Abstract
ADAMTS-13 was recently identified as a new hemostatic factor, von Willebrand factor (VWF)–cleaving protease. Either congenital or acquired defects of the enzymatic activity lead to thrombotic thrombocytopenic purpura (TTP). ADAMTS-13 specifically cleaves a peptidyl bond between Y1605 and M1606 in the A2 domain of VWF. Here, we determined the minimal region recognized as a specific substrate by ADAMTS-13. A series of partial deletions in the A2 domain flanked with N- and C-terminal tags were expressed in Escherichia coli and affinity-purified. These purified proteins were incubated with human plasma, subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and analyzed by Western blot. Judging from mobility shifts, all constructs except one were cleaved at the expected site. Data suggested that a minimal region as a functional substrate consisted of 73 amino acid residues from D1596 to R1668 of VWF, designated VWF73, and that further deletion of the E1660-R1668 region led to the loss of cleavage by ADAMTS-13. VWF73 was not cleaved by plasma from patients with congenital or acquired TTP, but cleaved by plasma from patients with hemolytic uremic syndrome, suggesting that VWF73 is a specific substrate forADAMTS-13. Thus, VWF73 will be a useful seed to develop a new rapid assay to determine ADAMTS-13 activity.
Introduction
Thrombotic thrombocytopenic purpura (TTP), a serious disease with high mortality, is typically characterized by 5 features: thrombocytopenia, microangiopathic hemolytic anemia, renal failure, fever, and neurologic dysfunction.1 In patients with TTP, formation of platelet thrombi within the microvasculature is observed. TTP can be categorized into congenital and acquired types. In congenital cases with neonatal onset, the patients quickly respond to infusion of fresh frozen plasma, but the episodes of thrombocytopenia and hemolytic anemia are repeated. TTP with neonatal onset and frequent relapses is often diagnosed as Upshaw-Schulman syndrome (USS).2 The majority of clinically observed TTP is acquired, often affecting adolescents and adults.
The 2 key molecules involved in the pathogenesis of TTP are the plasma proteins, von Willebrand factor (VWF)3-6 and ADAMTS-13.7-11 The platelet-adhesive blood-coagulation protein, VWF, is synthesized primarily in vascular endothelial cells and released into plasma as large multimeric forms, which are highly active in interactions with platelets and collagen.12,13 In patients with both congenital and acquired TTP, unusually large VWF multimers circulate in plasma, resulting in the promotion of microvascular thrombosis, platelet consumption, and hemolysis. In normal plasma, VWF multimers are rapidly cleaved into smaller forms ranging in size from 500 to 20 000 kDa. This physiologically important cleavage is achieved by a newly identified plasma protease, ADAMTS-13. Functional deficiency of ADAMTS-13 caused by genetic mutation,14-18 inhibitory autoantibodies,19,20 or other etiologies leads to the accumulation of unusually large VWF multimers in plasma.
Human ADAMTS-13 was purified from plasma21-23 and its cDNA was cloned.23-25 At the same time, the ADAMTS13 gene was also identified as a gene responsible for congenital TTP by linkage analysis.14 ADAMTS-13 mRNA is predominantly expressed in liver.14,23,24,26 The protease consists of 1427 amino acid residues, containing an N-terminal signal peptide, a propeptide, a reprolysin-like metalloprotease domain, a disintegrin-like domain, a thrombospondin type-1 motif (TSP1), a cysteine-rich domain, a spacer domain, 7 more TSP1 repeats, and 2 CUB domains. The only known physiologic substrate for ADAMTS-13 is VWF multimers. The spacer domain of ADAMTS-13 is necessary for normal VWF-cleaving activity, and the more C-terminal domains are dispensable for the catalytic activity in vitro.27,28
ADAMTS-13 specifically cleaves a peptidyl bond between Y1605 and M1606 in the A2 domain of VWF.29-32 Although several methods have been developed to measure plasma ADAMTS-13 activity, they are not widely used at the clinical level due to various difficulties. The symptoms of TTP are similar to hemolytic uremic syndrome (HUS), a syndrome that is also characterized by thrombocytopenia, microangiopathic hemolytic anemia, and renal failure. HUS occurs mostly in young children after Escherichia coli O157 infection, but in some cases, it is difficult to discriminate between TTP and HUS. Therefore, the diagnosis of ambiguous TTP/HUS is made occasionally. For adequate therapy, the establishment of a consistent diagnosis system for TTP is eagerly anticipated by physicians and patients. The clinical assay ofADAMTS-13 activity is the most effective instrument for the diagnosis of TTP.33,34
To develop a more rapid and convenient method than previously described, an artificial specific substrate that can be easily processed by ADAMTS-13 will be useful. Here, we report that the minimal substrate for ADAMTS-13 is composed of 73 amino acid residues, and we designate this substrate as VWF73.
Materials and methods
Materials
Human plasma was obtained by centrifugation from whole blood that had been anticoagulated with 1:10 volume of 3.8% sodium citrate. Plasma from 3 patients with USS (congenital TTP), 6 patients with acquired TTP, 3 patients with HUS, and healthy individuals were used to measure the ADAMTS-13 activity.
Construction of bacterial expression vectors
Plasmid DNA to express partial regions of human VWF tagged with N-terminal glutathione S-transferase (GST) and C-terminal 6xHis (H) were constructed as follows. First, the D1459-R1668 region of VWF was amplified by reverse transcription–polymerase chain reaction (RT-PCR) using total RNA prepared from cultured human umbilical vein endothelial cells. We used 2 primers for amplification: 5′-cgggatccGACCTTGCCCCTGAAGCCCCTC-3′ and 5′-cggaattcTCAGTGATGGTGATGGTGATGCCTCTGCAGCACCAGGTCAGGA-3′. Lowercase letters indicate added restriction enzyme sites, and the underlined sequence is the inserted C-terminal H-tag. The PCR product was digested with BamHI and EcoRI and ligated into the corresponding site of pGEX-6P-1 (Amersham Biosciences, Buckinghamshire, England), a Schistosoma japonicum GST fusion expression vector. The other plasmids for E1554-R1668, D1587-R1668, D1596-R1668, and D1596-R1659 regions of VWF were also prepared in the same way by combinational use of primers as follows: 5′-cgggatccGAGGCACAGTCCAAAGGGGACA-3′,5′-cgggatccGACCACAGCTTCTTGGTCAGCC-3′, 5′-cgggatccGACCGGGAGCAGGCGCCCAACC-3′, and 5′-cggaattcTCAGTGATGGTGATGGTGATGTCGGGGGAGCGTCTCAAAGTCC-3′.
Expression and purification of recombinant proteins
To obtain the different recombinant proteins, expression vectors encoding GST-D1459R1668-H, GST-E1554R1668-H, GST-D1587R1668-H, GST-D1596R1668-H, and GST-D1596R1659-H were introduced into E coli, BL21 (Stratagene, La Jolla, CA). After isopropyl-β-d-thiogalactoside (IPTG) induction in liquid culture, bacterial cells were collected and lysed with CelLytic B (Sigma, St Louis, MO), followed by centrifugation to separate soluble and insoluble fractions. GST-D1587R1668-H, GST-D1596R1668-H, and GST-D1596R1659-H were collected in soluble fractions, whereas GST-D1459R1668-H and GST-E1554R1668-H were in insoluble fractions. First, all these proteins were purified by Ni-NTA Spin Kit (Qiagen, Hilden, Germany) in a denaturing condition containing 8 M urea and 20 mM 2-mercaptoethanol according to the instruction. The eluates (pH 4.3) were diluted to a 40-times volume of phosphate-buffered saline and left overnight at 4°C for refolding. Then, the proteins were purified by MicroSpin GST Purification Module (Amersham Biosciences) according to the instruction. Eluted proteins (10 mM glutathione) were dialyzed against 20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 8.0) and quantified by DC Protein Assay Kit (Bio-Rad, Hercules, CA) using bovine serum albumin as standard.
Cleavage of recombinant proteins by plasma
Purified recombinant proteins (300 ng) were incubated with 1 μL plasma in 40 μL reaction buffer (5 mM Tris-HCl, 10 mM BaCl2, and 1 mM amidinophenylmethanesulfonyl fluoride hydrochloride, pH 8.0) at 37°C for the indicated time. The reaction was stopped by adding 10 μL sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (50 mM Tris-HCl, 10% SDS, 250 mM dithiothreitol, 10 mM EDTA (ethylenediaminetetraacetic acid), 0.1% bromophenol blue, and 30% glycerol; pH 6.8). Alternatively, to detect inhibitors of ADAMTS-13 in plasma from patients, normal plasma was preincubated with an equal volume of heat-inactivated patient plasma for one hour at room temperature, and then incubated with recombinant substrate proteins at 37°C for 1 hour.
Western blot analysis
The samples were subjected to SDS-PAGE (10%-20% gradient gel) and transferred to a polyvinylidene fluoride membrane (Bio-Rad). Following blocking with 3% skim milk, the membrane was incubated with 1 μg/mL anti-GST (Molecular Probes, Eugene, OR) and then with 0.1 μg/mL peroxidase-labeled anti–rabbit immunoglobulin G (IgG; Kirkegaard & Perry Laboratories, Gaithersburg, MD). Chemiluminescence was developed using the Western Lightning Chemiluminescence Reagent Plus (PerkinElmer, Warrington, United Kingdom) and detected on an image analyzer LAS-1000plus (Fujifilm, Tokyo, Japan).
Results
Preparation of substrate proteins
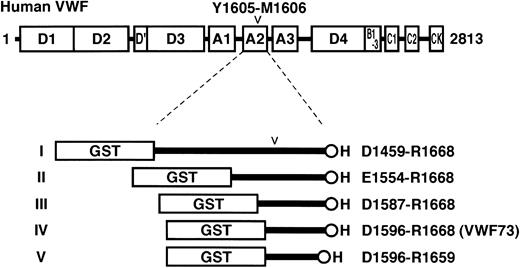
To identify the minimal region of VWF recognized as a substrate by ADAMTS-13, we prepared 5 recombinant proteins containing a partial region of human VWF. First, 2 criteria were set: (1) The region should contain the cleavage site by ADAMTS-13, Y1605 and M1606, in the A2 domain of VWF. (2) It should not contain any cysteine residues that often interfere with the proper folding of artificially engineered proteins. The longest region that satisfied the criteria ranged from D1459 to R1668 of VWF. These 210 amino acid residues were flanked with N-terminal GST and C-terminal H tags for convenient purification and detection, and designated GST-D1459R1668-H or substrate I (Figure 1). The other 4 substrates, GST-E1554R1668-H (substrate II), GST-D1587R1668-H (substrate III), GST-D1596R1668-H (substrate IV), and GST-D1596R1659-H (substrate V), were shorter derivatives of this VWF region.
Structures of VWF and fusion proteins for ADAMTS-13 substrate. The domain structure of human preproVWF is shown above the structures of recombinant fusion proteins designed in the present study. Amino acid residues of preproVWF are numbered from the initiating Met codon. The locations of 5 kinds of structural domains (A, B, C, D, and CK) are indicated. The mature VWF secreted from cells consists of 2050 residues (S764-K2813) from the D′ domain to the C-terminal CK domain. ADAMTS-13 cleaves the Y1605-M1606 peptidyl bond in the A2 domain (D1498-L1664). We made 5 different recombinant proteins flanked with GST- and H-tags: GST-D1459R1668-H (I), GST-E1554R1668-H (II), GST-D1587R1668-H (III), GST-D1596R1668-H (IV), and GST-D1596R1659-H (V).
Structures of VWF and fusion proteins for ADAMTS-13 substrate. The domain structure of human preproVWF is shown above the structures of recombinant fusion proteins designed in the present study. Amino acid residues of preproVWF are numbered from the initiating Met codon. The locations of 5 kinds of structural domains (A, B, C, D, and CK) are indicated. The mature VWF secreted from cells consists of 2050 residues (S764-K2813) from the D′ domain to the C-terminal CK domain. ADAMTS-13 cleaves the Y1605-M1606 peptidyl bond in the A2 domain (D1498-L1664). We made 5 different recombinant proteins flanked with GST- and H-tags: GST-D1459R1668-H (I), GST-E1554R1668-H (II), GST-D1587R1668-H (III), GST-D1596R1668-H (IV), and GST-D1596R1659-H (V).
When expressed in E coli, a band corresponding to the expected size of each substrate was visualized (substrate I, 50.8 kDa; II, 40.4 kDa; III, 36.7 kDa; IV, 35.7 kDa; V, 34.7 kDa) after IPTG induction (Figure 2A). Substrates I and II were collected from the insoluble fractions (inclusion bodies), whereas substrates III, IV, and V were mainly recovered in soluble fractions (Figure 2B). All of the recombinant proteins were purified by 2 steps, nickel-ion chelating column chromatography and glutathione-affinity column chromatography, using C-terminal H and N-terminal GST tags, respectively (Figure 2C).
Preparation of bacterial recombinant proteins. (A) Expression. Arrowheads indicate 5 recombinant proteins expressed in E coli after IPTG induction: GST-D1459R1668-H (I), GST-E1554R1668-H (II), GST-D1587R1668-H (III), GST-D1596R1668-H (IV), and GST-D1596R1659-H (V). Gels after SDS-PAGE were stained with GelCode Blue (Pierce, Rockford, IL). The sizes of the protein standards are indicated at the left. (B) Fractionation. Recombinant proteins I and II were collected in pellet fractions (P) after centrifugation, whereas III, IV, and V were in soluble fractions (S). (C) Purification. All the recombinant proteins were purified by 2 sequential column-chromatography procedures, nickel-ion chelating chromatography and glutathione-affinity chromatography. The representative pattern of GST-D1596R1668-H is shown. S indicates soluble fraction of bacterial lysate; F1, flow-through of nickel-ion column; W1, wash; E1, eluate; F2, flow-through of glutathione column; W2, wash; and E2, eluate.
Preparation of bacterial recombinant proteins. (A) Expression. Arrowheads indicate 5 recombinant proteins expressed in E coli after IPTG induction: GST-D1459R1668-H (I), GST-E1554R1668-H (II), GST-D1587R1668-H (III), GST-D1596R1668-H (IV), and GST-D1596R1659-H (V). Gels after SDS-PAGE were stained with GelCode Blue (Pierce, Rockford, IL). The sizes of the protein standards are indicated at the left. (B) Fractionation. Recombinant proteins I and II were collected in pellet fractions (P) after centrifugation, whereas III, IV, and V were in soluble fractions (S). (C) Purification. All the recombinant proteins were purified by 2 sequential column-chromatography procedures, nickel-ion chelating chromatography and glutathione-affinity chromatography. The representative pattern of GST-D1596R1668-H is shown. S indicates soluble fraction of bacterial lysate; F1, flow-through of nickel-ion column; W1, wash; E1, eluate; F2, flow-through of glutathione column; W2, wash; and E2, eluate.
Cleavage of substrate proteins by normal plasma
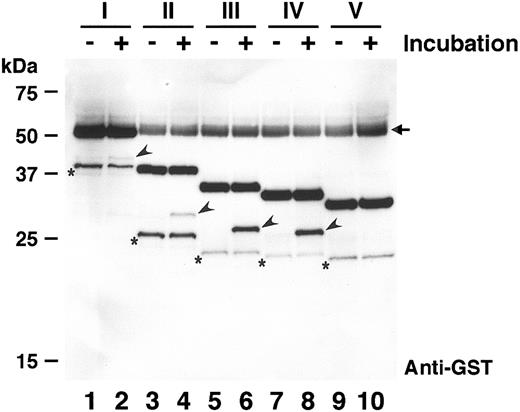
If ADAMTS-13 cleaves the expected site of substrates I, II, III, IV, and V, the sizes of N-terminal portion including the GST-tag will be 43.1, 32.7, 29.0, 28.0, and 28.0 kDa, respectively. To explore the proteolytic effects of human plasma, these substrate proteins were incubated with normal plasma and analyzed by Western blot using an anti-GST antibody (Figure 3). When substrate I was incubated with normal human plasma for one hour, a very faint band (arrowhead in lane 2) appeared with the apparent size of approximately 43 kDa; this band was not detected before incubation (lane 1). This implied that substrate I was cleaved by some protease in plasma. In the presence of 50 mM EDTA, the substrate was not cleaved (data not shown), suggesting that this cleavage was catalyzed by a metalloprotease, possibly ADAMTS-13. For substrates II, III, and IV, the N-terminal fragments with expected sizes were also detected only after incubation with plasma (arrowheads in lanes 4, 6, and 8). Substrates III and IV were cleaved more effectively than I and II. This might be caused by different refolding efficiency during purification, because substrates I and II were recovered from inclusion bodies. Adding urea to the reaction, which is expected to expose the proper cleavage site of these substrates, did not enhance the cleavage (data not shown). Interestingly, substrate V was not cleaved by plasma, suggesting that it was not recognized as a substrate by ADAMTS-13. Thus, the shortest cleavable substrate in these, IV (GST-D1596R1668-H), was characterized further. Hereinafter, the peptide with 73 amino acid residues corresponding to the region from D1596 to R1668 of VWF is referred to as VWF73.
Cleavage of recombinant proteins by normal plasma. The recombinant substrates (I-V) were incubated with normal plasma at 37°C for 0 hours (lanes 1, 3, 5, 7, and 9) or 1 hour (lanes 2, 4, 6, 8, and 10). Both substrates and products were detected by Western blot using anti-GST. The product bands including N-terminal GST-tag are indicated by arrowheads. Substrates III and IV were cleaved more efficiently than I and II, and substrate V was not cleaved. The arrowed bands observed in all lanes are nonspecific signals derived from plasma albumin. The bands with asterisks, probably contaminating degradation products, are reproducible background signals.
Cleavage of recombinant proteins by normal plasma. The recombinant substrates (I-V) were incubated with normal plasma at 37°C for 0 hours (lanes 1, 3, 5, 7, and 9) or 1 hour (lanes 2, 4, 6, 8, and 10). Both substrates and products were detected by Western blot using anti-GST. The product bands including N-terminal GST-tag are indicated by arrowheads. Substrates III and IV were cleaved more efficiently than I and II, and substrate V was not cleaved. The arrowed bands observed in all lanes are nonspecific signals derived from plasma albumin. The bands with asterisks, probably contaminating degradation products, are reproducible background signals.
Characterization of cleavage
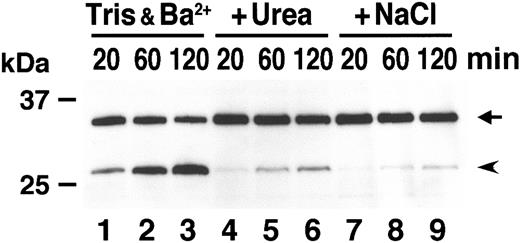
It was previously reported that ADAMTS-13 cleaves VWF in vitro preferentially in the presence of urea and in low ionic strength.4 We examined the effect of urea and NaCl on the cleavage efficiency of GST-VWF73-H. In hypotonic buffer including 5 mM Tris-HCl and 10 mM BaCl2, GST-VWF73-H was efficiently cleaved by normal plasma in a time-dependent manner (Figure 4, lanes 1-3). In the presence of either 1.5 M urea or 150 mM NaCl, however, the production of the N-terminal fragment was quite low (lanes 4-9). The inhibitory effect of physiologic ionic strength was consistent with a previous report.4 No requirement of urea for efficient cleavage suggests that the structure surrounding the Y1605-M1606 peptidyl bond is different between GST-VWF73-H and intact VWF multimers.
Effect of urea and ion strength on cleavage. GST-VWF73-H was incubated with normal plasma for the indicated time in reaction buffer (5 mM Tris-HCl, 10 mM BaCl2, pH 8.0) (lanes 1-3) or in the same buffer supplemented with either 1.5 M urea (lanes 4-6) or 150 mM NaCl (lanes 7-9). The substrate and product bands are shown by an arrow and an arrowhead, respectively.
Effect of urea and ion strength on cleavage. GST-VWF73-H was incubated with normal plasma for the indicated time in reaction buffer (5 mM Tris-HCl, 10 mM BaCl2, pH 8.0) (lanes 1-3) or in the same buffer supplemented with either 1.5 M urea (lanes 4-6) or 150 mM NaCl (lanes 7-9). The substrate and product bands are shown by an arrow and an arrowhead, respectively.
Cleavage of GST-VWF73-H by patient plasma
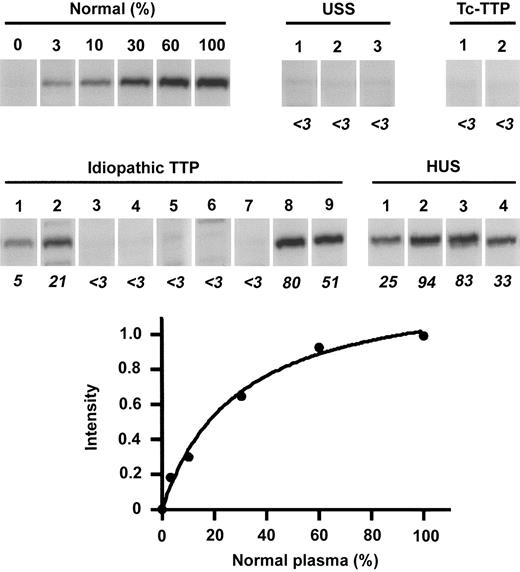
To confirm that the cleavage of GST-VWF73-H is catalyzed by ADAMTS-13, the substrate was incubated with plasma prepared from patients with congenital TTP, USS (Figure 5). Previously, we confirmed that these 3 patients have no VWF-cleaving activity,2 and that USS patients 2 and 3 are a homozygote of the ADAMTS-13 Q449X mutation and a compound heterozygote of R268P/C508Y, respectively.15 In the present assay, none of their plasma cleaved GST-VWF73-H, strongly suggesting that the cleavage of GST-VWF73-H by normal plasma is catalyzed by ADAMTS-13. The plasma derived from patients with ticlopidine-associated TTP also showed no cleavage of the substrate (Figure 5), consistent with our previous reports.35,36 Among 9 patients with idiopathic TTP examined, 5 patients had no ADAMTS-13 activity, but the remaining 4 patients had some apparent activity. This result was also consistent with previous data.37 Conversely, the plasma from 4 patients with HUS produced a fragment of the substrate. Thus, the recombinant substrate, GST-VWF73-H, was confirmed to be a specific substrate for ADAMTS-13.
Cleavage of GST-VWF73-H by patients' plasma. GST-VWF73-H was incubated with serially diluted normal plasma (0%-100%) or with plasma from patients with USS, ticlopidine-associated TTP (Tc-TTP), idiopathic TTP, and HUS. The band intensities of reaction products were measured by chemiluminescence on Western blot, and the relative activities of patients' plasma (%, shown by italic numbers) to normal plasma were calculated from nonlinear regression by serially diluted normal plasma. USS patients 1 to 3 correspond to ST-III-4, SY-III-1, and KI-III-2 by previous report.2 Tc-TTP patients 1 and 2 were reported by Sugio et al35 and Orimo et al,36 respectively. The idiopathic TTP patients 1 to 9 correspond to the case numbers 1-3, 5, 6, 11-13, and 17, and HUS patients 1 to 4 correspond to the case numbers 2, 4, 6, and 9 in the previous report.37
Cleavage of GST-VWF73-H by patients' plasma. GST-VWF73-H was incubated with serially diluted normal plasma (0%-100%) or with plasma from patients with USS, ticlopidine-associated TTP (Tc-TTP), idiopathic TTP, and HUS. The band intensities of reaction products were measured by chemiluminescence on Western blot, and the relative activities of patients' plasma (%, shown by italic numbers) to normal plasma were calculated from nonlinear regression by serially diluted normal plasma. USS patients 1 to 3 correspond to ST-III-4, SY-III-1, and KI-III-2 by previous report.2 Tc-TTP patients 1 and 2 were reported by Sugio et al35 and Orimo et al,36 respectively. The idiopathic TTP patients 1 to 9 correspond to the case numbers 1-3, 5, 6, 11-13, and 17, and HUS patients 1 to 4 correspond to the case numbers 2, 4, 6, and 9 in the previous report.37
Inhibitors of ADAMTS-13 in plasma from patients
Most patients with acquired TTP have autoantibodies that inhibit ADAMTS-13 activity in their plasma.19,20 No inhibitors are detected in plasma from patients with USS.19 After incubation of normal plasma with plasma from the patients with USS or acquired TTP, the cleavage of GST-VWF73-H was examined (Figure 6). Preincubation with plasma from 3 patients with acquired TTP inhibited the cleavage of GST-VWF73-H, whereas preincubation with plasma from 3 USS patients had no effect. This indicates that the assay system using recombinant substrate VWF73 can be also useful to measure inhibitors of ADAMTS-13.
Inhibitory activity of plasma from patients. Normal plasma was preincubated with equal volumes of heat-inactivated plasma from patients with USS (lanes 2-4), Tc-associated TTP (lanes 5-6), and idiopathic TTP (lane 7). Then, cleavage of GST-VWF73-H was compared with normal plasma without preincubation (lane 1). Plasma from patients with acquired TTP but not with USS inhibited substrate cleavage.
Inhibitory activity of plasma from patients. Normal plasma was preincubated with equal volumes of heat-inactivated plasma from patients with USS (lanes 2-4), Tc-associated TTP (lanes 5-6), and idiopathic TTP (lane 7). Then, cleavage of GST-VWF73-H was compared with normal plasma without preincubation (lane 1). Plasma from patients with acquired TTP but not with USS inhibited substrate cleavage.
Discussion
Several assay methods have been reported to measure plasma ADAMTS-13 activity. The original method was developed by Furlan et al31 and Tsai,32 independently. They purified human VWF and incubated it with plasma in the presence of urea or guanidine-HCl as well as divalent cations such as Ba2+ and Ca2+. Subsequently, Furlan et al separated the degraded material by SDS-agarose gel electrophoresis followed by Western blot using anti-VWF antibodies to detect a decrease in VWF-multimer ladders. Alternatively, Tsai separated the reaction materials by SDS-PAGE and detected the degraded products by Western blot. The former is visually attractive and sensitive, but time and skill are required. From an enzymologic viewpoint, the latter approach is superior in that it visualizes the product of the reaction, and not the disappearance of the substrate.
Gerritsen et al developed a different method based on the preferential binding of high–molecular-weight forms of VWF to collagen.38 The proteolytic degradation of VWF leads to low-molecular-weight forms of VWF, which show impaired binding to microtiter plates coated with collagen. The collagen-bound VWF is quantified using antibodies against VWF. Obert et al reported an immunoradiometric assay using 2 site-directed monoclonal antibodies to VWF.39 In this assay, the residual full-length VWF after proteolytic incubation was estimated by a sandwich enzyme-linked immunosorbent assay. Böhm et al recently reported a method based on the positive correlation between VWF multimeric size and Ristocetin cofactor activity.40 After digestion of VWF with plasma, the residual cofactor activity of the samples was assessed to calculate the ADAMTS-13 activity of the samples. Although these assay methods may be more suitable for clinical applications because they require less time to complete, they provide only an indirect detection of the cleavage reaction compared with the original methods developed by Furlan et al31 and Tsai.32
In the present study, we provide a new substrate for ADAMTS-13, VWF73, by which convenient clinical assays can be developed. Compared with the previous methods, VWF73 has several advantages. First, it is the only ADAMTS-13–specific substrate obtained by bacterial expression system. For an enzymatic assay to measure ADAMTS-13 activity, protease-free VWF should be purified from human plasma. To overcome this obstacle, the bacterial recombinant expression system is one of the most convenient alternative methods. Whole VWF, however, is not suitable because of its large size and many disulfide bonds. Therefore, short and soluble VWF73 will be a good molecule for this purpose. Second, VWF73 can be used with N- and C-terminal tag sequences, which are often used for convenient purification and detection. Here, we used both an N-terminal GST-tag and C-terminal H-tag for purification and the GST-tag for immunodetection. These tags could be used to develop a new assay system suitable for clinical usage. Third, no denaturing reagents such as urea or guanidine-HCl as used in the previous methods are needed to cleave VWF73 efficiently. To use whole VWF as a substrate, pretreatment with high concentrations of urea or guanidine-HCl and/or carrying out the proteolytic reaction in the presence of the denaturing reagents is required. VWF73 is efficiently cleaved by ADAMTS-13 in the absence of these reagents, therefore undesired damage on the enzyme can be avoided.
As far as we examined, no significant discrepancy in the plasma ADAMTS-13 activity could be found between assays using intact VWF multimers (multimer analysis) and recombinant VWF73. The discrepancies, however, could be found in the future, because the ADAMTS-13 mutants with different activity against intact VWF and VWF73 may be identified. Alternatively, the autoantibody inhibitors in acquired TTP patients might bind the protease and interfere with recognition of large VWF but not VWF73.
In general, a specific chromogenic assay for each protease is useful for routine clinical measurement. Therefore, trials to find a chromogenic oligopeptide substrate for ADAMTS-13 were carried out but were not successful,41 suggesting that the cleavage at Y1605-M1606 of VWF depends on not only specific residues in the close vicinity of the scissile bond but also some more remote sequences in the VWF subunit. The present study was quite consistent with this assumption. VWF73 (D1596-R1668, substrate IV in Figure 1) was a good substrate for ADAMTS-13, whereas D1596-R1659 (substrate V) was not degraded, indicating that 9 residues between E1660 and R1668 contain essential residues for cleavage. This region may contribute to the structural preservation of the cleavage site for ADAMTS-13 or interact directly with the protease. This will be interesting from the viewpoint of the enzymology of metalloproteases. In order to further define the role of residues E1660 to R1668, we tested whether substrate V could be cleaved by normal plasma in the presence of 1 to 100 μM nonapeptide, EAPDLVLQRR (corresponding to E1660-R1668 of VWF), but substrate V was not still cleaved (data not shown). The nonapeptide also had no effect on the cleavage of VWF73, suggesting that the region may not bind directly to ADAMTS-13 but contribute to proper presentation of the cleavage site to ADAMTS-13.
Causative mutations of the ADAMTS13 gene have been identified in patients with congenital TTP.14-18 In addition, we identified a common missense single-nucleotide polymorphism, P475S, with approximately 5% allele frequency in the Japanese population.15 When this mutant was transiently expressed in cultured cells, it was efficiently secreted from cells like the wild-type molecule but exhibited low VWF-cleaving activity. This suggested that approximately 10% of the Japanese population (heterozygotes of P475S) may possess significantly reduced activity of ADAMTS-13 with the normal antigen level. Other unknown common genetic variations or environmental factors might be involved in abnormal activity of ADAMTS-13. In these cases, enzymatic assays to measure the ADAMTS-13 activity will be more important than the measurement of the antigen levels. Although the almost complete loss of the ADAMTS-13 activity results in TTP, the weakened ADAMTS-13 activity may also be a risk factor for some thrombotic complications due to circulating large VWF multimers. In fact, a recent report suggested decreased levels of the ADAMTS-13 activity in coronary heart disease.42 Well-designed and large-scale studies to assess the relation between ADAMTS-13 and disease will be one of the most important issues in this field.
In conclusion, we here identified the minimal specific substrate for ADAMTS-13, VWF73, which could be a powerful tool to establish clinical enzymatic assays. We strongly hope that it will be widely used and contribute to improving the prognosis and prevention of TTP.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-08-2861.
Supported by grants-in-aid from the Ministry of Health, Labour, and Welfare of Japan; the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Yuko Nobe for her technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal