Abstract
To derive an efficient system for gene silencing in human hematopoietic stem cells (HSCs) we modified a lentiviral vector for small interfering RNA (siRNA) delivery. For this purpose, an H1 promoter-driven siRNA expression cassette was introduced into a lentiviral vector, and the p53 mRNA was chosen as a target for siRNA-mediated gene silencing. Using the recombinant lentivirus we infected human cord blood-derived CD34+ cells and obtained a transfection efficiency of up to 50%, as determined by expression of enhanced green fluorescent protein (EGFP). In EGFP-positive long-term culture-initiating cell (LTC-IC)- and colony-forming unit cell (CFU-C)-derived cells, we observed a reduction of p53 mRNA of up to 95%. Importantly, this reduction remained stable during several weeks of cell culture. Furthermore, p53 gene silencing resulted in decreased p21 mRNA levels and reduced the sensitivity of CD34+ cells toward the cytotoxic drug etoposide. Thus, lentiviral delivery of siRNA can allow for efficient and stable gene silencing in human HSCs and will be very valuable for further gene function studies. (Blood. 2004;103:4511-4513)
Introduction
Gene silencing by small interfering RNA (siRNA) has become a powerful and rapidly evolving experimental method for studying gene function in mammalian cells.1,2 The use of siRNA in hematopoietic stem cells (HSCs) is limited by the difficulty of delivering RNA or DNA into HSCs by conventional transfection methods. Lentiviral vectors have been shown to efficiently transduce human HSCs.3-6 Lentiviruses are able to infect nondividing primary cells, and transcription from integrated viruses remains stable over time.7,8 Lentiviral vectors have been used to deliver siRNA in some primary mouse and human tissues.9,10 Here we show that efficient gene silencing can be achieved in human HSCs by a lentiviral system designed for delivering siRNA.
Materials and methods
Constructs
To allow efficient transfer of the H1 promoter/siRNA cassette, we introduced a second ClaI site into pSUPER11 by ligating the adaptor 5′-AATTATCGATGTTGTAAAAC-3′ and 5′-AATTGTTTTACAACATCGAT-3′ into the unique EcoRI site. The template for human p53 siRNA was generated by ligating the annealed primers 5′-GATCCCCGACTCCAGTGGTAATCTACTTCAAGAGAGTAGATTACCACTGGAGTCTTTTTGGAAC-3′ and 5′-TCGAGTTCCAAAAAGACTCCAGTGGTAATCTACTCTCTTGAAGTAGATTACCACTGGAGTCGGG-3′ into the BglII and HindIII sites of pSUPER. As a nonrelevant control we used the primers 5′-GATCCCCCTGGCATCGGTGTGGATGATTCAAGAGATCATCCACACCGATGCCAGTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAACTGGCATCGGTGTGGATGATCTCTTGAATCATCCACACCGATGCCAGGGG-3′, which were derived from the mouse SMAD4, but were nonfunctional (ie, did not change SMAD4 mRNA levels) in mouse and human cells (data not shown). The expression cassette for p53 siRNA was excised from the modified pSUPER as a ClaI fragment and subcloned into the ClaI site of the lentiviral vector pWPXL. All constructs were verified by sequence analysis. Lentiviral production with pWPXL, the envelope vector pMD.G, and the packaging vector pCMVR8.91 (all kindly provided by Dr Didier Trono, University of Geneva) were carried out as described before.6,12,13
Cell culture and virus infection
293T cells were seeded in 6-well plates (3 × 105 cells per well) and after 24 hours were incubated with concentrated virus for 6 hours. The medium was changed and the cells were grown for the indicated times. Human cord blood CD34+ cells were purified as previously described.6 CD34+ cells were seeded in a 96-well plate at 1 × 105 cells per well and virus was added twice for 6 hours with a multiplicity of infection (MOI) of 10 to 30. Enhanced green fluorescent protein (EGFP)-positive cells were isolated using a fluorescence-activated cell-sorter (FACSVantage; Becton Dickinson Biosciences, San Diego, CA) and cultured in methylcellulose as described before14 or maintained in liquid culture in Iscoves modified Dulbecco medium (IMDM) and 10% fetal calf serum supplemented with human recombinant Flt-3 ligand (Amgen, Seattle, WA) and PEGylated megakaryocyte growth and development factor (MGDF; Amgen, Thousand Oaks, CA). Growth factors were used at concentrations of 50 ng/mL for MGDF and 100 ng/mL for Flt-3 ligand. Etoposide (Sigma-Aldrich, St Louis, MO) was used at 25 nM in methylcellulose cultures and at 20 μM for induction of apoptosis. Long-term culture-initiating cell (LTC-IC) cultures were performed as described.14
Western blot analysis
p53 protein was detected by immunoblot using the anti-p53 rabbit polyclonal antibody FL 393 (Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were reprobed using the monoclonal anti-β-actin antibody AC-15 (Sigma-Aldrich). Densitometry was performed on a ChemiImager 5500 (Alpha Inotech, San Leandro, CA) using the Alpha Ease Software.
Real-time
Total RNA (2 μg) from EGFP-positive CD34+ cells at days 7 and 20 of liquid culture was isolated with Trizol (Invitrogen, Carlsbad, CA) and reverse transcribed after random hexamer priming. Reverse transcriptase-polymerase chain reaction (RT-PCR) was carried out using the SYBR Green PCR Master Mix chemistry (Applied Biosystems, Warrington, United Kingdom). Primers for p53 were 5′-TTCACCCTTCAGATCCGTGG-3 and 5′-CAGCTCTCGGAACATCTCGAA-3′, for p21 5′-GGCAGACCAGCATGACAGATT-3′ and 5′-AGAAGATCAGCCGGCGTTT-3′. The conditions for RT-PCR of the ribosomal protein L19 (RPL19) were described elsewhere.15 All reactions were run in duplicate using the ABI 7000 Sequence Detection System (Applied Biosystems, Foster City, CA).
Quantilification of adoptosis by flow cytometry
Apoptosis was assessed using the annexin V-phycoerythrin (PE) Apoptosis Detection Kit I (Becton Dickinson Biosciences).
Results and discussion
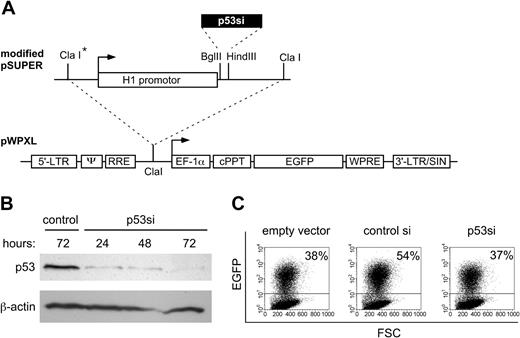
Since lentiviruses can efficiently transduce human hematopoietic cells,13 we tested whether the lentiviral vector pWPXL can be used for siRNA-mediated gene silencing in human HSCs. To allow for a convenient transfer of a cassette comprising the H1 promoter and the siRNA template into pWPXL, we modified the polylinker of the vector pSUPER by introducing a second ClaI site (Figure 1A). As a target gene we chose the p53 mRNA, because the conditions for efficient p53 gene silencing have already been established in other cell types.11 The resulting lentiviral vector pWPXL-p53si was packaged and first tested on 293T cells, a human embryonal kidney-derived cell line. p53 protein expression was clearly reduced in cells infected with pWPXL-p53si, compared with parental 293T cells (Figure 1B). Densitometric analysis at 72 hours after infection revealed that p53 protein was reduced by pWPXL-p53si to 17% of the control. Since 293T cells express high levels of p53 protein due to the presence of simian virus 40 large T antigen, which stabilizes and inactivates p53, the observed reduction of protein levels by pWPXL-p53si should be considered as very effective.
Construction of a lentiviral vector for the expression of siRNA. (A) The template DNA for p53 siRNA (p53si) was directionally inserted into the Bgl II and HindIII sites of pSUPER. A second ClaI restriction site (asterisk) was created by adaptor ligation. The complete p53si expression cassette was excised from the modified pSUPER as a ClaI fragment and inserted into the lentiviral vector pWPXL (dotted line). LTR indicates long terminal repeat; ψ, psi packaging signal; RRE, Ref-responsive element; cPPT, central polypurine tract; EF1-α, human elongation factor alpha; EGFP, enhanced green fluorescent protein; WPRE, posttranscriptional cis-acting regulatory element of the woodchuck hepatitis virus; and LTR/SIN, self-inactivating 3′ long terminal repeat. (B) Western analysis of p53 protein expression in 293T cells. Control indicates nontransduced 293T cells; p53si, 293T cells transduced with pWPXL-p53si. (C) Assessment of lentiviral infection efficiency. CD34+ cells infected with pWPXL (empty vector), pWPXL-control-si (control si), or pWPXL-p53si (p53si) were analyzed for EGFP expression by flow cytometry. Cutoff used for cell sorting of EGFP-positive cells is shown (horizontal lines). FSC indicates forward side scatter. The percentages of EGFP-positive cells are given.
Construction of a lentiviral vector for the expression of siRNA. (A) The template DNA for p53 siRNA (p53si) was directionally inserted into the Bgl II and HindIII sites of pSUPER. A second ClaI restriction site (asterisk) was created by adaptor ligation. The complete p53si expression cassette was excised from the modified pSUPER as a ClaI fragment and inserted into the lentiviral vector pWPXL (dotted line). LTR indicates long terminal repeat; ψ, psi packaging signal; RRE, Ref-responsive element; cPPT, central polypurine tract; EF1-α, human elongation factor alpha; EGFP, enhanced green fluorescent protein; WPRE, posttranscriptional cis-acting regulatory element of the woodchuck hepatitis virus; and LTR/SIN, self-inactivating 3′ long terminal repeat. (B) Western analysis of p53 protein expression in 293T cells. Control indicates nontransduced 293T cells; p53si, 293T cells transduced with pWPXL-p53si. (C) Assessment of lentiviral infection efficiency. CD34+ cells infected with pWPXL (empty vector), pWPXL-control-si (control si), or pWPXL-p53si (p53si) were analyzed for EGFP expression by flow cytometry. Cutoff used for cell sorting of EGFP-positive cells is shown (horizontal lines). FSC indicates forward side scatter. The percentages of EGFP-positive cells are given.
We next examined whether pWPXL-p53si can inhibit p53 expression in human cord blood-derived CD34+ cells. An advantage of pWPXL is that infected cells can be detected by presence of EGFP. Using concentrated virus, up to 54% of ow cytometry cells were EGFP positive, as determined by fl the CD34+ (Figure 1C). Silencing of p53 gene expression was assessed in infected cells sorted for EGFP expression. Since p53 protein in CD34+ cells was undetectable by Western analysis (data not shown), we used quantitative real-time PCR to assess the expression of p53 mRNA. In cells sorted for EGFP and CD34, we found that p53 mRNA levels were reduced to 3% (Figure 2A), whereas p53 mRNA levels in the EGFP-negative cell fraction remained unchanged (not shown). After 5 weeks in liquid culture, more than 90% of cells remained EGFP positive and 2% to 4% were CD34+/EGFP+ (not shown). In sorted CD34+/EGFP+ cells, p53 expression was 11% of the controls (Figure 2A). These results demonstrate persistent gene silencing over time.
Gene silencing by lentiviral delivery of p53 siRNA. (A) Reduction of p53 mRNA expression by siRNA. CD34+ cells were infected and sorted for EGFP-positive cells. Expression of p53 mRNA was assessed by quantitative RT-PCR at the day of sorting (left panel) and after 5 weeks of liquid culture (right panel). The value for the control cells infected with pWPXL virus (▪) was set as 100% and compared with cells infected with the pWPXL-control-si virus (▦) and the pWPXL-p53si virus (□). Error bars indicate the SEM when triplicate measurements were done. (B) Sorted CD34+/GFP+ cells were cultured in methylcellulose and single colonies were picked after 14 days for detection of p53 mRNA. Note that ΔCT (cycles threshold) represents a binary logarithmic scale and higher numbers correspond to lower levels of p53 expression. Each dot represents the result obtained from one single colony. transd. indicates transduced. (C) Effects of etoposide on CFU-C formation. Sorted CD34+/EGFP+ cells were grown in methylcellulose in the presence (+) or absence (-) of etoposide (25 nM). Error bars indicate SEM of triplicate cultures. * indicates a statistically significant difference (P < .01). (D) Measurement of etoposide-induced apoptosis by flow cytometry. CD34+ cells infected with pWPXL-p53si or the empty vector were sorted for EGFP expression by FACS and cultured for 4 days in liquid culture. After exposure to 20 μM etoposide (Eto) for 6 hours, apoptosis was measured by staining with PE-coupled annexin V. The percentages of Annexin V/EGFP-double-positive cells are given. (E) p53 target gene expression in silenced versus control cells. p21 mRNA levels were determined by real-time PCR in sorted EGFP-positive cells after 10 days in liquid culture. Annotation as in panel A. (F) Silencing of p53 expression in sorted CD34+/GFP+ cells after 5 weeks of LTC-IC stroma-cell culture. Annotation as in panel A. (G) Silencing of p53 expression in LTC-IC-derived colonies. Annotation as in panel B.
Gene silencing by lentiviral delivery of p53 siRNA. (A) Reduction of p53 mRNA expression by siRNA. CD34+ cells were infected and sorted for EGFP-positive cells. Expression of p53 mRNA was assessed by quantitative RT-PCR at the day of sorting (left panel) and after 5 weeks of liquid culture (right panel). The value for the control cells infected with pWPXL virus (▪) was set as 100% and compared with cells infected with the pWPXL-control-si virus (▦) and the pWPXL-p53si virus (□). Error bars indicate the SEM when triplicate measurements were done. (B) Sorted CD34+/GFP+ cells were cultured in methylcellulose and single colonies were picked after 14 days for detection of p53 mRNA. Note that ΔCT (cycles threshold) represents a binary logarithmic scale and higher numbers correspond to lower levels of p53 expression. Each dot represents the result obtained from one single colony. transd. indicates transduced. (C) Effects of etoposide on CFU-C formation. Sorted CD34+/EGFP+ cells were grown in methylcellulose in the presence (+) or absence (-) of etoposide (25 nM). Error bars indicate SEM of triplicate cultures. * indicates a statistically significant difference (P < .01). (D) Measurement of etoposide-induced apoptosis by flow cytometry. CD34+ cells infected with pWPXL-p53si or the empty vector were sorted for EGFP expression by FACS and cultured for 4 days in liquid culture. After exposure to 20 μM etoposide (Eto) for 6 hours, apoptosis was measured by staining with PE-coupled annexin V. The percentages of Annexin V/EGFP-double-positive cells are given. (E) p53 target gene expression in silenced versus control cells. p21 mRNA levels were determined by real-time PCR in sorted EGFP-positive cells after 10 days in liquid culture. Annotation as in panel A. (F) Silencing of p53 expression in sorted CD34+/GFP+ cells after 5 weeks of LTC-IC stroma-cell culture. Annotation as in panel A. (G) Silencing of p53 expression in LTC-IC-derived colonies. Annotation as in panel B.
To determine whether colony-forming unit cell (CFU-C) progenitors can be targeted, we plated sorted CD34+/EGFP+ cells in methyl cellulose. By real-time PCR, we observed an 8- to 10-fold decrease in p53 mRNA in single colonies derived from p53sitransduced progenitors (Figure 2B). Cells that have lost p53 function are less sensitive to agents that normally cause apoptosis.16-18 A reduction in, but not complete resistance to, apoptosis can be expected in cells transduced with p53 siRNA, as cells from p53 knock-out mice and human p53-deficient cell lines remained partially sensitive to cytotoxic drugs, such as etoposide, indicating that apoptosis can still be induced through p53-independent pathways.18-20 To provide evidence that gene silencing by pWPXL-p53si interfered with p53 function, we measured apoptosis in response to etoposide. CD34+ cells infected with p53si, control-si, and empty vector were EGFP-sorted and plated in semisolid media in the presence or absence of 25 nM etoposide, and colonies were counted after 14 days. In the presence of etoposide, CD34+ cells infected with the empty vector yielded 6%, and with control-si vector yielded only 3%, of colonies that were observed in the absence of etoposide. In contrast, pWPXL-p53si-infected CD34+ cells were more resistant to etoposide, as 36% of colonies survived in the presence of etoposide (Figure 2C). To demonstrate that this difference in survival is due to apoptosis, we exposed CD34+ cells in liquid culture to etoposide and assessed apoptosis by cell surface expression of annexin V. CD34+ cells infected with the pWPXL-p53si virus displayed reduced sensitivity to etoposide-induced apoptosis than the controls: the percentage of annexin-positive cells in pWPXL-p53si-infected cells increased by only 17%, compared with an increase of 31% in the controls (Figure 2D). To further verify that the observed effects result from p53 gene silencing, we analyzed the expression levels of p21 mRNA, a p53 transcriptional target, in the EGFP-positive fraction of the virus infected cells by real-time PCR. We found that in p53-silenced cells the levels of p21 mRNA were reduced to 16% of the controls (Figure 2E). Thus, p53 gene silencing by siRNA in human CD34+ cells resulted in the expected reduction of p53 function.
To demonstrate that not only CFU-Cs but also earlier hematopoietic progenitors can be transduced by our vectors, we analyzed p53 expression in sorted CD34+/EGFP+ cells grown for 5 weeks on feeder cells under LTC-IC conditions and in single methyl cellulose colonies derived from LTC-ICs. After 5 weeks of culture, more than 90% of cells remained EGFP positive and 0.3% were CD34+/EGFP+ (not shown). We sorted these CD34+/EGFP+ cells and found p53 mRNA expression to be reduced to 9% in pWPXL-p53si-transduced cells (Figure 2F). Furthermore, single methylcellulose colonies derived from LTC-ICs displayed an up to 16-fold reduction in p53 mRNA (Figure 2G). These results show that early hematopoietic progenitors of the LTC-IC type were transduced and that siRNA expression was persistent in LTC-IC-derived cells.
In summary, we demonstrate that lentiviral delivery of siRNA can be used for efficient and stable gene silencing in human hematopoietic progenitors. This system will be very valuable to study the function of key regulatory genes in human hematopoiesis.
Prepublished online as Blood First Edition Paper, February 26, 2004; DOI 10.1182/blood-2003-07-2397.
Supported by the Swiss National Science Foundation (3100-066 949.01, 3100-67072.01), the Swiss Cancer League (OCS-01163-09-2001, OCS-01282-08-2002), and the Stiftung für Hämatologische Forschung.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Silvia Sendelov and Verena Dalle Carbonare for technical assistance; Verena Jäggin for cell sorting; Robert Kralovics for helpful comments on the manuscript; Didier Trono for the lentiviral vectors; and Siegfried Heinzl for providing cord blood samples.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal