Abstract
The precise role of intravascular tissue factor (TF) remains poorly defined, due to the limited availability of assays capable of measuring circulating TF procoagulant activity (PCA). As a model of inflammation-associated intravascular thrombin generation, we studied 18 volunteers receiving an infusion of endotoxin. A novel assay that measures microparticle (MP)-associated TF PCA from a number of cellular sources (but not platelets) demonstrated an 8-fold increase in activity at 3 to 4 hours after endotoxin administration (P < .001), with a return to baseline by 8 hours. TF antigen-positive MPs isolated from plasma were visualized by electron microscopy. Interindividual MP-associated TF response to lipopolysaccharide (LPS) was highly variable. In contrast, a previously described assay that measurestotal (cell and MP-borne) whole-blood TF PCA demonstrated a more modest increase, with a peak in activity (1.3-fold over baseline; P < .000 01) at 3 to 4 hours, and persistence for more than 24 hours. This surprisingly modest increase in whole-blood TF activity is likely explained by a profound although transient LPS-induced monocytopenia. MP-associated TF PCA was highly correlated with whole-blood TF PCA and total number of circulating MPs, and whole-blood TF PCA was highly correlated with TF mRNA levels. (Blood. 2004;103:4545-4553)
Introduction
Tissue factor (TF), a 47 kDa transmembrane glycoprotein, is the principal activator of coagulation in vivo. The procoagulant activity (PCA) of factor VIIa (FVIIa) is markedly enhanced after binding to TF, with the subsequent activation of factors IX and X. Current evidence suggests that basal activation of the coagulation system in vivo is mediated by the TF/FVIIa complex. Specifically, plasma levels of factors IX and X activation peptides are markedly suppressed following administration of a potent monoclonal antibody to TF in normal chimpanzees.1,2 Furthermore, endotoxin-induced activation of coagulation in these animals can be blocked by the same monoclonal antibody, indicating that this response is also mediated via the TF pathway.3
These observations do not address whether contact between TF and FVIIa occurs in the intravascular or extravascular compartment, either at baseline or during endotoxemia. TF antigen is not normally detectable in endothelial cells or monocytes in vivo.4,5 Despite the demonstrated ability of these cells to synthesize TF following exposure to endotoxin in vitro,6,7 TF antigen expression could only be demonstrated in splenic endothelial cells in baboons infused with lipopolysaccharide (LPS).8 However, recent evidence suggests that intravascular TF may be important in both the initiation and propagation phases of coagulation.9-12 In these studies, whole blood was shown to contain circulating TF-bearing microparticles (MPs) derived from leukocytes, which may be transferred to a growing platelet-rich thrombus. Numerous studies have documented the presence of intravascular TF-bearing MPs in selected disease states.13-16 TF may also be present in the alpha-granules of circulating platelets.17
MPs are derived from cells undergoing activation or apoptosis. The most rigorous criteria for definition of MPs are by size (< 1.0 μm-1.5 μm), and by expression of negatively charged phosphatidylserine in the outer leaflet of the membrane bilayer.18 The physiologic and pathophysiologic functions of these MPs have yet to be defined, although evidence suggests that they may be able to facilitate cell-cell cross-talk.19,20
The purpose of this study was to characterize the time course of intravascular TF expression in both the cellular and MP fractions of blood during human endotoxemia, an accepted model of the early coagulation/inflammatory changes during sepsis.21 Whole-blood TF PCA was measured using a previously described method.22,23 This assay is very sensitive and specific for TF PCA and avoids the potential for artifactual increase in TF activity, which may occur during the isolation of monocytes.24 However, since all blood cells are converted to subcellular fragments and MPs by freeze-thawing, the assay does not distinguish cell-borne TF from MP-associated TF. We therefore developed a new assay that specifically quantifies MP-associated TF PCA in platelet-free plasma (PFP). Our data suggest that after endotoxin exposure, there is an early and robust increase in MP-associated TF PCA, accompanied by a more modest but sustained elevation in whole-blood TF PCA.
Materials and methods
Materials
LPS from Escherichia coli 055:B5 and A23187 were from Sigma Chemical (St Louis, MO). Human FVIIa and FX were from Enzyme Research Laboratories (South Bend, IN). S2756 was purchased from Chromogenix (Molndal, Sweden). Immunoglobulin M (IgM) monoclonal mouse antihuman fibroblast surface protein (1B10), mouse IgM isotype control for 1B10, and alkaline phosphatase-conjugated goat antimouse IgM were from Sigma. Rhodamine red-X goat antimouse IgG plus IgM heavy and light chain [H+L], fluorescein isothiocyanate (FITC)-conjugated goat antirabbit IgG (H+L), FITC-labeled goat antimouse IgG plus IgM (H+L), 12 nm colloidal gold goat antimouse IgG plus IgM (H+L), and 6 nm colloidal gold donkey antirabbit IgG (H+L) antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). CY5-annexin V and anti-CD144 (VE cadherin) for endothelial cells (55-7H1) were from Pharmingen (BD Biosciences, Lexington, KY), phycoerythrin (PE)-labeled anti-CD14 (IgG1) was from Biosource (Camarillo, CA), and labeled isotype control antibodies (IgG1-FITC and IgG1-PE) were from R&D Systems (Minneapolis, MN). FITC-labeled monoclonal antibody (MoAb) against TF (CJ4068) was obtained from American Diagnostica (Greenwich, CO). The rabbit polyclonal blocking antibody to TF (and preimmune IgG control) have been previously described.25 Innovin (recombinant relipidated human TF) was from Dade (Dade International, Miami, FL).
Study design and subjects
The study design was approved by the Institutional Human Subjects Committee of Vienna University. Written informed consent was obtained from all participants. Criteria for selection of volunteers and study protocol were as previously described.26-28 Eighteen healthy male volunteers ages 18 to 35 (body mass index range: 19 to 27.4 kg/m2 ) were invited to participate in this study. These subjects were admitted to the study ward after an overnight fast. After baseline blood sampling, 2 ng LPS/kg body weight (national reference endotoxin, Escherichia coli; USP Convention, Rockville, MD) was administered as an intravenous infusion over 1 to 2 minutes. Venous blood samples were collected into EDTA [ethylenediaminetetraacetic acid] Vacutainer tubes (Becton Dickinson, San Jose, CA) at baseline (3 hours before) and at 1, 2, 3, 4, 8, and 24 hours after LPS administration, applying minimal venostasis. After discarding the first 2 mL, aliquots were transferred immediately to polypropylene tubes and frozen at -70°C for later assay of whole-blood TF PCA. Platelet-free plasma (PFP) was obtained by double centrifugation, first at 1200g for 15 minutes, and subsequently at 12 000g for 12 minutes at 20°C. PFP was stored at -70°C for future assay of MP-associated TF PCA, as described under “Capture assay for MP-associated TF PCA.”
Healthy volunteer samples, prepared and stored identically, were used for comparison. These individuals had similarly not taken any medication for at least 10 days prior to blood collection.
In vitro stimulation of whole blood with LPS
Following informed consent, heparinized whole blood was collected from healthy donors using a 19-gauge needle (after discarding the first 3 mL). Whole-blood samples were stimulated with LPS (10 ng/mL) at 37°C, with gentle rocking, for up to 24 hours. PFP was then obtained by sequential centrifugation as described above.
Cell cultures
Human umbilical vein endothelial cells (HUVECs) were isolated and cultured as previously described.29 Human dermal microvascular endothelial cells (MVECs) were isolated from newborn human foreskin and cultured as previously described.30 A culture of human blood outgrowth endothelial cells (BOECs) was established from venous blood of a healthy donor from which buffy coat mononuclear cells were isolated using Histopaque-1077 (Sigma Chemical), as previously described.31 Human aortic smooth muscle cells and human skin fibroblasts were from ATCC (Manassas, VA). Smooth muscle cells were routinely identified by their appearance on light microscopy, and by immunostaining with antibody (1:200 dilution) to human smooth muscle actin (Oncogene Research Products, Boston, MA).
Peripheral blood cell isolation
Platelets were obtained from citric acid-citrate-dextrose (CACD)-anticoagulated blood by centrifugation at 130g for 20 minutes. PRP was then diluted 1:1 with 0.1 M CACD, pH 6.5 and centrifuged at 830g for a further 20 minutes. The pelleted platelets were washed in CACD and resuspended in HEPES buffer. Erythrocytes, mononuclear, and polymorphonuclear (PMN) cells were isolated from EDTA-anticoagulated blood using PMN isolation medium (Robbins Scientific, Sunnyvale, CA) by density separation. Monocytes were isolated by CD14 immunomagnetic beads and a magnetic column (MACS Miltenyi) according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA).
Cellular antigen detection by flow cytometry
Binding of 1B10 antibody to cells of interest was assessed by flow cytometry. Briefly, 106 cells were incubated with 1B10 or control murine IgM monoclonal antibody in the dark at room temperature for 30 minutes, washed once, and incubated with FITC-labeled goat antimouse IgG plus IgM. After washing twice with phosphate-buffered saline (PBS) and fixing with 2% paraformaldehyde, samples were acquired on a Becton Dickinson FACS Calibur and analyzed using Cellquestpro software. Mean fluorescent intensity (MFI) of the positive cell populations for 1B10 antigen were recorded.
Generation of microparticles following in vitro cellular activation
Cultured fibroblasts and smooth muscle cells, and isolated platelets and red blood cells were induced to generate MPs by exposure to calcium ionophore (10 μM A23 187) for 10 minutes.32,33 Control cells were exposed to vehicle alone. Supernatants were then removed and subjected to double centrifugation to remove contaminating intact cells. Similarly, MPs were harvested from the supernatant of HUVECs following exposure to 10 ng/mL tumor necrosis factor α (TNF-α) (or vehicle control) for 4 hours,34 and from monocytes following exposure to 10 ng/mL LPS (or vehicle control) for 4 hours.35 The MP-rich supernatant was stored at -80°C for assay of MP-associated TF PCA. Baseline MP-associated TF PCA was defined as the TF activity in the supernatant of cells that had been exposed to neither activator nor relevant vehicle control.
Isolation of MPs from PFP
PFP was diluted (1:400) with 0.2 μ-filtered wash buffer (10 mM HEPES, 144 mEq NaCl, 5.3 mEq KCl, 2.5 mM CaCl, 0.5% bovine serum albumin [BSA], and 0.01% sodium azide, pH 7.4) and ultracentrifuged twice at 100 000g for 60 minutes at 20°C. After careful removal of all but 1 milliliter of the supernatant, MPs were then resuspended by gentle vortexing and pipetting. MP suspensions were stored at -80°C for flow cytometry.
Flow cytometric analysis of MPs
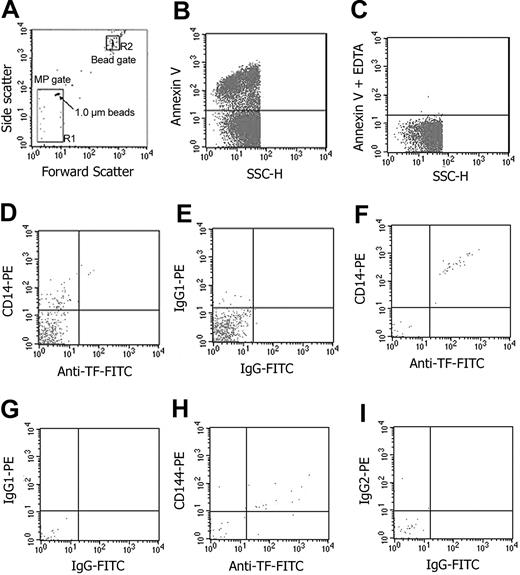
Samples of MPs isolated from blood were analyzed by flow cytometry using a Becton Dickinson FACS Calibur, and analyzed using Cellquestpro software (San Jose, CA) using described methods.36 For MP quantification, we used an internal standard consisting of calibrated polypropylene beads (Bangs Laboratories, Fisher, IN) added to the sample before acquisition. A known quantity (250 000) of 7.2 μm beads was added to each sample and acquisition was terminated after 10 000 beads were counted. Events falling in the predefined MP gate (ie, ≤ 1.0 μm; Figure 1A) that were annexin V-positive (Figure 1B) were defined and enumerated as MPs. Similar enumeration of cell-derived MPs was performed on blood samples that been stimulated with LPS both in vitro and in vivo. The number of MPs per mL of plasma was calculated as described elsewhere.36 To enumerate total MPs and monocyte-derived MPs, aliquots of the isolated washed MPs were incubated with Cy5-labeled annexin V-positive anti-CD14-PE (Figure 1D,F). For each sample, control labeling was performed in parallel by incubating an isotype control MoAb or annexin V Cy5 in EDTA-containing buffer (to prevent Ca2+-dependent annexin V binding). Endothelial cell-derived MPs were similarly defined as those events that were specifically labeled with both annexin V Cy5 and anti-CD144-PE (Figure 1H).
Flow cytometric analysis and quantification of MPs in both ex vivo LPS-treated whole blood and in vivo endotoxin volunteers. Determination of forward (FSC) and side-scatter (SSC) characteristics of light by 1.0-μm latex beads in buffer was used to establish the MP (or R1) gate (A). The R2 (or bead) gate includes 7.2-μm latex beads used for enumeration of MPs as described in “Materials and methods.” Detection of phosphatidyl-serine (PS)-positive MPs by annexin V-Cy5 labeling on the y-axis in relation to light scatter (SSC) on the x-axis (B). Determination of the limit for negative fluorescence, performed in the presence of EDTA, was performed as a negative control for annexin V (C). MPs isolated from whole blood following ex vivo stimulation by LPS are shown in panels D and E. In the remaining panels (F-I), MPs isolated from whole blood of volunteers exposed to endotoxin are shown. In all cases, MPs were triple-labeled for annexin V (not shown) with anti-CD14-PE (y-axis) and anti-TF-FITC (x-axis) (D,F), or anti-CD144-PE (y axis) and anti-TF-FITC (x-axis) (H) or relevant isotype control IgG-PE (y-axis) and IgG-FITC (x-axis) (E,G,I).
Flow cytometric analysis and quantification of MPs in both ex vivo LPS-treated whole blood and in vivo endotoxin volunteers. Determination of forward (FSC) and side-scatter (SSC) characteristics of light by 1.0-μm latex beads in buffer was used to establish the MP (or R1) gate (A). The R2 (or bead) gate includes 7.2-μm latex beads used for enumeration of MPs as described in “Materials and methods.” Detection of phosphatidyl-serine (PS)-positive MPs by annexin V-Cy5 labeling on the y-axis in relation to light scatter (SSC) on the x-axis (B). Determination of the limit for negative fluorescence, performed in the presence of EDTA, was performed as a negative control for annexin V (C). MPs isolated from whole blood following ex vivo stimulation by LPS are shown in panels D and E. In the remaining panels (F-I), MPs isolated from whole blood of volunteers exposed to endotoxin are shown. In all cases, MPs were triple-labeled for annexin V (not shown) with anti-CD14-PE (y-axis) and anti-TF-FITC (x-axis) (D,F), or anti-CD144-PE (y axis) and anti-TF-FITC (x-axis) (H) or relevant isotype control IgG-PE (y-axis) and IgG-FITC (x-axis) (E,G,I).
Capture assay for MP-associated TF PCA
1B10 antibody (50 μL; 40 μg/mL in PBS) was added to each well of a 96-well microtiter plate (Nunc MaxiSorp; Fisher Scientific, Hanover Park, IL) and incubated overnight at 4°C. After 2 washes with PBS, 365 μLof 3% bovine serum albumin in PBS was added to each well, and incubated overnight at 4°C.
Sample (PFP or conditioned cell supernatant; 50 μL) was added to each well and incubated for 24 hours at 4°C. Plates were then washed 4 times with 360 μL/well PBS. Tissue factor activity associated with the captured MPs was evaluated by the activation of FX (150 nM) by FVIIa (5 nM) in the presence of 5 mM CaCl2. After a 6-hour incubation at 37°C, the reaction was terminated by the addition of 25 mM EDTA (25 μL). The prewarmed S-2765 chromogenic substrate for FXa was then added at a final concentration of 1.47 mM and incubated for one hour at 37°C. The absorbance was measured in an enzyme-linked immunosorbent assay (ELISA) plate reader at 405 nm. A standard curve was constructed using known concentrations of FXa incubated for one hour with the chromogenic substrate. Confirmation that the PCA was indeed due to TF was established by including an inhibitory polyclonal antibody to TF in separate duplicate wells prior to addition of factors VIIa and X. For all samples, residual “nonspecific” PCA (usually < 5%) was subtracted from “total” PCA, and the remaining activity was taken as a measure of TF-dependent PCA.
Electron microscopy (EM) and immunogold labeling of MPs
The MP pellet was prefixed with 2% glutaraldehyde, postfixed with 2% OsO4, and then washed and dehydrated in graded alcohols. The pellet was then embedded in Epon resin. Sections were cut and photographed on a Philips 400 T electron microscope (Phillips Electronic Instruments, Mahwah, NJ).
For immunogold labeling, the MP pellet was resuspended in wash buffer and incubated with a rabbit polyclonal anti-TF antibody (1:500 dilution), followed by 6 nm antirabbit IgG gold (1:250 dilution). After immunolabeling, the samples were layered for 10 minutes on 0.1% polylysine-treated, formbar-coated grids. Grids with adherent vesicles were rinsed in PBS and fixed with 0.1% glutaraldehyde in PBS. Grids were then examined in the electron microscope. For CD14 labeling of MPs, the pellet was incubated with a mouse anti-CD14 IgG antibody (1:100 dilution), followed by 12 nm antimouse IgG (1:100). For 1B10 labeling of MPs, the pellet was incubated with 1B10 antibody (1:250), followed by 12 nm gold-labeled antimouse IgM (1:100). For double-labeling to TF and 1B10 or CD14, anti-TF antibody followed by 1B10 or anti-CD14 antibody were sequentially added. Prior to addition of the secondary antibodies, grids were washed 3 times with PBS. The specificity of immunolabeling was verified using control rabbit IgG, mouse IgG, mouse IgM, or secondary gold-tagged antibody alone.
Whole-blood TF PCA
Whole-blood TF PCA was assayed on frozen whole-blood samples exactly as previously described,22,23 except for the use of a commercially available thromboplastin, Innovin, in place of relipidated human brain TF as the standard in the 2-stage clotting assay. The concentration of TF apoprotein in the Innovin reagent was first measured using a previously described ELISA assay.25 Repeated estimates of the reagent lot utilized in these experiments gave a value of 93.5 ng/mL.
Whole-blood TF mRNA expression
TF mRNA expression was performed according to Franco et al37 with modifications. After preparing total RNA with the QuiAmp RNA easy kit (Quiagene, Valencia, CA), mRNA was directly transcribed into cDNA using the RT-Reagent kit (Applied Biosystems, Foster City, CA) and stored at -80°C until analysis. For TF mRNA quantification the Abi Prism 7700 (Applied Biosystems) was used. Primers were designed by Primer Express Software (Applied Biosystems) and synthesized based on the human TF cDNA sequence (Applied Biosystems): 680F 5-CCCGAACAGTTAACC GGAAGA-3 and 773R 5-GCTCCAATGATGTAGAATATTTCTCTGA-3, TaqMan probe 711T FAM CTCCTGGCCCATACACTCTACCGGG TAMRA. 18s was used as a housekeeping gene for multiplexing (Applied Biosystems) because of its stable expression under endotoxemia (B.J., unpublished data, 2003). TF mRNA was normalized against the reference gene (18s) and data expressed as fold-increase over baseline values. Dilution curves of TF mRNA obtained from LPS-incubated blood samples revealed linearity (r = 0.999) of the assay up to 37.5 cycles, which was set as the limit of sensitivity (and also as a baseline for conservative statistical calculations).
Statistics
Data are expressed as the mean plus or minus the standard error of the mean (SEM), unless otherwise indicated. Non-normally distributed data were analyzed with the Mann-Whitney U test. The Bonferroni method was used to correct for the multiple comparisons. Bivariate correlations between parameters were calculated with the use of the Spearman rank correlation test. P < .05 was considered to be statistically significant. Statistical analysis was performed with SPSS (version 10.0, Chicago, IL).
Results
Characterization of the MP-associated TF PCA capture assay
Since the solid-phase capture assay was dependent on broad cellular recognition of MPs by the 1B10 antibody, we first determined whether the antigen recognized by 1B10 is expressed by cell types that could be the source of circulating intravascular MP-borne TF. 1B10 is a complement fixing murine IgM monoclonal antibody that was originally produced by immunization of mice with cultured human thymic fibroblasts.38 The antigen recognized by this antibody was known to be expressed on fibroblasts, as well as tissue macrophages and peripheral blood monocytes, but not epithelial cells or lymphocytes.38,39
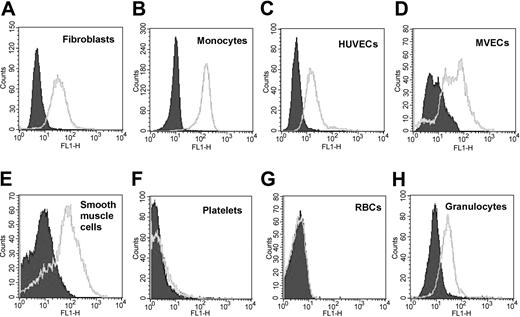
By flow cytometry, we found that 1B10 bound not only to fibroblasts and monocytes, but also to smooth muscle cells, granulocytes, and endothelial cells, regardless of whether the latter were of large vessel (umbilical vein) or small vessel (foreskin) origin (Figure 2). On the other hand, red blood cells and platelets were not recognized. Direct demonstration of 1B10 binding to MPs by flow cytometry could not be satisfactorily demonstrated due to high background fluorescence in filtered buffer samples containing unlabeled 1B10 (or control murine IgM) in the presence of the secondary antimouse antibody. No such fluorescence was detected with buffer with or without secondary antibody. This artifact was presumed to be due to aggregation or multimerization of IgM antibody.
Expression of antigen recognized by 1B10 by a variety of cell types. Fibroblasts (A), human umbilical vein endothelial cells (HUVECs) (C), microvascular endothelial cells (MVECs) (D), and smooth muscle cells (E) were cultured and CD14+ monocytes (B), granulocytes (H), platelets (F), and red cells (RBCs) (G) were isolated as described in “Materials and methods.” These cells were then stained with 1B10 MoAb or a control murine monoclonal IgM, followed by rabbit antimouse IgM-FITC and analyzed by flow cytometry. Labeling with control IgM antibody is shown in solid filled curve. Open histograms denote cell-specific antibody.
Expression of antigen recognized by 1B10 by a variety of cell types. Fibroblasts (A), human umbilical vein endothelial cells (HUVECs) (C), microvascular endothelial cells (MVECs) (D), and smooth muscle cells (E) were cultured and CD14+ monocytes (B), granulocytes (H), platelets (F), and red cells (RBCs) (G) were isolated as described in “Materials and methods.” These cells were then stained with 1B10 MoAb or a control murine monoclonal IgM, followed by rabbit antimouse IgM-FITC and analyzed by flow cytometry. Labeling with control IgM antibody is shown in solid filled curve. Open histograms denote cell-specific antibody.
In order to determine whether the MP capture assay would detect MP-associated TF PCA produced by the individual cell types of interest, we prepared MPs from each cell type after in vitro activation. As previously described, TF-bearing MPs were easily detectable in the supernatant of smooth muscle cells and fibroblasts, even without cellular activation32,33 (Table 1). Constitutive generation of TF-bearing MPs was barely detectable in supernatants from monocytes and endothelial cells, but increased after activation by LPS and TNF-α, respectively, as previously described.34,35 Red cells and platelets, which produce MPs in response to calcium ionophore stimulation, but were not recognized by 1B10, were included as a negative control.
Expression of microparticle-associated tissue factor procoagulant activity on in vitro-generated microparticles
Cellular origin of MPs . | MP-associated TF PCA in supernatant [Xa], pM/h per 106 cells . |
|---|---|
| Fibroblasts | |
| Ionophore− | > 300 |
| Ionophore+ | > 300 |
| Smooth muscle cells | |
| Ionophore− | > 300 |
| Ionophore+ | > 300 |
| HUVECs | |
| TNF− | 4 |
| TNF+ | 26 |
| CD14+ monocytes | |
| LPS− | 3 |
| LPS+ | 20 |
| Platelets | |
| Ionophore− | 0 |
| Ionophore+ | 0 |
| Red blood cells | |
| Ionophore− | 0 |
| Ionophore+ | 1 |
Cellular origin of MPs . | MP-associated TF PCA in supernatant [Xa], pM/h per 106 cells . |
|---|---|
| Fibroblasts | |
| Ionophore− | > 300 |
| Ionophore+ | > 300 |
| Smooth muscle cells | |
| Ionophore− | > 300 |
| Ionophore+ | > 300 |
| HUVECs | |
| TNF− | 4 |
| TNF+ | 26 |
| CD14+ monocytes | |
| LPS− | 3 |
| LPS+ | 20 |
| Platelets | |
| Ionophore− | 0 |
| Ionophore+ | 0 |
| Red blood cells | |
| Ionophore− | 0 |
| Ionophore+ | 1 |
Cells were isolated as described in “Materials and methods.” Fibroblasts, smooth muscle cells, red blood cells, and platelets (106 cells in each case) were activated by exposure to 10 μM ionophore (or vehicle control) for 10 minutes at 37°C. Monocytes were activated by exposure to 10 ng/mL LPS (or vehicle control) for 4 hours at 37°C and HUVECs were activated by exposure to 10 ng/mL TNF-α (or vehicle control) for 4 hours at 37°C. In every case, the supernatant was removed and assayed for MP-associated TF PCA as described in “Materials and methods.”
MP-associated TF PCA is detectable in healthy subjects
PFP was obtained from 40 healthy volunteers (25 male, 15 female; age range: 19 to 54 years) and assayed in duplicate for MP-associated TF PCA. All samples had detectable TF PCA, with a mean Xa generation of 3.96 ± 2.58 pM/h (mean ± SD). No significant gender difference in TF activity was detected (3.70 ± 2.28 vs 4.40 ± 3.04 pM/h, males vs females, respectively).
In vitro stimulation of whole blood by LPS leads to increased MP-associated TF PCA
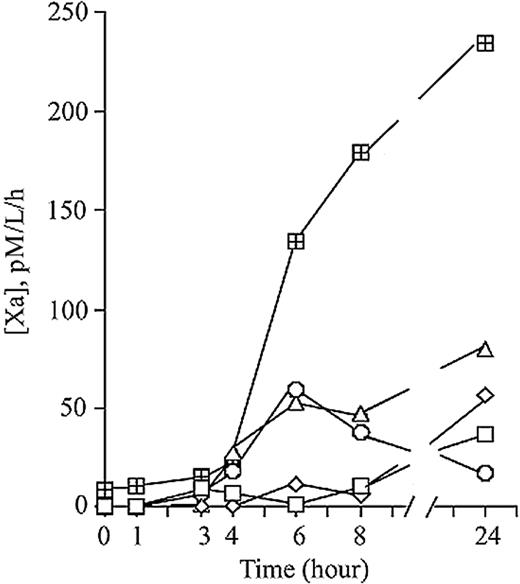
Heparin-anticoagulated whole blood from 5 healthy volunteers was exposed to 10 ng/mL LPS in vitro for up to 24 hours at 37°C. PFP was prepared from these samples, and assayed for MP-associated TF PCA. As shown in Figure 3, there was a time-dependent increase in TF activity in the PFP fraction, although the magnitude of the response varied markedly between individuals. Since monocytes are presumably the sole source of inducible activity, the variability in MP-borne TF response to LPS is seemingly analogous to the previously described variability in monocyte-borne TF, which has been termed the “high/low LPS responder phenomenon.”40-42 On repeated testing, we found the MP tissue factor response to be highly consistent for any given individual (data not shown).
Time-dependent increase in MP-associated TF PCA in platelet-free plasma following exposure of whole blood to LPS ex vivo. Heparinized whole-blood samples from 5 healthy controls were stimulated with 10 ng/mL LPS at 37°C for 24 hours. At various time points after LPS exposure, platelet-free plasma was prepared (as described in “Materials and methods”) and assayed for MP-associated TF PCA. Each line represents a different individual.
Time-dependent increase in MP-associated TF PCA in platelet-free plasma following exposure of whole blood to LPS ex vivo. Heparinized whole-blood samples from 5 healthy controls were stimulated with 10 ng/mL LPS at 37°C for 24 hours. At various time points after LPS exposure, platelet-free plasma was prepared (as described in “Materials and methods”) and assayed for MP-associated TF PCA. Each line represents a different individual.
Circulating in vivo MP-associated TF PCA increases after endotoxin administration
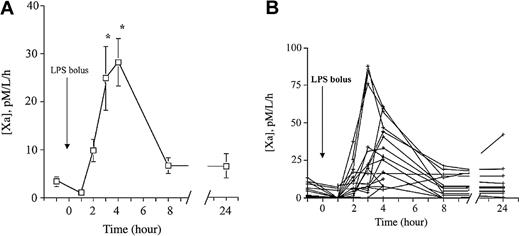
MP-associated TF PCA was detectable in all volunteers at baseline, and increased in most subjects after endotoxin administration. Mean TF activity increased approximately 8-fold with a peak at 3 to 4 hours, and a return to baseline levels by 8 hours (Figure 4A). Similar to the in vitro experiment, considerable interindividual variability of TF expression in MPs was observed (Figure 4B), probably reflecting the “high” and “low” LPS responder phenomenon. It should be noted that a marked (≥ 90%) but transient decrease in peripheral blood monocyte counts occurs between 1.5 and 6 hours after LPS administration in this model.27,28 The rapid disappearance of MP-borne TF in vivo compared with the in vitro experiment probably reflects clearance of MPs. Visual inspection of Figure 4A produces a crude estimate of the in vivo half-life of MPs of 2 hours or less.
LPS increases circulating MP-associated TF PCA in vivo. (A) Time-dependent increase in MP-associated TF PCA in vivo after LPS administration. EDTA-anticoagulated blood samples for assay of MP-associated TF PCA were collected at baseline and various time points after LPS infusion (2 ng/kg) in 18 male volunteers. MP-associated TF PCA was measured in platelet-free plasma as described in “Materials and methods.” Data are presented as the means ± SEM. *P < .05 versus baseline. (B) The MP-borne TF response following LPS exposure is highly variable between subjects. The data from panel A are replotted by individual subject to illustrate the variability of response.
LPS increases circulating MP-associated TF PCA in vivo. (A) Time-dependent increase in MP-associated TF PCA in vivo after LPS administration. EDTA-anticoagulated blood samples for assay of MP-associated TF PCA were collected at baseline and various time points after LPS infusion (2 ng/kg) in 18 male volunteers. MP-associated TF PCA was measured in platelet-free plasma as described in “Materials and methods.” Data are presented as the means ± SEM. *P < .05 versus baseline. (B) The MP-borne TF response following LPS exposure is highly variable between subjects. The data from panel A are replotted by individual subject to illustrate the variability of response.
Increase in total numbers of circulating MPs occurs after endotoxin administration
We determined whether endotoxemia produced an increase in the total number of circulating MPs in a time course that mirrored that of MP-associated TF PCA. Flow cytometry was used to enumerate MPs in blood obtained from 13 randomly selected study participants at baseline and at various time intervals up to 8 hours. Additionally, we specifically enumerated monocyte-derived MPs, defined as those events that were also CD14+. As shown in Table 2, 3 to 4 hours after LPS administration, the total number of MPs had increased approximately 2-fold (from 727 ± 131 × 103 MPs/mL at baseline to 1469 ± 267 × 103 MPs/mL at 3 hours; P < .05). A parallel increase in the number of CD14+ monocyte-derived MPs (from 104 ± 42 × 103 MPs/mL at baseline to 227 ± 57 × 103 MPs/mL at 3 hours; P < .05) was also apparent. Both the total number of MPs, as well as the number of CD14+ MPs had returned to baseline values by 8 hours. There was a significant linear correlation between the total number of MPs and the number of CD14+ monocyte-derived MPs (Table 3). In addition, MP-associated TF PCA was highly correlated with the total number of MPs, though not with the number of CD14+ MPs.
Number of circulating microparticles (MPs × 103/mL) in endotoxin-treated volunteers
Time, h . | Total MPs, n = 13 . | CD14+ MPs, n = 13 . | VE cadherin+ MPs, n = 3 . | TF+ MPs, n = 3 . |
|---|---|---|---|---|
| −3 | 727 ± 131* | 104 ± 42 | 3 ± 3 | 26 ± 8 |
| 3 | 1469 ± 267† | 227 ± 57† | 48 ± 35 | 83 ± 27 |
| 4 | 1333 ± 235† | 192 ± 52† | — | — |
| 8 | 890 ± 537 | 91 ± 33 | 84 ± 84 | 100 ± 81 |
Time, h . | Total MPs, n = 13 . | CD14+ MPs, n = 13 . | VE cadherin+ MPs, n = 3 . | TF+ MPs, n = 3 . |
|---|---|---|---|---|
| −3 | 727 ± 131* | 104 ± 42 | 3 ± 3 | 26 ± 8 |
| 3 | 1469 ± 267† | 227 ± 57† | 48 ± 35 | 83 ± 27 |
| 4 | 1333 ± 235† | 192 ± 52† | — | — |
| 8 | 890 ± 537 | 91 ± 33 | 84 ± 84 | 100 ± 81 |
— indicates no data.
Data are expressed as mean plus or minus SEM.
P < .05 versus baseline.
Spearman Rho correlations in endotoxin-treated volunteers
. | MP-associated TF PCA, n = 18 . | Whole-blood TF PCA, n = 18 . | Total no. of MPs, n = 13 . | CD14+ MPs, n = 13 . |
|---|---|---|---|---|
| Whole-blood TF PCA (P) | 0.34 (.0001) | — | — | — |
| Total no. of MPs (P) | 0.56 (.0002) | 0.03 (.88) | — | — |
| CD14+ MPs (P) | 0.24 (.14) | 0.04 (.80) | 0.61 (.00003) | — |
| TF mRNA (P) | 0.27 (.06) | 0.54 (.0001) | 0.40 (.09) | 0.54 (.02) |
. | MP-associated TF PCA, n = 18 . | Whole-blood TF PCA, n = 18 . | Total no. of MPs, n = 13 . | CD14+ MPs, n = 13 . |
|---|---|---|---|---|
| Whole-blood TF PCA (P) | 0.34 (.0001) | — | — | — |
| Total no. of MPs (P) | 0.56 (.0002) | 0.03 (.88) | — | — |
| CD14+ MPs (P) | 0.24 (.14) | 0.04 (.80) | 0.61 (.00003) | — |
| TF mRNA (P) | 0.27 (.06) | 0.54 (.0001) | 0.40 (.09) | 0.54 (.02) |
Data are shown as r values. — indicates.
Because monocyte-derived MPs always accounted for less than 20% of total MPs (Table 2), we wondered whether LPS-activated endothelial cells (ECs) might contribute to the expanded pool of circulating MPs at 3 to 4 hours. In fact, while an increase in EC-derived (annexin V-positive/VE cadherin-positive) MPs was detected after LPS administration in 3 individuals, the absolute numbers were relatively small (3 ± 3 × 103 MPs/mL at baseline, 48 ± 35 × 103 MPs/mL at 3 hours, and 84 ± 84 × 103 MPs/mL at 8 hours; Table 2). In a previous study,36 we found that platelets account for the majority of circulating MPs in plasma from healthy individuals, although we did not systematically enumerate platelet-derived MPs in this study.
The number of TF-positive (annexin V-positive/anti-TF-positive) MPs was enumerated in 3 individuals (Table 2). Unlike our experience in patients with sickle cell disease,36 our attempts to define the cellular origin of TF-positive MPs by triple-labeling experiments produced numbers that were too small to accurately quantify.
Identification of TF on circulating MPs from endotoxin-treated volunteers
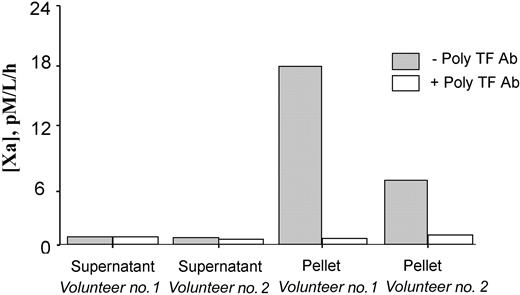
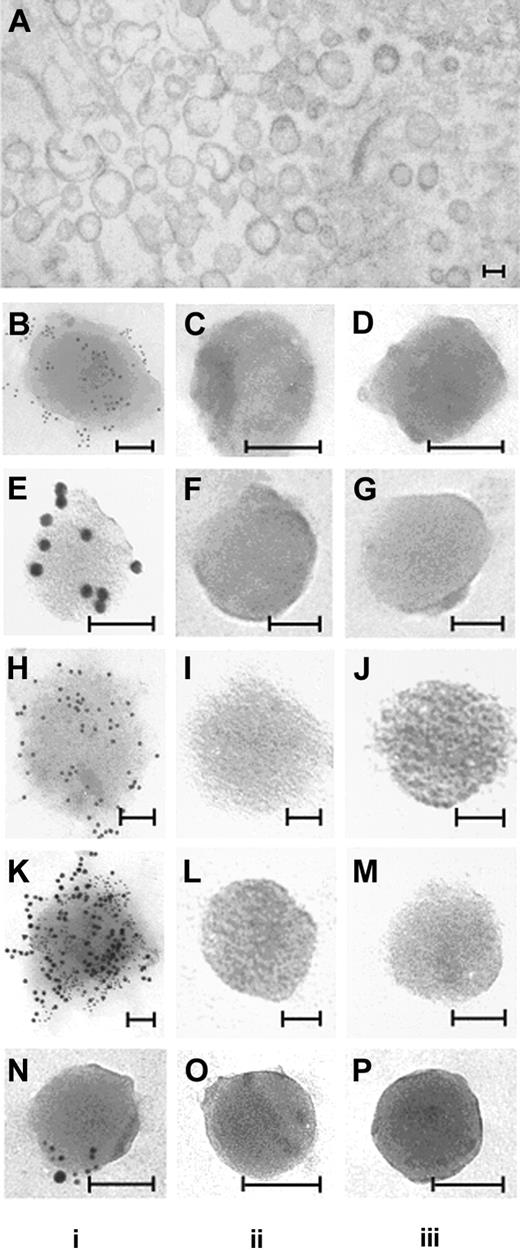
Additional experiments were performed to confirm that the TF PCA detected by the capture assay was in fact associated with circulating MPs. First, PFP from 2 of the highest LPS-responding individuals was ultracentrifuged (400 000g for one hour), after which the supernatant plasma was carefully decanted, and assayed for MP-associated TF PCA. As seen in Figure 5, this maneuver removed essentially all of the detectable TF PCA from solution. Electron microscopic examination of the pellet obtained by ultracentrifugation demonstrated the presence of TF antigen on MPs as shown in Figure 6B. These in vivo-generated MPs are seen as vesicular structures with diameters ranging from 0.1 μm to 0.5 μm, which do not contain mitochondria or other recognizable cellular organelles (Figure 6A). Immunogold labeling of MPs demonstrated positive labeling with anti-TF (Figure 6B), anti-CD14 (Figure 6E), and 1B10 (Figure 6H) antibodies as well as double-labeling with 1B10 and anti-TF (Figure 6K). No labeling was observed when MPs were incubated with control isotype or secondary gold particle-labeled antibodies alone. The presence of MPs that were positive for both CD14 and TF (Figure 6N) establishes that at least some circulating TF-positive MPs originated from monocytes.
MP-associated TF PCA is recovered in the pellet of ultracentrifuged platelet-free plasma following LPS exposure in vivo Platelet-free plasma from 2 volunteers, obtained 3 to 4 hours after LPS exposure, was subjected to high-speed ultracentrifugation. The resuspended pellet and supernatant fractions were then independently assayed for MP-associated TF PCA, as described in “Materials and methods.” An inhibitory polyclonal antibody to TF (with or without poly TF Ab) was included in some wells.
MP-associated TF PCA is recovered in the pellet of ultracentrifuged platelet-free plasma following LPS exposure in vivo Platelet-free plasma from 2 volunteers, obtained 3 to 4 hours after LPS exposure, was subjected to high-speed ultracentrifugation. The resuspended pellet and supernatant fractions were then independently assayed for MP-associated TF PCA, as described in “Materials and methods.” An inhibitory polyclonal antibody to TF (with or without poly TF Ab) was included in some wells.
Ultrastructure and immunogold labeling of MPs. Preparation of samples for electron microscopy is described in “Materials and methods.” MPs were isolated from PFP of a volunteer receiving endotoxin. In vivo-generated MPs are seen as vesicular structures with diameters ranging from 0.1 μm to 0.5 μm (A). MPs obtained by ultracentrifugation were incubated with rabbit polyclonal anti-TF antibody and/or anti-CD14 antibody or 1B10 anibody with appropriate secondary gold-labeled antibodies. In column i, positive gold-labeling to TF is demonstrated (B; 6 nm gold), CD14 (E; 12 nm gold), 1B10 (H; 12 nm gold). Microparticles double-labeled for 1B10 and TF (K), and CD14 and TF (N) are also shown. No labeling was observed with control antibodies (column ii) or secondary gold particle-labeled antibodies alone (column iii). Bars = 100 nm.
Ultrastructure and immunogold labeling of MPs. Preparation of samples for electron microscopy is described in “Materials and methods.” MPs were isolated from PFP of a volunteer receiving endotoxin. In vivo-generated MPs are seen as vesicular structures with diameters ranging from 0.1 μm to 0.5 μm (A). MPs obtained by ultracentrifugation were incubated with rabbit polyclonal anti-TF antibody and/or anti-CD14 antibody or 1B10 anibody with appropriate secondary gold-labeled antibodies. In column i, positive gold-labeling to TF is demonstrated (B; 6 nm gold), CD14 (E; 12 nm gold), 1B10 (H; 12 nm gold). Microparticles double-labeled for 1B10 and TF (K), and CD14 and TF (N) are also shown. No labeling was observed with control antibodies (column ii) or secondary gold particle-labeled antibodies alone (column iii). Bars = 100 nm.
Cell-surface PCA may be regulated by several mechanisms, including encryption of membrane-bound TF.43 Encryption is defined as the ability of TF to bind its ligand, FVIIa, while failing to express full PCA. This activity may be “decrypted” by maneuvers such as trypsinization, freeze-thawing, or exposure to calcium ionophore.43-45 The phenomenon may at least in part be explained by loss of phospholipid asymmetry in the membrane bilayer43 and is linked to the process of MP generation.45 We thus sought to determine whether expression of MP-associated TF PCA could be further enhanced by “decryption” maneuvers. Unlike cell-bound TF in cultured cells or freshly isolated monocytes, where a 10- to 100-fold increase in apparent TF PCA is typically seen after freeze-thawing or ionophore stimulation,44 no incremental rise in TF PCA could be demonstrated in MPs derived from either cultured cell lines or isolated from PFP of endotoxin recipients (data not shown). Therefore, by these criteria, MP-borne TF circulates in a form that is already maximally decrypted, a finding consistent with the fact that membrane phospholipid asymmetry is frequently lost on circulating MPs.18 However, it cannot be assumed that decryption necessarily implies greater functional importance of the TF in vivo, since inhibition by TF pathway inhibitor (TF PI) may provide a counter-balancing anticoagulant effect.
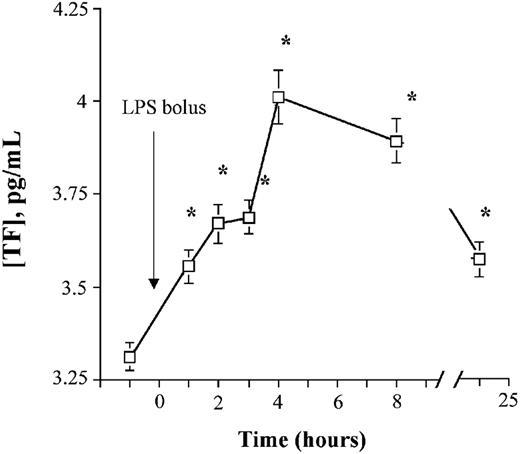
Whole-blood TF PCA and TF mRNA increase after endotoxin administration
Whole-blood TF PCA also increased in a time-dependent manner, with a more prolonged duration than MP-associated TF PCA (to at least 24 hours) after endotoxin exposure. Although peak activity was also observed at 4 hours, the absolute increase was relatively modest (≈ 30% over baseline) compared with MP-associated TF (Figure 7). An approximate 25-fold increase (95% confidence interval [CI]: 12-to 39-fold) in TF mRNA was noted. This activity peaked at about 3 hours (P < .000 01), and was still elevated at 5 hours (P < .0001) but had returned to baseline by 24 hours. The increase in whole-blood TF PCA was highly correlated with TF mRNA and MP-associated TF activity, but not with the total number of MPs, nor the number of monocyte-derived CD14+ MPs (Table 3).
Whole-blood TF PCA increases after LPS exposure. EDTA-anticoagulated whole-blood samples from LPS-treated volunteers were frozen at -70°C immediately after collection. Whole-blood TF PCA was measured as described in “Materials and methods.” Data are presented as geometric means ± 95% CI.*P < .05 versus baseline.
Whole-blood TF PCA increases after LPS exposure. EDTA-anticoagulated whole-blood samples from LPS-treated volunteers were frozen at -70°C immediately after collection. Whole-blood TF PCA was measured as described in “Materials and methods.” Data are presented as geometric means ± 95% CI.*P < .05 versus baseline.
Discussion
In these studies, we used 2 different functional assays to measure circulating intravascular TF PCA in human volunteers exposed to endotoxin. One was designed to measure MP-associated TF PCA in PFP, following the capture of these MPs by the monoclonal antibody 1B10. This antibody recognizes an unidentified antigen on the surface of various cell types including monocytes, granulocytes, endothelial cells, smooth muscle cells, and fibroblasts, but not platelets. Any or all of these cells could be a potential source of circulating TF-bearing MPs in vivo. Although technical problems precluded flow cytometry demonstration of 1B10 binding to MPs derived from some or all of these cells, direct binding was shown by electron microscopy (Figure 6). Furthermore, the ability of 1B10 to capture MPs is strongly supported by the fact that measurable TF activity can be pelleted by ultracentrifugation of plasma (Figure 5), or detected in the cell-free supernatants of cultured cell lines (Table 1).
Circulating MP-associated TF PCA was present in normal controls at baseline, consistent with the findings of one group,46 but in contrast to another study in which platelet, red cell, granulocyte, and endothelial cell-derived MPs were found to support coagulation in a TF-independent manner.47 We did not routinely examine MPs isolated by ultracentrifugation for non-TF-dependent PCA as others have done. However, in preliminary experiments, we did note that the PCA on MPs isolated from plasma by ultracentrifugation is often significantly less TF-dependent than the PCA on MPs captured by the 1B10 antibody (data not shown). While the reason(s) for this discrepancy remain(s) unclear, we postulate that MP-associated TF PCA (the focus of these studies) is likely to be augmented by non-TF-dependent PCA in vivo.
Endotoxin stimulation of monocytes in vitro leads to the generation of TF-bearing MPs seen using our MP-associated TF PCA assay (Figure 3). However, the lack of correlation between MP-associated TF PCA and number of monocyte-derived MPs in vivo requires explanation. One possibility is that monocyte-derived MPs are selectively enriched in TF,48 or that the increase in MP-associated TF activity is due to release of MPs bearing TF by cells other than monocytes. Endothelial cells could certainly contribute to the TF-positive MP pool, and indeed we have demonstrated that they do so in other disease states.36 Others have shown that platelet-derived MPs may contain TF,17 although we are unable to confirm this observation using our methods.36 In this study, we identified only a modest number of EC-derived MPs, although because of low event numbers, we were unable to accurately quantify the proportion of EC (or monocyte)-derived MPs that were TF-positive by triple-labeling flow cytometry experiments. We did note a significantly larger pool of flow cytometry events that were less than one μm in size, TF-positive, and either VE-cadherin-positive or CD14+ (data not shown). However, because these events were annexin V-negative—and therefore failed to meet our criteria for MPs—we did not enumerate them. Thus, the possibility also exists that the lack of correlation between the number of MPs on flow cytometry and MP-associated TF activity may be explained by the precise criteria we chose to define MPs.
The increase in MP-associated TF PCA coincides with the profound monocytopenia (> 90% reduction compared with baseline) that occurs from about 1.5 to 6 hours following endotoxin exposure in this model.27,28 However, individual monocytes remaining in the circulation at 6 hours following endotoxin exposure demonstrate elevated TF antigen expression,27 and about a 15-fold increase in monocyte-associated TF PCA.49 The monocytopenia appears to be of a sufficiently severe degree to account for the modest rise in whole-blood TF PCA that we observed. The persistent increase in whole-blood TF PCA at a time when MP-associated TF PCA and total number of MPs had returned to baseline (8-24 hours) suggests that the majority of this activity resides in the cell-bound, rather than the non-cell-bound (ie, microparticle) fraction of blood. This impression is supported by the tight correlation between TF mRNA and whole-blood TF PCA levels, but not MP-associated TF PCA.
Although it is now accepted that coagulation is activated via the TF pathway in endotoxemia and sepsis, much of the supporting evidence is indirect, originating from therapeutic intervention studies. Administration of TF inhibitors—such as TFPI,50 active site-inhibited factor VIIa,51 or recombinant nematode anticoagulant protein C252 —to human endotoxin volunteers dose-dependently inhibits thrombin generation in vivo. Direct evidence for the up-regulation of TF expression includes the demonstration that whole-blood TF mRNA levels increase after endotoxin administration.37 Osterud and Flaegstad41 have demonstrated that monocyte TF activity is increased in certain forms of sepsis, but the more widespread use of their assay has been limited by the fact that it is technically cumbersome and prone to artifactual ex vivo activation of monocytes during isolation.24 Functional assays of TF based on modified whole-blood clotting times have also been proposed.28,53 Using one such assay, Marsik et al28 recently demonstrated that a proportion of the total PCA in whole blood following endotoxin administration is TF-dependent, although the presence of “downstream” activated clotting factors also contribute to the shortening of the clotting time.
Plasma TF antigen by ELISA has also been used as a measure of TF expression in vivo, although this assay does not provide any information on the origin or functional competence of circulating TF. It has been reported that plasma TF antigen levels remain persistently elevated between 8 and 48 hours after endotoxin administration,54 although we were unable to confirm these findings.27 Since only one third to one half of plasma TF antigen can be sedimented by high-speed centrifugation, it has been proposed that the assay may in fact measure 2 forms of TF antigen in plasma: soluble and MP-associated.55 This view is supported by a recent study showing that 45% to 77% of “non-cell-bound” TF in pericardial blood of patients undergoing cardiac surgery is MP-associated and functionally active, whereas the remaining fluid-phase TF antigen is nonfunctional.56 Because soluble TF lacking the transmembrane domain is not fully functional,57 the correlation between TF antigen levels and our assay for whole-blood TF PCA is likely to be poor.
The suggestion that MP-associated TF plays an important role in the coagulopathy of sepsis has been previously addressed using other approaches. Nieuwland and colleagues15 demonstrated that patients with meningococcal sepsis have elevated numbers of circulating MPs that were derived from multiple cell types, especially granulocytes and platelets. However, 85% or more of TF-positive MPs were of monocytic origin.15 Curiously however, the same group found that the number of TF-positive MPs was decreased in patients with multiple organ dysfunction syndrome and (nonmeningococcal) sepsis compared with healthy controls.47 Whether this apparent paradox reflects the acuity of the various forms of sepsis is unclear.
We also found that the endotoxin-induced increase in TF PCA on circulating MPs was highly variable between individuals. We believe this finding represents the in vivo analog of the previously described “high versus low responder” phenotype phenomenon, which is used to describe the variable monocyte responsiveness to LPS in whole blood in vitro.40-42 The precise explanation for this phenomenon is unclear, but may reflect individual differences in plasma,40 leukocyte,42 or platelet58 factors. The absolute increase in TF activity in peripheral blood monocytes was demonstrated to have prognostic significance in meningococcal sepsis, with higher levels portending a poorer outcome.41 Whether the same holds true for whole-blood and/or MP-associated TF PCA is an eminently testable hypothesis.
Prepublished online as Blood First Edition Paper, February 26, 2004; DOI 10.1182/blood-2003-03-0713.
Supported by grant HL65578 (N.S.K.) and HL5552 (R.P.H.) from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, and grant SAF 2003-05780 from the government of Spain (G.E.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Dr Gary Nelsestuen for helpful discussions. The technical assistance of Julia Nguyen in the isolation and culture of endothelial cells, Marcie Krumwiede in the preparation of the EM mounts, Nicole Henderson in sample collection and processing, and Carol Taubert in manuscript preparation is gratefully acknowledged.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal