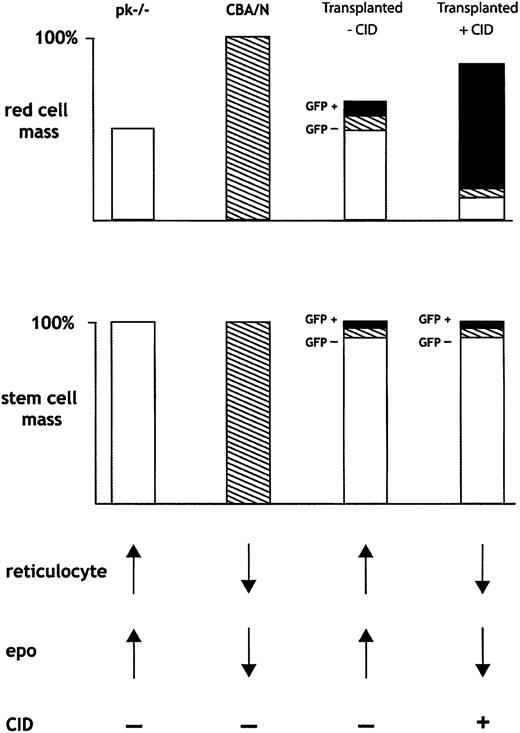
Selectively expanding the numbers of transduced, normal donor RBCs allowed for the expansion of total RBC mass and a consequent suppression of erythropoietin. In this manner, the recipient, abnormal erythropoiesis was further suppressed. Figure 7 provides a schematic overview of the hematologic consequences of CID-mediated expansion of normal donor RBCs. Mice that underwent transplantation resulting in chimerism had 3 populations of marrow stem cells: (1) CBA-Pk-1slc/Pk-1slc recipient cells (PK-/-; open boxes); (2) nontransduced CBA/N donor cells (hatched); and (3) transduced, CID-responsive, CBA/N donor cells (black boxes). In the absence of CIDs, the donor RBCs had a survival advantage and increased relative to recipient cells. CID treatment expanded the transduced RBC population, resulting in a decrease in erythropoietin and a concomitant reduction in abnormal erythropoiesis, further enhancing selection of the normal donor cell population. Nevertheless, we cannot exclude the possibility of an erythropoietin-independent process in which the CID-responsive RBC population directly suppresses production from the nontransduced, CID-insensate, RBC compartment. One erythropoietin-independent process could be the marked splenomegaly that occurs during CID administration and could result in nonspecific RBC sequestration. In the setting of a fixed number of nontransduced RBCs and increasing numbers of GFP-positive RBCs, total numbers of GFP-negative RBCs would appear to decrease. We did not observe prolonged selection after CID withdrawal, arguing against an effect at the stem cell level. This result is in contrast to other selection strategies that rely on cytotoxic agents to select for stem cells that express chemoprotectant proteins from drug resistance genes.32,35,36
Schematic of proposed method whereby CID-mediated expansion of transduced erythrocytes results in in vivo selection. Selective expansion of GFP-positive RBCs results in decreased erythropoietin and decreased production of nontransduced RBCs from donor and recipient cells. See “Discussion” for a complete description of Figure 7.
Schematic of proposed method whereby CID-mediated expansion of transduced erythrocytes results in in vivo selection. Selective expansion of GFP-positive RBCs results in decreased erythropoietin and decreased production of nontransduced RBCs from donor and recipient cells. See “Discussion” for a complete description of Figure 7.
However, drug resistance genes have encountered 2 types of obstacles. First, stem cells and progenitors are relatively resistant to many cytotoxic drugs, resulting in relatively limited in vivo efficacy for drug resistance genes such as dihydrofolate reductase37-39 and MDR1.40,41 Second, the drugs used to achieve selection harbor considerable hematologic and nonhematologic toxicities.39,42,43 In addition to immediate toxicities, the mutagenicity of many cytotoxic agents, most notably alkylating drugs used in association with methylguanine methyltransferase (MGMT), adds a significant burden of secondary malignancies, especially leukemias.44,45
Compared with selection using chemoprotectant proteins, CID-dependent expansion is reversible and does not require the systemic administration of DNA-damaging agents. However, limiting the proliferative response to the progeny of transduced stem cells would require continuous CID administration to maintain the therapeutic effect. Leukemia is generally thought to originate in cells with unlimited capacity for self-renewal, such as B cells, T cells, and hemopoietic stem cells.46 Based on this principle, directing a growth signal to cells with a limitless capacity for self-renewal may raise a greater concern for the development of leukemia than if the growth signal were confined to committed progenitors, which are generally considered to have finite capacity for self-renewal. This view is supported by the abundance of clinical safety data that exist for chronically administered growth factors such as erythropoietin, granulocyte macrophage-colony-stimulating factor (GM-CSF) and G-CSF.47,48 Although the requirement for ongoing CID administration constitutes an inconvenience and poses the risk for long-term drug exposure, the ability to withdraw selection may provide a crucial safety valve if leukemia were to occur. Testing for the development of leukemias in mice treated with CID may require performing secondary transplantation, similar to previous reports of leukemogenesis in mouse transplantation models.34
This method could be applied as adjunct therapy to gene transfer-based therapies for erythrocyte disorders and for transplantation strategies aimed at establishing mixed chimerism. Improvements to this approach include the use of erythrocyte-specific promoters such as spectrin or ankyrin to limit expression of the selectable protein to erythroid progenitors.49-51 In addition, we are testing other signaling molecules, including the JH1 domain of Janus kinase 2 (Jak2), which may provide a more selective signal for directing RBC expansion (Shengming Zhao, M.W., Kenji Ihara, R.E.R., and C.A.B., manuscript submitted, January 2004). The combination of reduced-intensity conditioning with in vivo selection could prove useful for eventual gene therapy for the erythrocyte disorders.32
Prepublished online as Blood First Edition Paper, February 26, 2004; DOI 10.1182/blood-2003-10-3705.
Supported by grants 5R01DK52997, 1R01DK57525, 2P01HL53750, 1P01DK55820, and 2P01DK47754 from the National Institutes of Health and grant MOP-14663 from the Canadian Institutes of Health Research.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank James Yan for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal