Abstract
SOCS1 and SHP1 negatively regulate the Janus kinase/signal transducer and activator of transcription (Jak/STAT) signaling pathway. The role of promoter hypermethylation leading to epigenetic inactivation of SOCS1 and SHP1 in myeloma was investigated. The methylation-specific polymerase chain reaction (MSP) was used to define SOCS1 and SHP1 methylation in 34 diagnostic myeloma samples. For SOCS1, MSP primers 3′ to the translation start site were unreliable and gave positive results in normal controls. However, primers in the 5′ promoter region were specific, although no myeloma samples showed methylation. For SHP1, 27 of 34 (79.4%) myeloma samples showed SHP1 hypermethylation. The biologic significance of SHP1 methylation was investigated in the U266 human myeloma line. U266 contained completely methylated SHP1. Furthermore, there was constitutive STAT3 phosphorylation. Treatment with 5-azacytidine led to progressive demethylation of SHP1 on days 2 to 5, with consequent increasing reexpression of SHP1 as shown by reverse transcription-polymerase chain reaction (RT-PCR). Concomitant with increasing SHP1, a parallel down-regulation of phosphorylated STAT3 occurred, so that by day 5 phosphorylated STAT3 was barely detectable. The overall survivals of patients with and without SHP1 methylation were similar. SHP1 methylation leading to epigenetic activation of the Jak/STAT pathway might have a tentative role in the pathogenesis of myeloma, which should be further confirmed by functional studies in primary myeloma samples. (Blood. 2004;103:4630-4635)
Introduction
Multiple myeloma is characterized by neoplastic proliferation of monoclonal plasma cells. These neoplastic plasma cells are thought to be derived from a postgerminal center B cell, which migrates to the bone marrow, adheres to the marrow stroma, and triggers subsequent bone resorption and a paracrine cytokine loop that involves interleukin-6 (IL-6).1,2 The natural course of the disease may progress through monoclonal gammopathy of undetermined significance (MGUS), smoldering myeloma, intramedullary myeloma, and eventually extramedullary myeloma. MGUS is a precursor of myeloma, and the median interval from discovery of the M protein to the development of myeloma might be up to 10 years.3,4
The binding of cytokines to their receptors results in the dimerization of receptor complexes and activation of the Janus family of protein tyrosine kinases (Jak's),5-7 followed by phosphorylation of the cytoplasmic signal transducers and activators of transcription (STATs). Upon phosphorylation, STATs form homodimers or heterodimers, migrate to the nucleus, and activate gene transcription. In myeloma, binding of the IL-6 to its membrane receptor induces dimerization of receptor subunit gp130 and results in activation of Jak by cross-phosphorylation.8 This Jak/STAT pathway is subject to negative regulation by 3 families of proteins, the protein inhibitors of activated STATs (PIAS), the suppressors of cytokine signaling (SOCS), and the SH2-containing phosphatases (SHP).6,7,9
The SOCS family comprises at least 8 members characterized by the presence of a central Src homology (SH2) domain and a conserved carboxy-terminal “SOCS box.”6,7 SOCS members are cytokine-inducible negative regulators of the cytokine signaling. An important member is SOCS1. The SOCS1 gene can be induced by a multitude of cytokines, including IL-1, IL-3, IL-6, erythropoietin, granulocyte-macrophage colony-stimulating factor, and γ-interferon.10-12 Human SOCS1 is a single-exon gene encoding 211 amino acids and lies within a CpG island spanning 2.5 kilobase (kb). The expression of SOCS1 impairs cellular response to IL-6 through direct interaction with Jak proteins.13
SHP1, a member of the SHP family of proteins, is a 68-kd, cytoplasmic protein tyrosine phosphatase (PTP).14 The human SHP1 gene consists of 17 exons and spans approximately 17 kb of DNA. It contains 2 tandem Src homology (SH2) domains, a catalytic domain, and a C-terminal tail of about 100 amino acid residues.14 In contrast to the ubiquitous expression of the structurally related SHP2, SHP1 is primarily expressed in hematopoietic cells, and considered a putative tumor suppressor gene in lymphoma and leukemias, because it antagonizes the growth-promoting and oncogenic potentials of protein tyrosine kinase.14
DNA methylation, catalyzed by DNA methyltransferase, involves the addition of a methyl group to the carbon 5 position of the cytosine ring in the CpG dinucleotide, leading to a conversion to methylcytosine.15-17 In many cancers, the CpG islands of selected genes are aberrantly methylated (hypermethylated), resulting in transcriptional repression of these genes.18 This may serve as an alternative epigenetic mechanism of gene inactivation.19,20
Dysregulation of the IL-6/Jak/STAT pathway has been shown to be involved in hematologic cancers. For instance, activation of STAT3 is frequent in primary human myeloma or myeloma cell line,13 and blocking of either IL-6 receptor, Jak, or STAT3 inhibits the expression of Bcl-XL and promotes apoptosis in myeloma cells. Moreover, a TEL-Jak2 fusion protein is expressed as a consequence of t(9;12)(p24;p13) in some T-cell acute lymphoblastic leukemias (T-ALLs).21 Recently, hypermethylation of SOCS1 has been separately reported in hepatoma.22,23 In this study, we investigated the potential involvement of SOCS1 and SHP1 promoter hypermethylation in the pathogenesis of myeloma.
Patients, materials, and methods
Patients, diagnosis, and treatment
The diagnosis of myeloma was made according to standard criteria with marrow plasmacytosis, presence of paraprotein, and osteolytic bone lesions.24 Complete staging work-up included bone marrow examination, skeletal survey, serum and urine protein electrophoresis, serum immunoglobulin (IgG, IgA, and IgM) levels, renal function tests, and serum calcium level.25 Monoclonal immunoglobulins were identified by cellulose acetate or agarose-gel electrophoresis. When an abnormal band or equivocal pattern was detected, immunoelectrophoresis or immunofixation was performed. All blood tests were repeated every 6 months, and skeletal survey was repeated yearly. Primary treatment included either VAD (vincristine, 0.4 mg/d, 4 times daily; adriamycin, 9 mg/m2/d, 4 times daily; and dexamethasone, 40 mg/d, days 1 to 4, 9 to 12, and 17 to 20) or MP (melphalan, 0.15 mg/kg/d, for 7 days; prednisolone, 60 mg/d, for 7 days). On reaching a plateau phase, eligible patients received either allogeneic or autologous hematopoietic stem cell transplantation (HSCT). Patients who were not candidates for HSCT, and who showed disease progress from the plateau phase, received melphalan or cyclophosphamide depending on initial treatment. Involved field radiotherapy was given to relieve local symptoms. Patients gave informed consent to treatment, and the study was approved by the institutional review board of Queen Mary Hospital, Hong Kong.
Methylation-specific polymerase chain reaction (MSP)
High-molecular-weight genomic DNA was isolated by standard protocols from diagnostic bone marrow aspirates, myeloma cell line AF10 (kindly provided by Professor Zelig Eshhar, Department of Immunology, Weiz-mann Institute of Science, Israel), and leukemic cell lines (HL60, U937, Jurkat, and Raji). The methylation-specific polymerase chain reaction (MSP) for promoter methylation was performed as described.26-28 Briefly, treatment of DNA with bisulfite (which resulted in conversion of unmethylated cytosine to uracil, but unaffecting methylated cytosine) was performed with a commercially available kit (CpGenome DNA modification kit; Intergen, New York, NY). MSP primers were designed to amplify the methylated (M-MSP) and unmethylated (U-MSP) alleles. For SOCS1, 2 sets of primers (MSP3′ and MSP5′) were adopted from previous studies.22,23 These 2 sets of MSP primers were located 5′ (MSP5′)23 and 3′ (MSP3′)22 to the translation start site of SOCS1. Both sets of primers could be identified on NT_010393.11, and the nucleotide positions of these 2 sets of SOCS1 primers with reference to GenBank sequence GI: 27486099 (NT_010393.11), subregion: complement (2121832 to 2123597) are listed in Table 1. The relative positions with respect to the translation start site are depicted in Figure 1. Notably, the forward and reverse primers of MSP3′ (adopted from Yoshikawa et al22 ) mapped to nucleotide positions 400 to 423 and 537 to 559 for methylated DNA and 391 to 423 and 537 to 565 for unmethylated DNA in GenBank sequence U88326. The MSP primers for SHP1 are listed in Table 1. There are 2 isoforms of SHP1, one expressed in nonhematopoietic tissues and the other in hematopoietic tissues,30 which use different promoters, P1 and P2, located upstream of exon 1 and exon 2, respectively. MSP primers were selected for the hematopoietic-specific P2 promoter. For all experiments, DNA from the peripheral blood of 12 healthy blood donors and 3 healthy bone marrow donors was used as negative control, and methylated DNA (CpGenome Universal Methylated DNA; Intergen) was used as positive control. MSP was performed in a thermal cycler (9700; PE Biosystems, Foster City, CA) with the following cycling conditions: 95°C for 12 minutes, 35 to 45 (Table 1) cycles of 95°C for 45 seconds, specific annealing temperature (Table 1) for 30 seconds, 72°C for 30 seconds, and a final extension of 10 minutes at 72°C. The polymerase chain reaction (PCR) mixture contained 50 ng bisulfite-treated DNA, 0.2 mM deoxyribonucleoside triphosphates (dNTPs), 2 mM MgCl2, 10 pmol of each primer, 1 × PCR Buffer II, and 2.5 units of AmpliTaq Gold (PE Biosystems) in a final volume of 50 μL. Ten microliters of PCR products were loaded onto 6% nondenaturing polyacrylamide gels, electrophoresed, and visualized under ultraviolet light after staining with ethidium bromide. Previous experiments had shown that the MSP had a sensitivity of 10-3 to 10-5 for detecting the methylated allele.27,31
Methylation-specific polymerase chain reaction (MSP): primer sequences and reaction conditions
Gene . | Forward primer: 5′-3′ . | Reverse primer: 5′-3′ . | Temperature, °C (no. cycles) . | PCR products, bp . | Reference no. . |
|---|---|---|---|---|---|
| SOCS1: MSP3′* | |||||
| M-MSP | TTC GCG TGT ATT TTT AGG TCG GTC (nt: 1081 - 1104) | CGA CAC AAC TCC TAC AAC GAC CG (nt: 1218 - 1240) | 63 (35) | 159 | Yoshikawa et al22 |
| U-MSP | TTA TGA GTA TTT GTG TGT ATT TTT AGG TTG GTT (nt: 1072 - 1104) | CGA CAC AAC TCC TAC AAC GAC CG (nt: 1218 - 1246) | 63 (35) | 174 | |
| SOCS1: MSP5′* | |||||
| M-MSP | GTT GTA GGA TGG GGT CGC GGT CGC (nt: 186 - 209) | CTA CTA ACC AAA CTA AAA TCC ACA (nt: 335 - 312) | 63 (35) | 149 | Nagai et al23 |
| U-MSP | GTT GTA GGA TGG GGT TGT GGT TGT (nt: 186 - 209) | CTA CTA ACC AAA CTA AAA TCC ACA (nt: 335 - 312) | 63 (35) | 149 | |
| SHP1† | |||||
| M-MSP | GAA CGT TAT TAT AGT ATA GCG TTC (nt: 6857 - 6880) | TCA CGC ATA CGA ACC CAA ACG (nt: 6995 - 7015) | 60 (40) | 158 | Oka et al29 |
| U-MSP | GTG AAT GTT ATT ATA GTA TAG TGT TTG G (nt: 6857 - 6882) | TTC ACA CAT ACA AAC CCA AAC AAT (nt: 6993 - 7015) | 59 (35) | 158 |
Gene . | Forward primer: 5′-3′ . | Reverse primer: 5′-3′ . | Temperature, °C (no. cycles) . | PCR products, bp . | Reference no. . |
|---|---|---|---|---|---|
| SOCS1: MSP3′* | |||||
| M-MSP | TTC GCG TGT ATT TTT AGG TCG GTC (nt: 1081 - 1104) | CGA CAC AAC TCC TAC AAC GAC CG (nt: 1218 - 1240) | 63 (35) | 159 | Yoshikawa et al22 |
| U-MSP | TTA TGA GTA TTT GTG TGT ATT TTT AGG TTG GTT (nt: 1072 - 1104) | CGA CAC AAC TCC TAC AAC GAC CG (nt: 1218 - 1246) | 63 (35) | 174 | |
| SOCS1: MSP5′* | |||||
| M-MSP | GTT GTA GGA TGG GGT CGC GGT CGC (nt: 186 - 209) | CTA CTA ACC AAA CTA AAA TCC ACA (nt: 335 - 312) | 63 (35) | 149 | Nagai et al23 |
| U-MSP | GTT GTA GGA TGG GGT TGT GGT TGT (nt: 186 - 209) | CTA CTA ACC AAA CTA AAA TCC ACA (nt: 335 - 312) | 63 (35) | 149 | |
| SHP1† | |||||
| M-MSP | GAA CGT TAT TAT AGT ATA GCG TTC (nt: 6857 - 6880) | TCA CGC ATA CGA ACC CAA ACG (nt: 6995 - 7015) | 60 (40) | 158 | Oka et al29 |
| U-MSP | GTG AAT GTT ATT ATA GTA TAG TGT TTG G (nt: 6857 - 6882) | TTC ACA CAT ACA AAC CCA AAC AAT (nt: 6993 - 7015) | 59 (35) | 158 |
Tm indicates annealing temperature; M-MSP, methylation-specific polymerase chain reaction for the methylated allele; U-MSP, methylation-specific polymerase chain reaction for the unmethylated allele; nt, nucleotide; and bp, base pairs.
Accession no.: Gl: 27486099 (NT_010393.11), subregion: complement (2121832 to 2123597).
Accession no.: X82818.
Schematic diagram of the location of the 2 sets of MSP primers (MSP5′ and MSP3′) for SOCS1 in relation to the translation start site.
Schematic diagram of the location of the 2 sets of MSP primers (MSP5′ and MSP3′) for SOCS1 in relation to the translation start site.
DNA sequencing
The identity of the methylated and unmethylated sequences was confirmed by automated DNA sequencing. PCR products were gel purified, sequenced bidirectionally, and analyzed on an automated DNA sequence analyser (3700 ABI Prism; PE Biosystems).
5-Azacytidine (5-AC) treatment of the myeloma cell line U266
The human myeloma cell line U266 was obtained from the American Type Culture Collection (Manassas, VA) and cultured in RPMI supplemented with 10% fetal calf serum. For treatment with 5-AC, U266 cells were seeded in 25 cm2 culture flasks at a density of 106/mL and treated with 3 μM 5-AC (Sigma, St Louis, MO) for 5 days, with fresh medium containing 5-AC replenished on day 2. U266 cells were harvested on days 1, 2, 4, and 5 for genomic DNA, total cellular RNA, and whole cell protein extractions.
Reverse transcription polymerase chain reaction (RT-PCR) for SHP1
One microgram of total cellular RNA was reverse transcribed with Moloney murine leukemia virus (M-MLV) reverse transcriptase (Life Technologies, Rockville, MD), and 2 μL of the resulted cDNA was amplified by PCR with SHP1-specific primers (forward: 5′-GGC ACT GGG AGC TGC ATC TGA GGC-3′; and reverse: 5′-CTC GCA CAT GAC CTT GAT GTG-3′30 ). The identity of the RT-PCR product was confirmed by DNA sequencing.
Western blot
Whole-cell lysate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane (PALL, Pensacola, FL). The membrane was blocked with 5% bovine serum albumin (Sigma) and immunoblotted with antibodies against phosphorylated STAT3 (Tyr705) (Cell Signaling Technology, Beverly, MA) and nonphosphorylated STAT3 (Zymed Laboratories, South San Francisco, CA). Equal loading of protein (7 μg for each lane) was confirmed by immunoblotting with an anti-β-actin antibody (Sigma).
Statistical analysis
Overall survival (OS) was measured from the date of diagnosis to the date of death or last follow-up. For patients undergoing HSCT, OS was censored at the time of transplantation. Survival curves were computed by the Kaplan-Meier method and compared by the log-rank test. All P values were 2-sided.
Results
Patients
Thirty-four patients (19 male, 15 female) at a median age of 62 years (range, 25 to 87 years) were studied. In these patients, the bone marrow plasma cells ranged from 38% to 89% (median, 56%). The types of myeloma were IgG (n = 21, 62%), IgA (n = 7, 21%), light chain (n = 5, 14%), and IgD (n = 1, 3%). The Durie-Salmon staging was stage 1 (n = 5, 14.7%), stage 2 (n = 12, 35.3%), and stage 3 (n = 17, 50%). Six patients (17.6%) had impaired renal function at diagnosis. The median diagnostic paraprotein level was 29.8 g/L (range, 10 to 78 g/L). Eighteen patients received 3 courses of VAD and then MP until plateau phase or maximum response, whereas 16 patients received MP until maximum response or plateau phase. Eight patients underwent HSCT (2 allogeneic, 6 autologous) during the plateau phase.
MSP for SOCS1
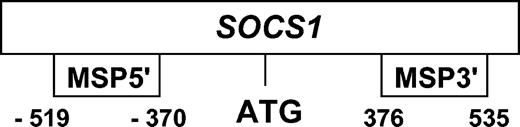
With primers MSP3′ (selected from the CpG islands inside exon 1 of the SOCS1 gene), M-MSP was positive in 6 of 12 normal peripheral blood DNAs, 2 of 3 normal marrows, and the methylated positive control DNA (Figure 2A). In 1 of the normal marrow samples and 4 of the 6 normal peripheral blood samples showing positive methylation signals, DNA sequencing of the M-MSP product confirmed authentic SOCS1 amplification (Figure 2B). Several observations were made from the sequence analysis of these M-MSP products from normal marrow and blood samples. First, the authenticity of methylation was confirmed by the finding of multiple unconverted cytosine molecules at “CpG” dinuleotides (Figures 2B). Second, not all CpG dinucleotides within this amplified region were methylated. Third, there was variable methylation of the individual CpG dinucleotides in different samples. Fourth, some CpG dinucleotides showed comparable signal intensity of C and T, suggesting hemizygous methylation (NG dinucleotides in Figure 2B). Sequence analysis of the M-MSP product from the methylated control DNA, on the other hand, showed complete methylation of all the CpG islands (Figure 2C). With the primer set MSP5′ (selected from the 5′ promoter region), none of the normal peripheral blood and marrow samples showed positive M-MSP (Figure 2D). The methylated control DNA, however, showed positive methylation (Figure 2D) with the expected sequence alterations (Figure 2E). These data suggested that methylation occurred in the 3′ coding region, but not the 5′ promoter region, of SOCS1 in normal DNA. Therefore, analysis of the 5′ region of SOCS1 might truly reflect promoter methylation, so that MSP of the myeloma samples was performed with primer set MSP5′ instead of MSP3′.
Methylation-specific polymerase chain reaction (MSP) for SOCS1 (A) MSP for the unmethylated allele (U-MSP) and methylated allele (M-MSP), showing that with the MSP3′ primers, 6 normal peripheral bloods (N2, N4, N7, N8, N9, and N10) and 2 marrows (Ma1 and Ma3) showed methylation signals. MW indicates molecular weight control; B, blank; P, positive control of methylated DNA; U, U-MSP; M, M-MSP; N, normal peripheral blood; and Ma, normal marrow. (B) Sequencing of SOCS1 in 5 bisulfite-converted peripheral blood controls (N7 to N10 and Ma3) showing methylation signals with primer set MSP3′. The DNA sequence of the “methylated” (Me) PCR product was aligned and compared with the germ line sequence of the wild-type DNA (WT). Methylated cytosine residues in CpG dinucleotide remained as C, whereas unmethylated cytosine read as T after bisulfite conversion. Underlined N suggested hemizygous methylation in a CpG island. Note the presence of many TG islands, representing unmethylated CpG dinucleotides. (C) Sequencing of the methylated PCR product with the MSP3′ primers of the methylated positive control. (D) MSP for SOCS1 using the MSP5′ primers. None of the normal peripheral blood and marrow samples showed positive methylation signals. (E) Sequencing of the methylated PCR product with MSP5′ primers from the methylated control DNA, showing complete methylation.
Methylation-specific polymerase chain reaction (MSP) for SOCS1 (A) MSP for the unmethylated allele (U-MSP) and methylated allele (M-MSP), showing that with the MSP3′ primers, 6 normal peripheral bloods (N2, N4, N7, N8, N9, and N10) and 2 marrows (Ma1 and Ma3) showed methylation signals. MW indicates molecular weight control; B, blank; P, positive control of methylated DNA; U, U-MSP; M, M-MSP; N, normal peripheral blood; and Ma, normal marrow. (B) Sequencing of SOCS1 in 5 bisulfite-converted peripheral blood controls (N7 to N10 and Ma3) showing methylation signals with primer set MSP3′. The DNA sequence of the “methylated” (Me) PCR product was aligned and compared with the germ line sequence of the wild-type DNA (WT). Methylated cytosine residues in CpG dinucleotide remained as C, whereas unmethylated cytosine read as T after bisulfite conversion. Underlined N suggested hemizygous methylation in a CpG island. Note the presence of many TG islands, representing unmethylated CpG dinucleotides. (C) Sequencing of the methylated PCR product with the MSP3′ primers of the methylated positive control. (D) MSP for SOCS1 using the MSP5′ primers. None of the normal peripheral blood and marrow samples showed positive methylation signals. (E) Sequencing of the methylated PCR product with MSP5′ primers from the methylated control DNA, showing complete methylation.
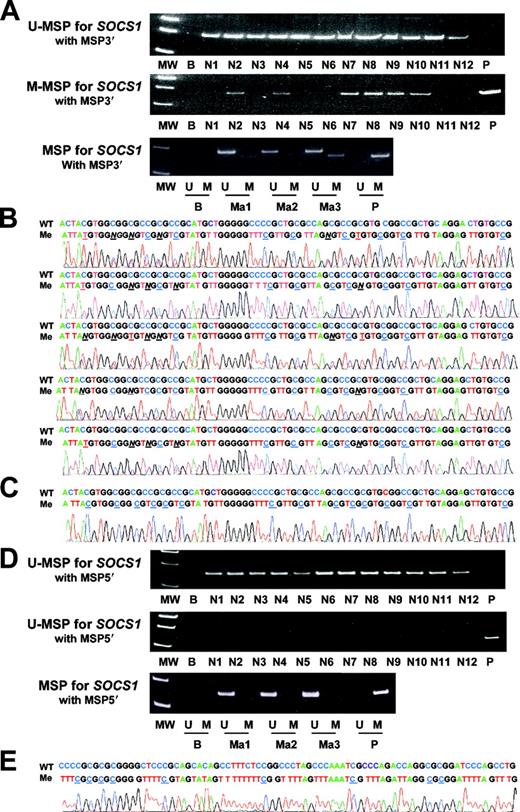
MSP of SHP1 in control DNA
M-MSP was negative in all of the normal peripheral blood and marrow samples but positive for the methylated control DNA. Sequencing of the M-MSP product from the methylated control DNA showed the expected nucleotide changes (Figure 3).
DNA sequencing of SHP1 in bisulfite-converted methylated positive control DNA.
DNA sequencing of SHP1 in bisulfite-converted methylated positive control DNA.
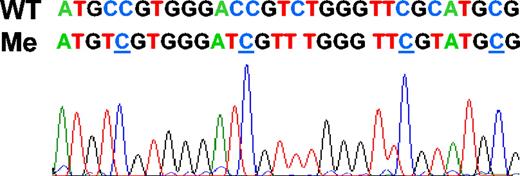
MSP of SOCS1 and SHP1 in cell lines and diagnostic myeloma samples
For the SOCS1 gene with the primers MSP5′, the cell lines AF10, HL60, U937, Jurkat, and Raji showed negative M-MSP but positive U-MSP that verified DNA integrity (Figure 4A). None of the myeloma samples showed SOCS1 methylation (Figure 4A). For the SHP1 gene, AF10, U937, Jurkat, and Raji showed hemizygous methylation, while HL60 was totally unmethylated (Figure 4B). On the other hand, 27 of 34 (79.4%) myeloma samples showed SHP1 methylation (Figure 4B). Sequencing of the SHP1 M-MSP product from 8 methylated myeloma samples confirmed authentic SHP1 amplification with the expected sequence changes.
MSP of cell lines and primary myeloma samples. (A) MSP for SOCS1 with primers MSP5′, showing that methylation was absent in the cell lines and primary myeloma samples tested. B indicates blank; P, positive control; MM, primary multiple myeloma samples. (B) MSP for SHP1, showing that with the exception of HL60 all the cell lines were methylated. All 5 of the myeloma samples (MM1 to MM5) were methylated.
MSP of cell lines and primary myeloma samples. (A) MSP for SOCS1 with primers MSP5′, showing that methylation was absent in the cell lines and primary myeloma samples tested. B indicates blank; P, positive control; MM, primary multiple myeloma samples. (B) MSP for SHP1, showing that with the exception of HL60 all the cell lines were methylated. All 5 of the myeloma samples (MM1 to MM5) were methylated.
5-AC treatment of the myeloma cell line U266
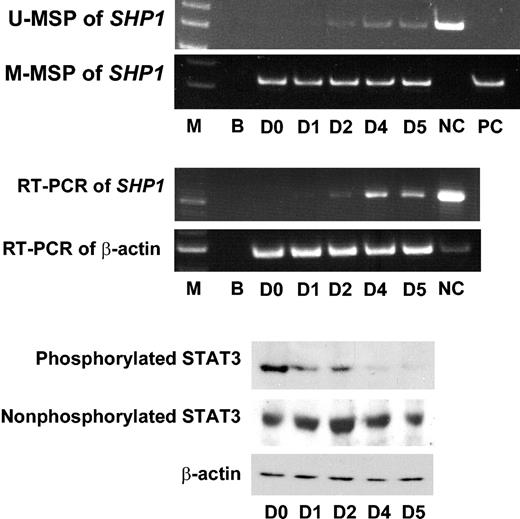
To study the biologic significance of SHP1 methylation, the myeloma cell line U266 was chosen as an in vitro model. U266 showed complete methylation of SHP1, with a consequent absence of SHP1 mRNA expression as shown by RT-PCR (Figure 5). A high level of phosphorylayted STAT3 was constitutively expressed. Treatment with 5-AC led to a progressive demethylation of SHP1 that started from day 2 onward, as shown by positive U-MSP with increasing amplification intensity. The progressive demethylation of SHP1 was associated with a parallel reexpression of SHP1 mRNA. This resulted in a corresponding down-regulation of phosphorylated STAT3. On day 5, phosphorylated STAT3 was almost undetectable. The level of nonphosphorylated STAT3 remained unchanged, showing that SHP1 reexpression interfered with phosphorylation of STAT3. Therefore, the biologic consequence of SHP1 gene methylation was repression of SHP1 expression and, hence, unopposed STAT3 phosphorylation. On the other hand, demethylation leading to reexpression of SHP1 resulted in down-regulation of phosphorylation of STAT3. These results implied that the epigenetic control of SHP1 expression might be critically involved in the regulation of STAT3 phosphorylation.
5-Azacytidine (5-AC) treatment of the myeloma line U266. The U266 cell line was totally methylated at SHP1, as shown by positive amplification only in M-MSP and not U-MSP at day 0 (D0) before treatment with 5-AC. On treatment with 5-AC, positive amplification appeared in U-MSP on day 2 (D2), indicating SHP1 demethylation. The U-MSP amplification became progressively stronger until day 5 (D5), indicating increasing demethylation of SHP1. The progressive demethylation of SHP1 was associated with increasing reexpression of SHP1, as shown by increasing amplification intensity of SHP1 mRNA by reverse transcription PCR (RT-PCR). Western blot analysis of STAT3 showed that before treatment (D0), phosphorylated STAT3 was constitutively expressed. With progressive demethylation and reexpression of SHP1, there was progressive down-regulation of phosphorylated STAT3, without significant changes in the amount of nonphosphorylated STAT3. By day 5 (D5), phosphorylated STAT3 was almost undetectable. Comparable protein loading was shown by β-actin. M indicates molecular weight marker; B, blank; D0 to D5, days 1 to 5 after 5-AC treatment; NC, normal control; PC, positive control with methylated DNA.
5-Azacytidine (5-AC) treatment of the myeloma line U266. The U266 cell line was totally methylated at SHP1, as shown by positive amplification only in M-MSP and not U-MSP at day 0 (D0) before treatment with 5-AC. On treatment with 5-AC, positive amplification appeared in U-MSP on day 2 (D2), indicating SHP1 demethylation. The U-MSP amplification became progressively stronger until day 5 (D5), indicating increasing demethylation of SHP1. The progressive demethylation of SHP1 was associated with increasing reexpression of SHP1, as shown by increasing amplification intensity of SHP1 mRNA by reverse transcription PCR (RT-PCR). Western blot analysis of STAT3 showed that before treatment (D0), phosphorylated STAT3 was constitutively expressed. With progressive demethylation and reexpression of SHP1, there was progressive down-regulation of phosphorylated STAT3, without significant changes in the amount of nonphosphorylated STAT3. By day 5 (D5), phosphorylated STAT3 was almost undetectable. Comparable protein loading was shown by β-actin. M indicates molecular weight marker; B, blank; D0 to D5, days 1 to 5 after 5-AC treatment; NC, normal control; PC, positive control with methylated DNA.
Survival analysis
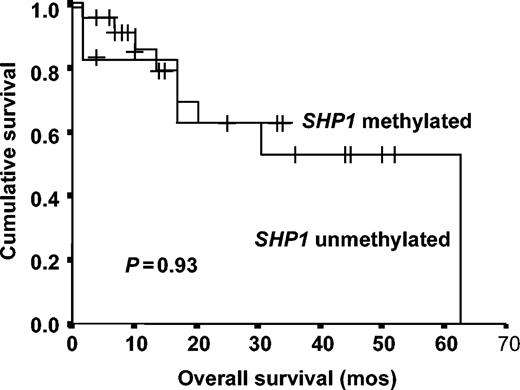
The projected 3-year OSs of patients with and without SHP1 methylation were 53% and 63%, respectively (P = .93) (Figure 6).
Overall survival of myeloma patients with and without SHP1 methylation.
Discussion
For the SOCS1 gene, with primer set MSP3′ designed inside exon 1, our study showed methylation signals in about half of the normal blood and marrow DNA. The possibility of mispriming was excluded by sequencing, which showed extensive methylation of most of the CpG dinucleotides in the amplified region. Moreover, the completeness of bisulfite conversion of the unmethylated cytosines to uracils was also demonstrated in the normal and methylated control samples. Incomplete bisulfite conversion is unlikely, because all non-CpG cytosines in these normal controls were converted to uracil and thus read as “T” in sequencing. These served as internal controls for the completeness of bisulfite conversion. Moreover, all non-CpG cytosine molecules in the methylated positive control were also successfully converted and thus also read as “T” in sequencing. Therefore, this region contained genuine methylated CpG islands in normal samples. On the other hand, with primer set MSP5′ selected from the 5′ promoter region, none of the normal blood and marrow samples showed methylation. Indeed, methylation as detected by MSP5′ primer has been demonstrated to be associated with down-regulation of, and thus silencing of, SOCS1 by immunohistochemistry in hepatoblastoma.23 Therefore, detection of methylation in CpG islands is more accurate in the 5′ promoter than the exon 1 region. This observation supported the occurrence of a methylation boundary in normal cellular DNA, as demonstrated in normal cellular DNA of certain genes.32,33 Using 6 sets of consecutive MSP primers spanning the 5′ untranslated region (UTR) to intron 1 of the E-cadherin gene in normal breast tissues, Graff et al showed that, while CpG dinucleotides in the 5′UTR promoter region were unmethylated, CpG dinucleotides toward the 3′ end of the intron 1 were methylated.32 This notion of methylation boundary had been further illustrated in another study of high-resolution methylation mapping of 45 CpG dinucleotides in the HIC1 gene.33 The results showed that in normal DNA significant CpG methylation occurred beyond exon 3 but not in intron 2, while for leukemic samples variable methylation of CpG dinucleotides occurred both 5′ and 3′ to exon 3. Therefore, a boundary sequence within the HIC1 CpG island existed that marked the junction between methylated and unmethylated DNA in normal hematopoietic cells.33 The presence of a methylation boundary underscores the importance of selecting MSP primers from the 5′UTR region. Conversely, MSP primers selected inside the coding regions might overestimate the frequency of methylation.
SOCS1 binds to the conserved regulatory tyrosine in the activation loop of the Jak2 kinase (JH1) domain through its SH2 domain and inhibits Jak kinase activity.34,35 As a negative regulator of the signaling pathway for IL-6, SOCS1 is an attractive target of dysregulation in myeloma. However, we showed that SOCS1, being a potential tumor suppressor in myeloma,36 was infrequently inactivated by methylation. This contrasted with a previous study using MSP3′ primers from the coding DNA region,37 which showed SOCS1 methylation in 63% of myeloma patients. This disparity might be partly explained by overestimation of the frequency of SOCS1 methylation using MSP primers in the coding region. Moreover, a less important role of SOCS1 in the pathogenesis of myeloma has also been demonstrated in SOCS1-deficient mice, which died of a myeloproliferative disease instead of plasma cell dyscrasia.7,10
On the other hand, SHP1, a PTP important in the negative regulation of Jak/STAT signaling, has been shown to be frequently silenced by methylation in leukemias and lymphomas.29,38 In this study, we showed frequent SHP1 methylation in our myeloma patients, suggesting that it might be involved in the pathogenesis through dysregulation of the Jak/STAT pathway. Ideally, SHP1 methylation is best demonstrated by purifying the myeloma cells by sorting for CD38- or CD138-positive cells. However, we have shown that the sensitivity of MSP for various genes ranges from 10-3 to 10-5. Moreover, because SHP1 methylation was undetectable in normal hematopoietic cells, and thus specific for the neoplastic plasma cells, our results were valid without a purified myeloma cell population.
To demonstrate that SHP1 methylation would lead to down-regulation of SHP1 with consequent activated phosphorylation of STAT3, immunohistochemical studies would be required to show concurrent down-regulation of SHP1 and up-regulation of phosphorylated STAT3 in the same cellular populations. However, this is technically difficult, the results are not always easy to interpret, and a qualitative relationship between SHP1 down-regulation and STAT3 activation cannot be demonstrated. Moreover, almost all the primary myeloma samples would be contaminated by residual normal hematopoietic cells that expressed SHP1, so that performing Western blot analysis to show a relationship between SHP1 and phosphorylated STAT3 would be unreliable. Therefore, the U266 myeloma cell line was used as an in vitro model. A previous study had shown that STAT3 was constitutively activated in U266, which expressed high levels of Bcl-XL and was resistant to Fas-mediated apoptosis.13 Our study further showed that SHP1 methylation was implicated in the constitutive activation of STAT3. The biallelic SHP1 gene methylation led to down-regulation of SHP1 and constitutive phosphorylation of STAT3. SHP1 reexpression consequent to demethylation resulted in down-regulation of the activated phosphorylated STAT3 protein. Because IL-6 is a major growth factor for myeloma cells, and IL-6 signaling acts through the Jak/STAT pathway, our results strongly suggest that SHP1 methylation might be an important epigenetic mechanism that collaborates with IL-6 in promoting the growth of the neoplastic plasma cells. However, a recent study showed that increased nuclear expression of phosphorylated STAT3, found in about 48% of myeloma patients, might not be related to the abundance of antiapoptotic proteins including Bcl-XL and Mcl-1.39 Furthermore, the expression of cyclin D1 and phosphorylated STAT3 appeared to be mutually exclusive. Therefore, mechanisms additional to constitutive activation of STAT3 might be involved in the pathogenesis of myeloma. Further experiments will thus be needed to define how modulation of the Jak/STAT pathway might interact with apoptotic mechanisms in myeloma.
In cancer, one hit of the cell cycle control pathway may be sufficient to result in dysregulation of cellular proliferation. For instance, selective, but not concurrent, inactivation of either p16, RB, or cyclin D1 has been reported in glioblastoma40 and melanoma.41 Moreover, reciprocal inactivation of either RB or p16 has been reported in small and non-small cell lung cancers.42,43 Similarly, our finding of frequent epigenetic inactivation of SHP1 but not SOCS1, both being negative regulators of the Jak/STAT pathway, in myeloma also lent support to this notion.
Previous studies in myeloma have demonstrated frequent epigenetic inactivation of the INK4/CDK/RB pathway by hypermethylation of p15 and p16. Others showed frequent hypermethylation of death-associated protein (DAP) kinase in the p14/MDM2/p53 pathway. In this study, we showed frequent epigenetic inactivation of SHP1, which potentially resulted in constitutive activation of the IL-6/Jak/STAT pathway. Notably, however, SHP1 methylation and STAT activation have only been demonstrated in vitro. Therefore, the functional relationship between SHP1 methylation and constitutive STAT activation in primary myeloma samples remains speculative and must be further evaluated. However, our findings together with results from previous studies suggest that gene hypermethylation might be a frequent genetic aberration in myeloma and may collaborate with translocation and other molecular aberrations in pathogenesis.1 Therefore, demethylating agents and histone deacetylase inhibitors are potentially useful in the treatment of myeloma.
Prepublished online as Blood First Edition Paper, February 19, 2004; DOI 10.1182/blood-2003-06-2007.
Supported by the Kadoorie Charitable Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Professor Z. Eshhar, Department of Immunology, Weizmann Institute of Science, Israel, for provision of the AF10 cell line and thank the Department of Pathology, Queen Mary Hospital, for pathologic diagnoses.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal