Abstract
Graft-versus-host disease (GVHD) occurs in an unpredictable fashion after 30% to 50% of matched-related transplantations. The presence of increased frequencies of CD4+CD25+ regulatory T cells in donor grafts has been shown to ameliorate GVHD after allogeneic transplantation in murine models. To determine whether a similar relationship exists in humans, we quantitated the coexpression of CD25 on CD4+ and CD8+ T cells within 60 donor grafts infused into matched siblings and examined GVHD incidence in the respective recipients. Recipients in whom GVHD developed received donor grafts containing significantly higher frequencies of CD4+ T cells coexpressing CD25 than those who did not (median, 9.26% vs 2.22%; P = .004). Frequencies of donor graft CD8+ T cells coexpressing CD25 were also higher (0.65% vs 0.14%; P = .002). Furthermore, transplant recipients who received grafts containing fewer CD4+CD25+ and CD8+CD25+ T cells were less likely to acquire acute GVHD, even though these donor-recipient pairs were similar to others with respect to relevant clinical variables. These data suggest that the coexpression of CD4 and CD25 may be insufficient to identify regulatory T cells in humans and that increased frequencies and numbers of CD25+ T cells in donor grafts is associated with GVHD in transplant recipients. (Blood. 2004;103:1140-1146)
Introduction
Allogeneic stem cell transplantation (SCT) remains an important therapeutic modality for many patients with cancer, especially those with relapsed leukemia and lymphoma (reviewed in Champlin et al1,2 ). Novel approaches to SCT, including the development of nonmyeloablative conditioning regimens and the use of peripheral blood stem cell grafts, have led to more rapid post-SCT engraftment and to reductions in morbidity and mortality, allowing allogeneic SCT to be applied to a wider range of patients, including the elderly and those with nonhematologic malignancies.1,2 Although these advances and improvements in supportive care have improved the prognosis for patients who have undergone SCT in the past 2 decades, acute and chronic graft-versus-host disease (GVHD) remains a major cause of sickness and death after allogeneic SCT.3,4 The incidence of GVHD, thought to be mediated by donor T cells recognizing major and minor histocompatibility antigens on recipient target cells,5,6 may be reduced by serologic and molecular HLA matching of donors and recipients. Despite close molecular matching, GVHD occurs in an unpredictable fashion in 30% to 50% of patients after matched-related transplantation and in a higher fraction of patients after transplantation performed using HLA-mismatched or matched-unrelated donors.3,4,7
Prior clinical studies have identified donor graft characteristics associated with an increased risk for GVHD in patients who have undergone SCT, including the infusion of increased numbers of total nucleated cells (TNCs), CD34+ cells, or CD3+ T cells.3,4,8,9 In addition, murine transplantation models have identified cellular elements and cytokines that modulate the development of GVHD, though none of these factors is routinely assessed in clinical practice.10,11 Recently, a resurgence of attention has been directed toward regulatory T cells (Treg) and their potential to attenuate GVHD and autoimmunity in murine models (reviewed in Bach,12 Shevach,13 and Wood and Sakaguchi14 ). Originally described as a cellular subset that could prevent autoimmunity after neonatal thymectomy in mice, Treg cells are thought to be contained within the subset of CD4+ T cells coexpressing CD25, the interleukin-2 receptor α chain (IL-2Rα).15,16 CD4+CD25+ Treg cells have been shown to suppress alloreactivity in a contact-dependent fashion, with conflicting evidence also suggesting a role for immunosuppressive cytokines such as IL-10 and transforming growth factor β (TGF-β) in Treg-mediated immunosuppression.17-19 In a murine SCT model, Taylor et al20 demonstrated that the depletion of CD25+ T cells accelerated the development of GVHD in recipients, an effect that was also seen after in vivo administration of a CD25 monoclonal antibody (mAb). Additionally, the infusion of ex vivo activated and expanded CD4+CD25+ T cells ameliorated the development of GVHD.20 Other murine studies have confirmed that infusing donor-derived CD4+CD25+ T cells may suppress the development of GVHD in a dose-dependent fashion after allogeneic SCT.21-23 These results have led many to propose that enrichment, expansion, or both of CD4+CD25+ T cells in donor grafts might similarly decrease the incidence of GVHD after SCT in humans, though this has not yet been studied.
Because human SCT grafts vary in composition with respect to the numbers and functions of CD4+ and CD8+ T cells, including putative Treg cells, we sought to determine the association between CD4+CD25+ T cells in donor grafts and the incidence of GVHD in patients who have undergone HLA-matched sibling SCT. No other studies published to date have examined the relationship between CD25+ T cells within donor stem cell grafts and GVHD in patients who have undergone SCT. We hypothesized that heterogeneity in the frequencies of Treg cells would be observed among T cells in donor grafts and that increasing frequencies of CD4+CD25+ T cells might be associated with a lower incidence of GVHD in patients who have undergone SCT. To test this hypothesis, we examined the immunophenotypic composition of donor peripheral blood stem cell (PBSC) grafts and their association with GVHD incidence in 60 HLA-matched sibling transplant recipients of these grafts. We focused on the incidence of acute GVHD as the primary end point of the study.
We found that patients who experienced GVHD after SCT had significantly higher frequencies and absolute numbers of CD4+ and CD8+ T cells coexpressing CD25 in their donor grafts than those who did not. Furthermore, after SCT recipients whose donors had the lowest fractions of CD4+CD25+ or CD8+CD25+ T cells were less likely to acquire acute GVHD than others. These data suggest that the coexpression of CD4 and CD25 may be insufficient to identify Treg in humans and that these cells may represent an activated T-cell phenotype. Importantly, CD25 coexpression on CD4+ or CD8+ donor T cells may be a predictor of GVHD incidence in recipients after HLA-matched SCT.
Patients, materials, and methods
Patient selection
This study used a convenience sample of 60 PBSC grafts from HLA-matched sibling donors obtained during allogeneic transplantations performed at the University of Texas MD Anderson Cancer Center between November 1997 and September 2000. These samples were collected in an unbiased fashion from a subset of allogeneic SCT donors at our center during this period. PBSC graft samples were studied after donors and recipients provided written, informed consent on a study approved by the M. D. Anderson Cancer Center institutional review board, consistent with the principles outlined in the Declaration of Helsinki. Healthy donors were mobilized using granulocyte colony-stimulating factor (G-CSF), and the resultant PBSC products were collected by apheresis using standard methods, with an aliquot of the donor graft cryopreserved for laboratory research. Investigators were blinded with respect to clinical outcome while analyzing CD25 expression on donor PBSC grafts.
Diagnosis, clinical management, and scoring of GVHD
All patients who underwent SCT received standard prophylaxis for GVHD using tacrolimus and, in most cases, low-dose methotrexate (Table 1).24 Clinical GVHD was graded according to standard criteria.3,25 These data, in addition to data related to patient and graft characteristics, are entered and validated in a departmental clinical database according to set standards. A retrospective analysis was performed of PBSC graft characteristics and of the incidence and severity of GVHD in Patients who underwent SCT. GVHD was managed with usual best practices and generally was treated initially with steroids; additional therapies were used for steroid-refractory GVHD.3,4 Because we sought to determine the relationship between T cells in the infused donor graft and the incidence of GVHD, patients who underwent SCT who received subsequent donor lymphocyte infusions (DLIs) for the treatment of relapse after SCT were censored at the time of DLI.
Patient and donor characteristics and GVHD incidence
. | No. . | Range . |
|---|---|---|
| Recipient-donor pairs studied, n | 60 | — |
| Recipient median age, y | 46.5 | 10-70 |
| Donor median age, y | 44.5 | 16-76 |
| Recipient sex, n, M/F | 43/17 | — |
| Donor sex, n, M/F | 35/25 | — |
| Male recipient of female donor, n (%) | 18 (30) | — |
| Recipient diagnosis, n | ||
| Non-Hodgkin lymphoma | 20 | — |
| Acute myeloid leukemia | 16 | — |
| Chronic myeloid leukemia | 11 | — |
| Acute lymphoid leukemia | 3 | — |
| Chronic lymphoid leukemia | 4 | — |
| Multiple myeloma | 2 | — |
| Hodgkin disease | 2 | — |
| Other | 2 | — |
| Conditioning regimen, n | ||
| Busulphan/cyclophosphamide | 17 | — |
| TB1/cyclophosphamide | 3 | — |
| BEAM | 14 | — |
| Fludarabine/melphalan | 6 | — |
| Fludarabine/busulphan | 1 | — |
| Fludarabine/idarubicin/cytarabine | 6 | — |
| Fludarabine/cyclophosphamide | 8 | — |
| Fludarabine/cisplatin/cytarabine | 5 | — |
| GVHD prophylaxis, n | ||
| Tacrolimus/low-dose methotrexate | 58 | — |
| Tacrolimus | 1 | — |
| Cyclosporine/prednisone | 1 | — |
| Median infused values | ||
| TNC, × 108/kg | 5.97 | 1.05-18.32 |
| CD34+, 106/kg | 4.47 | 2.56-7.1 |
| CD3+, 106/kg | 178.1 | 41.3-558.6 |
| CD4+, 106/kg | 109.3 | 23.1-268.2 |
| CD8+, 106/kg | 63.2 | 11.6-130.1 |
| Follow-up, d | 330 | 67-1310 |
| GVHD, n | ||
| Acute | 9 (15)* | — |
| Chronic | 11 (18)† | — |
| Acute + chronic | 13 (22)‡ | — |
| No GVHD, n | 27 (45) | — |
. | No. . | Range . |
|---|---|---|
| Recipient-donor pairs studied, n | 60 | — |
| Recipient median age, y | 46.5 | 10-70 |
| Donor median age, y | 44.5 | 16-76 |
| Recipient sex, n, M/F | 43/17 | — |
| Donor sex, n, M/F | 35/25 | — |
| Male recipient of female donor, n (%) | 18 (30) | — |
| Recipient diagnosis, n | ||
| Non-Hodgkin lymphoma | 20 | — |
| Acute myeloid leukemia | 16 | — |
| Chronic myeloid leukemia | 11 | — |
| Acute lymphoid leukemia | 3 | — |
| Chronic lymphoid leukemia | 4 | — |
| Multiple myeloma | 2 | — |
| Hodgkin disease | 2 | — |
| Other | 2 | — |
| Conditioning regimen, n | ||
| Busulphan/cyclophosphamide | 17 | — |
| TB1/cyclophosphamide | 3 | — |
| BEAM | 14 | — |
| Fludarabine/melphalan | 6 | — |
| Fludarabine/busulphan | 1 | — |
| Fludarabine/idarubicin/cytarabine | 6 | — |
| Fludarabine/cyclophosphamide | 8 | — |
| Fludarabine/cisplatin/cytarabine | 5 | — |
| GVHD prophylaxis, n | ||
| Tacrolimus/low-dose methotrexate | 58 | — |
| Tacrolimus | 1 | — |
| Cyclosporine/prednisone | 1 | — |
| Median infused values | ||
| TNC, × 108/kg | 5.97 | 1.05-18.32 |
| CD34+, 106/kg | 4.47 | 2.56-7.1 |
| CD3+, 106/kg | 178.1 | 41.3-558.6 |
| CD4+, 106/kg | 109.3 | 23.1-268.2 |
| CD8+, 106/kg | 63.2 | 11.6-130.1 |
| Follow-up, d | 330 | 67-1310 |
| GVHD, n | ||
| Acute | 9 (15)* | — |
| Chronic | 11 (18)† | — |
| Acute + chronic | 13 (22)‡ | — |
| No GVHD, n | 27 (45) | — |
Numbers in parentheses are percentages.—indicates no range possible.
One patient had stage 1 disease, and 8 had stages II-IV disease.
Two patients had limited disease, and 9 had extensive disease.
Three patients had stage I disease, and 10 had stages II-IV disease. Four patients had limited disease, and 9 had extensive disease.
Assessment of TNC, CD34+, and CD3+ cell numbers in PBSC grafts
TNC, CD34+ cell, and CD3+ cell numbers in the donor grafts were assessed in the clinical cellular processing laboratory before PBSC cryopreservation using standard methods.
Preparation of PBSCs for CD25 analysis
After apheresis, PBSC products were cryopreserved using standard methods of graduated freezing in 10% dimethyl sulfoxide (DMSO), followed by transfer to liquid nitrogen for long-term storage. Donor PBSC products were thawed and washed with cold phosphate-buffered saline (PBS) before resuspension in PBS containing 2% fetal bovine serum (FBS) in preparation for mAb staining. Cell counts were determined visually using a hemacytometer; viability was assessed by trypan blue exclusion and generally exceeded 90%. Aliquots of 106 cells were used for mAb staining.
Assessment of CD25 expression on PBSCs by flow cytometry
PBSCs were stained in a PBS buffer containing 2% FBS. Replicate aliquots of 106 cells were used to examine CD25 expression with a phycoerythrin (PE)-conjugated murine antihuman CD25 monoclonal mAb (BD Biosciences, San Jose, CA) using a matched, PE-conjugated isotype control mAb (immunoglobulin G1 [IgG1]; BD Biosciences) as a control. Initially, we confirmed that CD25 expression was similar when quantitation was performed using a distinct antihuman mAb clone (Coulter, Hialeah, FL) (data not shown). Cells were stained at 25°C for 15 minutes with the CD25-specific mAb and with additional mAbs specific for CD4 (conjugated to fluorescein isothiocyanate [FITC]) and CD8 (conjugated to PerCP) (all antibodies from BD Biosciences). After staining, cells were washed in PBS containing 2% FBS and were fixed in 1% paraformaldehyde. Staining was quantitated using a FACScan cytometer (BD Biosciences) and were analyzed using CellQuest software (BD Biosciences). The frequency of CD25-expressing T cells was calculated by sequential gating on lymphocytes (by scatter), CD4+ and CD8+ T cells, and the use of a histogram depicting CD25 expression within the respective CD4+- and CD8+-gated populations. The threshold for background fluorescence was set based on isotype control staining of 0.5% in a matched control sample, with the same threshold then used to determine the frequency of CD25+ T cells in the matched experimental sample.
Statistical analyses
The primary end point of the study was the incidence of acute GVHD. Patients and graft characteristics were compared using the χ2 and Fisher exact tests for categorical variables and the Mann-Whitney U test for continuous variables. The incidence of acute GVHD was estimated using the Kaplan-Meier method. We used the cumulative incidence method to estimate the incidence of chronic GVHD, considering death and relapse without GVHD as competing risks. A Cox proportional hazard model was used to compare the rates of acute and chronic GVHD according to patient and graft characteristics in univariate and bivariate analyses. All P values were 2-tailed and were considered significant at P < .05. Statistical analyses were performed using STATA (StataCorp 2001, v 7.0; College Station, TX). Results were displayed using Prism (GraphPad) and Illustrator (Adobe, Seattle, WA) using Macintosh computers (Apple Computer, Cupertino, CA).
Results
Patient characteristics and incidence of GVHD
We studied 60 recipients of allogeneic mobilized PBSC transplants who underwent SCT between November 1997 and October 2000, and we assessed the immunophenotypic characteristics of their respective donor grafts. Characteristics of the patient population are listed in Table 1. Most recipients had hematologic malignancies. A wide variety of diseases was represented, including non-Hodgkin lymphoma (20 patients), acute myeloid leukemia (16 patients), chronic myeloid leukemia (11 patients), chronic lymphoid leukemia (4 patients), acute lymphoid leukemia (3 patients), multiple myeloma (2 patients), Hodgkin disease (2 patients), malignant histiocytosis (1 patient), and breast cancer (1 patient). Twenty Patients who underwent SCT received standard ablative conditioning regimens containing cyclophosphamide (combined with intravenous busulphan in 17 recipients and total body irradiation in 3 recipients); 14 received conditioning with BEAM (carmustine, etoposide, cytosine arabinoside, and melphalan); 26 others received fludarabine-based reduced-intensity or nonmyeloablative conditioning regimens. In the first 100 days after SCT, 1 patient died (day 67) without acquiring GVHD, and 9 patients had relapses at a median of 76 days (range, 64-95 days) in the absence of GVHD. Acute GVHD developed in 22 (37%) of 60 recipients of stem cell transplants at a median of 22 days (range, 9-93 days), with 90% of cases occurring by day 63 after SCT. Thirteen of the 22 patients subsequently acquired chronic GVHD. Chronic GVHD developed in 11 more patients without antecedent acute GVHD. Data related to the frequency and severity of GVHD are outlined in Table 1.
CD25 expression may be assessed in CD4+ and CD8+ T cells using flow cytometry
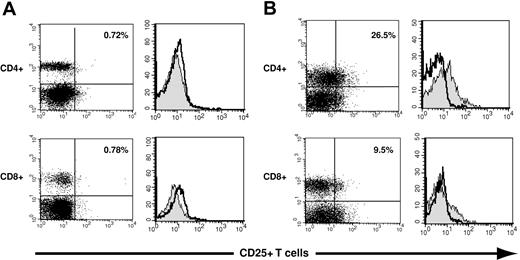
Flow cytometric methods were used to assess the expression of the IL-2 receptor α chain, or CD25, on human CD4+ and CD8+ T cells. Representative examples of staining of CD4+ T cells using an IgG1 isotype control antibody and a murine mAb specific for CD25 are shown in Figure 1. In the 60 PBSC grafts, CD25 expression on CD4+ T cells ranged from 0% to 31% (median, 7.2%), whereas fewer CD8+ T cells in PBSC grafts had demonstrable CD25 expression (range, 0% to 9.5%; median, 0.4%; data not shown).
Assessment of CD25 expression on donor graft CD4+ and CD8+ T cells. Three-color flow cytometry was used to assess CD25 expression on CD4+ and CD8+ T cells in donor PBSC grafts. (A) CD25 expression is illustrated using dot plots and histograms on a donor PBSC graft with minimal expression of CD25. The left panels illustrate the expression of CD25 on CD4+ T cells (top) and CD8+ T cells (bottom). The right panels depict histograms used to quantitate CD25 expression: The gray histograms represent staining with a CD25-specific antibody; open histograms, isotope control staining on a paired sample. CD25+ T cells constituted 0.72% of CD4+ T cells (top) and 0.78% of CD8+ T cells (bottom). (B) CD25 expression is similarly illustrated for a donor with relatively high CD25 expression on graft CD4+ and CD8+ T cells (26.5%, top panel, upper right quadrant; and 9.5%, bottom panel, upper right quadrant).
Assessment of CD25 expression on donor graft CD4+ and CD8+ T cells. Three-color flow cytometry was used to assess CD25 expression on CD4+ and CD8+ T cells in donor PBSC grafts. (A) CD25 expression is illustrated using dot plots and histograms on a donor PBSC graft with minimal expression of CD25. The left panels illustrate the expression of CD25 on CD4+ T cells (top) and CD8+ T cells (bottom). The right panels depict histograms used to quantitate CD25 expression: The gray histograms represent staining with a CD25-specific antibody; open histograms, isotope control staining on a paired sample. CD25+ T cells constituted 0.72% of CD4+ T cells (top) and 0.78% of CD8+ T cells (bottom). (B) CD25 expression is similarly illustrated for a donor with relatively high CD25 expression on graft CD4+ and CD8+ T cells (26.5%, top panel, upper right quadrant; and 9.5%, bottom panel, upper right quadrant).
Increased CD25 expression on donor CD4+ and CD8+ T cells is associated with GVHD in recipients after SCT
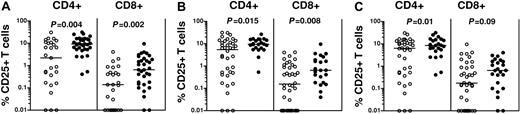
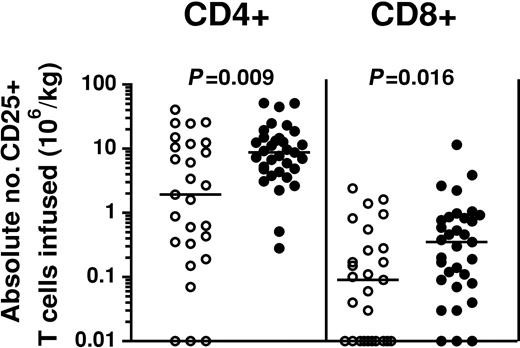
To determine whether CD25 coexpression on donor PBSC graft T cells was associated with the development of GVHD in patients who underwent SCT, we first compared the distribution of CD4+ and CD8+ T cells expressing CD25 in donor-recipient pairs stratified according to the occurrence of GVHD in the recipient. Patients with GVHD received significantly higher fractions of CD25-expressing CD4+ T cells in donor grafts than those in whom GVHD did not develop (respective medians, 9.26% and 2.22%; respectively; P = .004). Similarly, patients with GVHD received significantly higher fractions of CD25-expressing CD8+ T cells than those who did not (respective medians, 0.65% and 0.14%; P = .002). Statistically significant increases were seen in the distribution of CD4+ and CD8+ T cells coexpressing CD25 regardless of the subset of GVHD patients considered, including those with acute GVHD (P = .015 and P = .008 for CD4+25+ and CD8+CD25+ T cells, respectively) or chronic GVHD (P = .01 and P = .09 for CD4+25+ and CD8+CD25+ T cells, respectively) (Figure 2). We also performed a similar analysis examining the absolute numbers of CD4+CD25+ and CD8+CD25+ T cells infused. Recipients experiencing acute or chronic GVHD also received increased absolute numbers of CD25-expressing CD4+ and CD8+ T cells in donor PBSC grafts. Patients experiencing GVHD after SCT received higher numbers of CD4+CD25 T cells within donor grafts than those who did not (median, 8.75 × 106 CD4+CD25+ T cells/kg recipient weight vs 1.93 × 106; P = .009) and higher absolute numbers of CD8+CD25+ T cells (median, 0.35 × 106 CD8+CD25+ T cells/kg recipient weight vs 0.09 × 106; P = .016) (Figure 3).
Increased frequencies of CD4+CD25+ and CD8+CD25+ T cells are present in PBSC grafts of SCT recipients with GVHD. Increased frequencies of CD4+CD25+ and CD8+CD25+ T cells were present in donor PBSC grafts of those recipients who experienced GVHD (•) compared with those who did not (○). Horizontal bars indicate the mean. (A) Stratification by incidence of any GVHD after SCT. (B) Stratification by incidence of acute GVHD (grades I-IV). (C) Stratification by incidence of chronic GVHD. All P values were obtained using the Mann-Whitney U test.
Increased frequencies of CD4+CD25+ and CD8+CD25+ T cells are present in PBSC grafts of SCT recipients with GVHD. Increased frequencies of CD4+CD25+ and CD8+CD25+ T cells were present in donor PBSC grafts of those recipients who experienced GVHD (•) compared with those who did not (○). Horizontal bars indicate the mean. (A) Stratification by incidence of any GVHD after SCT. (B) Stratification by incidence of acute GVHD (grades I-IV). (C) Stratification by incidence of chronic GVHD. All P values were obtained using the Mann-Whitney U test.
Increased absolute numbers of CD4+CD25+ and CD8+CD25+ T cells are present in PBSC grafts of SCT patients with GVHD. Increased absolute numbers of CD4+CD25+ and CD8+CD25+ T cells were present in donor PBSC grafts of recipients who experienced GVHD (•) compared with those who did not (○). Values were calculated using actual infused cell doses of CD3+CD4+ and CD3+CD8+ T cells and quantitation of CD25 expression on donor graft T cells as assessed by flow cytometry. All P values were obtained using the Mann-Whitney U test.
Increased absolute numbers of CD4+CD25+ and CD8+CD25+ T cells are present in PBSC grafts of SCT patients with GVHD. Increased absolute numbers of CD4+CD25+ and CD8+CD25+ T cells were present in donor PBSC grafts of recipients who experienced GVHD (•) compared with those who did not (○). Values were calculated using actual infused cell doses of CD3+CD4+ and CD3+CD8+ T cells and quantitation of CD25 expression on donor graft T cells as assessed by flow cytometry. All P values were obtained using the Mann-Whitney U test.
Patients who received fewer donor CD4+ and CD8+ T cells coexpressing CD25 after allogeneic SCT were less likely to acquire GVHD
After the observation that median values of CD25-expressing CD4+ and CD8+ T cells were increased in donor PBSC grafts infused into recipients with GVHD, we sought to determine whether the infusion of PBSCs containing fewer CD25+ T cells was associated with a lower likelihood of GVHD developing after allogeneic SCT. We performed a quartile analysis to divide patients based on the extent of CD25 expression on donor PBSC grafts in the CD4+ and CD8+ T-cell compartments. We then examined the incidence of acute GVHD in the respective groups of SCT patients and calculated hazard ratios for acute and chronic GVHD incidence for those receiving the fewest CD25+ T cells (Table 2). Only 1 (7%) of 15 recipients who received donor PBSCs within the lowest quartile of CD25-expressing CD4+ T cells acquired GVHD, compared with 21 (47%) of 45 remaining patients (hazard ratio for acute GVHD for the lowest quartile of CD4+CD25+ expression was 0.1; 95% confidence interval [CI], 0.01-0.9). Similarly, acute GVHD developed in 2 (13%) of 15 recipients who received donor PBSCs within the lowest quartile of CD25 expression on CD8+ T cells compared with 20 (45%) of 45 remaining recipients (hazard ratio for acute GVHD for the lowest quartile of CD8+CD25+ expression was 0.3; 95% CI, 0.1-1.1). The actuarial incidence of acute GVHD in patients stratified with respect to the first quartile of CD4+CD25+ and CD8+CD25+ expression on donor grafts is depicted in Figure 4. Similar results were seen when stratification was performed with respect to absolute numbers of CD25+ T cells infused (data not shown).
Risk for GVHD compared with quartile of donor CD4+ and CD8+ T CD25 coexpression
. | Patients with acute GVHD (%) . | Hazard ratio vs quartiles 2-4 (95% CI) . | Patients with chronic GVHD (%) . | Hazard ratio vs quartiles 2-4 (95% CI) . |
|---|---|---|---|---|
| CD4+CD25+ T cells | ||||
| Quartile 1 | 1 of 15 (7) | 0.1 (0.01-0.9) | 2 of 15 (13) | 0.15 (0.02-1.2) |
| Quartile 2 | 6 of 15 (40) | — | 8 of 15 (53) | — |
| Quartile 3 | 8 of 15 (53) | — | 6 of 15 (40) | — |
| Quartile 4 | 7 of 15 (47) | — | 8 of 15 (53) | — |
| Quartiles 2-4 | 21 of 45 (47) | — | 22 of 45 (49) | — |
| CD8+CD25+ T cells | ||||
| Quartile 1 | 2 of 15 (13) | 0.2 (0.1-1.1) | 4 of 15 (27) | 0.3 (0.1-1.5) |
| Quartile 2 | 6 of 15 (40) | — | 4 of 15 (27) | — |
| Quartile 3 | 5 of 15 (33) | — | 9 of 15 (60) | — |
| Quartile 4 | 9 of 15 (60) | — | 7 of 15 (47) | — |
| Quartiles 2-4 | 20 of 45 (44) | — | 20 of 45 (44) | — |
. | Patients with acute GVHD (%) . | Hazard ratio vs quartiles 2-4 (95% CI) . | Patients with chronic GVHD (%) . | Hazard ratio vs quartiles 2-4 (95% CI) . |
|---|---|---|---|---|
| CD4+CD25+ T cells | ||||
| Quartile 1 | 1 of 15 (7) | 0.1 (0.01-0.9) | 2 of 15 (13) | 0.15 (0.02-1.2) |
| Quartile 2 | 6 of 15 (40) | — | 8 of 15 (53) | — |
| Quartile 3 | 8 of 15 (53) | — | 6 of 15 (40) | — |
| Quartile 4 | 7 of 15 (47) | — | 8 of 15 (53) | — |
| Quartiles 2-4 | 21 of 45 (47) | — | 22 of 45 (49) | — |
| CD8+CD25+ T cells | ||||
| Quartile 1 | 2 of 15 (13) | 0.2 (0.1-1.1) | 4 of 15 (27) | 0.3 (0.1-1.5) |
| Quartile 2 | 6 of 15 (40) | — | 4 of 15 (27) | — |
| Quartile 3 | 5 of 15 (33) | — | 9 of 15 (60) | — |
| Quartile 4 | 9 of 15 (60) | — | 7 of 15 (47) | — |
| Quartiles 2-4 | 20 of 45 (44) | — | 20 of 45 (44) | — |
—indicates not applicable.
Acute GVHD incidence is significantly reduced in SCT recipients who received donor PBSC grafts, with the lowest fractions of CD4+ and CD8+ T cells expressing CD25. (A) Actuarial incidence of acute GVHD was significantly lower in SCT patients receiving a lower fraction of CD4+CD25+ T cells than others (7% for recipients with donors in the lowest quartile of CD25 expression [solid line] vs 47% for others [dashed line]; P = .01). (B) Actuarial incidence of acute GVHD was also lower in SCT patients receiving a lower fraction of CD8+CD25+ T cells than others (13% for recipients with donors in the lowest quartile of CD25 expression [solid line] vs 44% for others [dashed line]; P = .05).
Acute GVHD incidence is significantly reduced in SCT recipients who received donor PBSC grafts, with the lowest fractions of CD4+ and CD8+ T cells expressing CD25. (A) Actuarial incidence of acute GVHD was significantly lower in SCT patients receiving a lower fraction of CD4+CD25+ T cells than others (7% for recipients with donors in the lowest quartile of CD25 expression [solid line] vs 47% for others [dashed line]; P = .01). (B) Actuarial incidence of acute GVHD was also lower in SCT patients receiving a lower fraction of CD8+CD25+ T cells than others (13% for recipients with donors in the lowest quartile of CD25 expression [solid line] vs 44% for others [dashed line]; P = .05).
The lower GVHD incidence in patients receiving fewer CD25+ donor T cells is not explained by differences in other GVHD-associated variables
Several variables related to the composition of PBSC grafts have been associated with an increased risk for GVHD in SCT recipients, including the dose of total nucleated cells, the number of CD34+ progenitor cells, and the number of CD3+ T cells.3,4,8,9 To ensure that the lower GVHD incidence seen in SCT patients receiving the fewest CD25+ T cells was not caused by differences in other GVHD-associated variables in this group, we examined GVHD-associated graft and clinical factors in matched donor-recipient pairs in the lowest quartiles of CD25 expression within CD4+ and CD8+ donor graft T cells. As shown in Table 3, we found no significant differences in recipient conditioning regimen, patient or donor sex, patient or donor age, or number of infused total nucleated cells, CD34+ cells, or CD3+ cells in recipients stratified by quartile of CD25 expression on donor CD4+ or CD8+ T cells.
GVHD-associated factors compared with quartile of donor graft T-cell CD25 expression
. | CD4+CD25+ T cells . | . | . | CD8+CD25+ T cells . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | Lowest quartile* . | Quartiles 2-4† . | P . | Lowest quartile* . | Quartiles 2-4† . | P . | ||||
| Conditioning intensity, n (%) | .9 | .6 | ||||||||
| Myeloablative | 4 (27) | 16 (36) | — | 4 (27) | 16 (36) | — | ||||
| Reduced-intensity | 6 (40) | 14 (31) | — | 7 (47) | 13 (29) | — | ||||
| Nonmyeloablative | 5 (33) | 15 (33) | — | 4 (27) | 16 (36) | — | ||||
| Sex compatibility, n (%) | .7 | .7 | ||||||||
| Female donor of male recipient | 3 (20) | 13 (29) | — | 3 (20) | 13 (29) | — | ||||
| Others | 12 (80) | 32 (71) | — | 12 (80) | 32 (71) | — | ||||
| Patient age, y, median (range) | 48 (10-72) | 45 (21-70) | .6 | 49 (10-66) | 45 (21-72) | .6 | ||||
| Donor age, y, median (range) | 49 (17-76) | 43 (16-70) | .2 | 51 (17-65) | 43 (16-76) | .2 | ||||
| Infused nucleated cells, × 108/kg, median (range) | 6.7 (1-20.2) | 5.9 (2.4-18.3) | .5 | 7.1 (1-9.8) | 5.9 (2.4-20.2) | .4 | ||||
| Infused CD34+ cells, × 106/kg, median (range) | 4.3 (2.6-6.9) | 4.7 (2.2-8) | .2 | 4.4 (2.6-6.9) | 4.7 (2.2-8) | .8 | ||||
| Infused CD3+ cells, × 106/kg, median (range) | 180 (79-360) | 176 (41-2342) | .6 | 180 (79-360) | 175 (41-559) | .6 | ||||
. | CD4+CD25+ T cells . | . | . | CD8+CD25+ T cells . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | Lowest quartile* . | Quartiles 2-4† . | P . | Lowest quartile* . | Quartiles 2-4† . | P . | ||||
| Conditioning intensity, n (%) | .9 | .6 | ||||||||
| Myeloablative | 4 (27) | 16 (36) | — | 4 (27) | 16 (36) | — | ||||
| Reduced-intensity | 6 (40) | 14 (31) | — | 7 (47) | 13 (29) | — | ||||
| Nonmyeloablative | 5 (33) | 15 (33) | — | 4 (27) | 16 (36) | — | ||||
| Sex compatibility, n (%) | .7 | .7 | ||||||||
| Female donor of male recipient | 3 (20) | 13 (29) | — | 3 (20) | 13 (29) | — | ||||
| Others | 12 (80) | 32 (71) | — | 12 (80) | 32 (71) | — | ||||
| Patient age, y, median (range) | 48 (10-72) | 45 (21-70) | .6 | 49 (10-66) | 45 (21-72) | .6 | ||||
| Donor age, y, median (range) | 49 (17-76) | 43 (16-70) | .2 | 51 (17-65) | 43 (16-76) | .2 | ||||
| Infused nucleated cells, × 108/kg, median (range) | 6.7 (1-20.2) | 5.9 (2.4-18.3) | .5 | 7.1 (1-9.8) | 5.9 (2.4-20.2) | .4 | ||||
| Infused CD34+ cells, × 106/kg, median (range) | 4.3 (2.6-6.9) | 4.7 (2.2-8) | .2 | 4.4 (2.6-6.9) | 4.7 (2.2-8) | .8 | ||||
| Infused CD3+ cells, × 106/kg, median (range) | 180 (79-360) | 176 (41-2342) | .6 | 180 (79-360) | 175 (41-559) | .6 | ||||
— indicates not calculated.
N = 15.
N = 45.
SCT patients with GVHD have high frequencies of CD25high T cells in donor grafts
It has been suggested that the intensity of CD25 expression on CD4+ T cells may be used to demarcate the subset of CD4+CD25+ T cells with regulatory activity.26 Baecher-Allan et al26 stained human PBMCs with a CD25-specific mAb and suggested that only CD4+CD25+ cells with the highest fluorescence intensity (ie, CD25high) after CD25 mAb staining were capable of suppressing the proliferation of CD4+CD25 T cells after stimulation using platebound anti-CD3 antibodies. To determine whether the presence of donor graft T cells expressing high levels of CD25 was associated with a lower incidence of GVHD in SCT patients, we also compared frequencies of CD4+CD25high T cells in donor grafts stratified by the occurrence of GVHD in recipients. Consistent with what we observed for total CD4+CD25+ T cells, CD4+CD25high T cells were also seen at higher frequencies within donor grafts of recipients experiencing acute or chronic GVHD compared with those in whom GVHD did not develop (data not shown).
Discussion
Although allogeneic SCT continues to be an important curative treatment for many patients with malignancy, GVHD remains a significant cause of morbidity and mortality in SCT patients.2-4 Indeed, despite the availability of molecular HLA typing and the use of matched sibling donors when available, it is difficult to identify patients at risk for GVHD. Better elucidation of clinical or graft-related variables that identify those at highest risk for GVHD may lead to targeted trials of prophylaxis and may also suggest strategies by which graft engineering might be used to limit the onset of GVHD. Recently, a large body of work in murine models has suggested that CD4+CD25+ Treg cells are important in regulating T-cell responses and autoimmunity in vivo16,19,27-31 and that such cells play a particularly important role in controlling allogeneic graft rejection and GVHD after tissue and bone marrow transplantation, respectively.20-23,32 Indeed, it has been shown that CD4+CD25+ T cells lower the incidence, and even completely suppress the development, of GVHD after major histocompatibility complex (MHC)-mismatched or even haploidentical murine SCT.20-23,33 Importantly, the ratio of CD4+CD25+ T cells to other cells in the donor graft has been shown to be predictive of GVHD risk.21 The suppressor phenotype of CD4+CD25+ Treg cells has also been shown to be preserved after ex vivo expansion and subsequent infusion into SCT recipients, providing a potential rationale for similar strategies in human SCT.20,22 Given the impressive potential of CD4+CD25+ T cells to control GVHD in the mouse, we sought to determine whether CD25 expression in donor PBSC grafts in humans could similarly influence the development of GVHD in recipients of matched allogeneic SCT grafts. This study is the first to delineate the association between CD25 expression on donor PBSC graft T cells and GVHD in human recipients after SCT.
We first examined the overall incidence of GVHD in our group of 60 SCT patients and the clinical features of donors and recipients previously known to have an increased incidence of GVHD. We also examined known graft variables associated with the development of GVHD in recipients, including higher total nucleated cell dose, higher CD34-expressing progenitor cells, and total CD3+ T cell dose.3,4,8,9 When we examined the expression of CD25 on CD4+ and CD8+ T cells among donor PBSC grafts, we found that higher frequencies of CD4+ T cells expressing CD25 are seen in patients with acute and chronic GVHD compared with those who did not experience GVHD and that SCT recipients receiving donor grafts with the fewest CD4+CD25+ or CD8+CD25+ T cells were less likely to have GVHD than others receiving grafts with higher frequencies of CD25-expressing T cells. Patients receiving the fewest CD25+ T cells in donor grafts were similar to other recipients with respect to previously defined GVHD-associated variables, including donor- and recipient-associated factors. This relationship was consistent whether we examined the frequencies of CD25+ T cells within donor grafts, as shown to influence the development of GVHD in murine models, or the absolute numbers of CD25+ CD4+ and CD8+ T cells. Similar associations were observed whether the total CD4+CD25+ population or the CD4+CD25high population, as defined by the fluorescence intensity of CD25 staining, was considered. These data do not support the hypothesis that most CD25+ T cells in donor PBSC grafts have suppressor/regulatory activity. Instead, they suggest that the adoptive transfer of increased frequencies or numbers of these cells in unmanipulated donor grafts may increase the likelihood of GVHD in recipients of stem cell transplants.
These data highlight an important problem in our understanding of activated and regulatory T-cell subsets—currently accepted phenotypic markers of regulatory T cells are also found on activated T cells. For example, the increased expression of the IL-2 receptor (CD25) is seen on activated T cells and on CD4+ Treg cells. Indeed, a consistent feature of other surface markers of Treg cells, including cytotoxic T-lymphocyte antigen 4 (CTLA4), the glucocorticoid-induced tumor necrosis factor receptor family-related gene (GITR), and CD122 (the IL-2 receptor β-chain), is that they are all up-regulated with cellular activation.14 Recently, it has been shown that expression of the forkhead transcription factor Foxp3 appears to be an important determinant of lineage commitment to a Treg phenotype in murine models.34-36 Overexpression of Foxp3 induced naive CD4+ T cells to function in a regulatory capacity.34-36 Although Foxp3 expression may serve as the best known discriminator of CD4+ Treg cells, the lack of an associated cell-surface phenotype leaves a selectable surface marker of Treg cells elusive. Because of these limitations, we could not confirm in this analysis that most CD25-expressing T cells in donors whose recipients experienced GVHD were activated, not regulatory, T cells. However, the parallel expression of CD25 on donor CD4+ and CD8+ T cells is consistent with this notion because regulatory activity is not thought to reside in CD8+CD25+ T cells. Events leading to the activation of donor T cells might be expected to induce the up-regulation of CD25 on CD4+ and CD8+ T cells, as we observed in association with recipient GVHD.
If CD25 expression on donor CD4+ and CD8+ T cells is a reflection of cellular activation, as we hypothesize, it appears that adoptively transferred activated T cells may be involved in the initiation phase of GVHD. Because our PBSC donors were mobilized with G-CSF and donor graft cells were subsequently collected by apheresis, it is possible that either the mobilization or the collection process could have contributed to cellular activation. However, systematic analysis of phenotypic and functional properties of donor immune cells demonstrated that though absolute numbers of CD3+CD25+ T cells were increased in peripheral blood after G-CSF administration, cell levels increased in proportion to increases in total CD3+ T cells.37 In fact, the proportion of CD25-expressing T cells within the overall CD3+ population actually declined slightly after G-CSF therapy.37 Although the impact of the apheresis process on T-cell activation is uncertain, there is no reason to suspect that some, but not other, donors would be preferentially affected. Similarly, though the disparity in minor histocompatibility antigens on recipient target cells is likely to be an important determinant of GVHD incidence in HLA-matched SCT recipients,5,38,39 there is no a priori reason to suspect that donors with T cells recognizing such antigens should have increased CD25 expression on graft T cells before infusion into recipients.
Our results support a model wherein GVHD is determined through allorecognition of major and minor antigen differences by SCT recipient antigens by donor T cells, but they may also be determined by the preexisting activation status of these T cells in the infused graft. This may predispose such activated T cells toward a lower threshold for effector response after T-cell receptor (TCR)-mediated recognition of their cognate target antigens after infusion into SCT recipients, perhaps by the elaboration of GVHD-mediating cytokines at the sites of tissue injury.10,11 Further studies, conducted in murine models of SCT and in human clinical subjects, will be needed to test these possibilities. Additional clinical studies, including larger and prospective studies of the association between donor PBSC CD25 expression and recipient GVHD and of the effects of G-CSF mobilization and apheresis on T-cell activation, should be conducted to confirm or extend our findings. If such studies confirm that CD25 expression on donor CD4+ and CD8+ T cells is a result of cellular activation that subsequently increases the risk for GVHD after SCT, it may be worthwhile to assess whether the active depletion of these relatively minor T-cell subsets may lessen the incidence of GVHD after PBSC infusion. Although the depletion of total CD3+ T cells is associated with an increased risk for infection and relapse,40 because of the loss of antigen-specific T cells capable of mediating protective immune responses to pathogens41 and leukemia-specific antigens,42 the depletion of a minor activated T-cell subset would still leave most of the TCR repertoire intact in the donor graft. In murine models43 and in human preclinical trials,44-46 the ex vivo depletion of CD25+ donor T cells after stimulation with recipient alloantigens using a toxin-conjugated CD25-specific mAb has been shown to limit the development of GVHD after SCT. To our knowledge, the depletion of CD25-expressing T cells without alloantigenic stimulation has not been tested as a strategy to limit GVHD. Ideally, because CD25 expression demarcates activated and Treg cells, such depletion would be executed using an alternative marker (or markers) of activated T cells that would spare Treg cells capable of GVHD suppression. Further studies to characterize the function and phenotype of Treg cells, perhaps using Foxp3 expression as a molecular marker to enable the segregation of surface phenotypes of such cells, should facilitate the development of such graft-engineering strategies.
In conclusion, we have demonstrated that CD4+CD25+ and CD8+CD25+ T cells were present at higher frequencies in donor PBSC grafts of patients who underwent allogeneic SCT and who acquired GVHD and that infusing grafts containing reduced numbers of such cells results in a decreased incidence of acute GVHD. Additional studies to define the function and phenotypes of regulatory and activated CD4+ and CD8+ T cells in donor PBSC grafts should enable the development of more rational strategies to attenuate the risk for GVHD after allogeneic SCT.
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2003-06-2085.
Supported in part by the Gillson-Longenbaugh Foundation (K.V.K.), the Leukemia and Lymphoma Society of America Translational Research Program Grant 6606-01 (K.V.K.), and National Institutes of Health grant RO1 CA81247 (J.J.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Safa Karandish and Jo Lauppe for assistance obtaining graft-related data and Elizabeth Shpall, Qing Ma, and Eric Wieder for helpful discussions. We also thank the clinicians, nurses, donors, and patients of the Department of Blood and Marrow Transplantation at the MD Anderson Cancer Center.

![Figure 4. Acute GVHD incidence is significantly reduced in SCT recipients who received donor PBSC grafts, with the lowest fractions of CD4+ and CD8+ T cells expressing CD25. (A) Actuarial incidence of acute GVHD was significantly lower in SCT patients receiving a lower fraction of CD4+CD25+ T cells than others (7% for recipients with donors in the lowest quartile of CD25 expression [solid line] vs 47% for others [dashed line]; P = .01). (B) Actuarial incidence of acute GVHD was also lower in SCT patients receiving a lower fraction of CD8+CD25+ T cells than others (13% for recipients with donors in the lowest quartile of CD25 expression [solid line] vs 44% for others [dashed line]; P = .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/3/10.1182_blood-2003-06-2085/6/m_zh80030455890004.jpeg?Expires=1769094985&Signature=OMkbaDXm35ttz9tKfILA3B9vP0LK8lHRcWOmuC27HCz5g3NALHPZWveizfWXcjgepdkx3PVdNB-drrlXf3xusUgyDhhb9Bt6Mq635TLzRjlywcrIeJkHWPhEdFgkjldUswrJOWL1glJz6YUUe-GQch9jCmGIa3G-rGcRi-h15TqylwDluDjJ0nYsalizEkXtGn0HY95eUO6DR4UdZ5i8lw1K16E1~OSIkXNTaYPC-Av6sdg92bB64Qb8NEllh8koat~KhazlAQR~s5cUWbfDe7fl6GJJ3MFdvmfHMtzISRIC4kSOBIleLnle65PNK1aw57VdvzNWdiBZTtxPDOYm9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal