Abstract
The syndrome of multiple intestinal atresia with immunodeficiency is a rare, invariably fatal congenital disorder. At 16 months of age, a child with this syndrome underwent liver-small bowel transplantation from a 1-of-6 HLA-matched donor. He acquired full enteral tolerance and normal liver function and has never shown evidence of allograft rejection. After mild graft-versus-host disease developed, studies revealed that more than 99% of his CD3+ lymphocytes and 50% of his CD19+ lymphocytes were of donor origin, whereas granulocytes and monocytes remained of recipient origin. He synthesizes polyclonal immunoglobulin G (IgG), IgA, and IgM and has developed antibodies to cytomegalovirus (CMV) and parainfluenza 3. His T lymphocytes are predominately CD3+CD4-CD8- with T-cell receptor γδ heterodimers and CD3+CD4-CD8+ with CD8αα homodimers, populations consistent with an intraepithelial lymphocyte phenotypic profile. We postulate that he has engrafted a donor intestine-derived immune system and is incapable of rejecting his engrafted organs. (Blood. 2004;103:1171-1174)
Introduction
Multiple intestinal atresia with immunodeficiency (MIAI) is a rare autosomal-recessive inherited disorder.1-3 Infants with MIAI need early surgical intervention, develop short bowel syndrome (SBS), require total parenteral nutrition (TPN), and develop progressive liver disease. The immunodeficiency affects T- and B-cell function, with lymphopenia, agammaglobulinemia, impaired mitogen responses, frequent opportunistic infections, and death occurring before the child is 2 years of age.2-4 Fatal transfusional graft-versus-host disease (GVHD) has been reported.2
Intestinal transplantation and combined liver-small bowel transplantation (LSBT) in the subset of patients with irreversible TPN liver disease is increasingly used for patients with SBS.5 The incidence of allograft rejection and patient survival at 1 year are reported as 71% and 65%, respectively.6 Despite the large number of lymphocytes present in the donor intestine, clinical GVHD is uncommon.7 It is routine to pretreat the donor, the engrafted organs, and the patient with multiple immunosuppressive modalities that include chemotherapeutic agents, antilymphocyte globulins, and irradiation of the graft to prevent rejection and GVHD.7-9 In the only reported LSBT for a child who had features similar to MIAI, fatal GVHD occurred on postoperative day (POD) 23.4
We report successful LSBT for a child with MIAI from a 1-of-6 HLA-antigen-matched cadaveric donor. Liver dysfunction has been corrected, portal hypertension has been resolved, and full enteral tolerance has been reestablished. No evidence of allograft rejection has been seen after 2 years of follow-up. Mild GVHD has been controlled with immunosuppression. He has engrafted unique populations of donor-derived T cells and has chimerism of B cells with persistent host myelopoiesis despite almost complete HLA disparity. The allograft-derived lymphoid reconstitution has a phenotypic profile of intraepithelial lymphocytes (IELs), which has resulted in the partial restoration of immune function.
Study design
Case report
An infant boy, born at 35 weeks' gestation, underwent imaging studies that showed complete bowel obstruction; multiple intestinal atresia was diagnosed at laparotomy. Resection of multiple atretic segments of small and large bowel was performed, and later an ileostomy was created. TPN was begun soon after birth, and enteral tolerance was never established.
At birth the absolute lymphocyte count was normal; however, lymphopenia rapidly developed. The absolute lymphocyte count at 11 weeks of age was 0.88×109/L, and characterization of lymphocyte frequency demonstrated the following cell marker counts: CD3+, 68%; CD4+, 58%; CD8+, 10%; and CD19+, 3%. The frequency of T-cell receptor (TCR) γδ+ cells was normal at 2%. Mitogen studies showed a normal response to phytohemagglutinin (113 174 counts per minute [cpm]/100 224 cpm control) and low or absent responses to pokeweed mitogen, concanavalin A, OKT3, and interleukin-2. Immunoglobulin levels at 8 weeks revealed depressed immunoglobulin G (IgG) (1.67 g/L; reference range, 4.53-9.16 g/L) and IgM (0.21 g/L; reference range, 0.17-1.46 g/L) and undetectable IgA (reference range, 0.20-1.00 g/L). At 10 weeks of age, periodic intravenous immunoglobulin (IVIg) was initiated. After immunization, T-cell proliferation to tetanus and diphtheria antigens was observed.
The child developed progressive liver disease secondary to TPN and at 16 months of age underwent LSBT. The donor, a 3-month-old boy, was matched for size and blood group. The HLA typing of the donor was A1, A24, B8, B51, DR3, and DR11, and for the recipient it was A1, A2, B44, B57, DR4, and DR7. Both donor and recipient were cytomegalovirus (CMV) antibody-positive at transplantation. The donor received a CMV-unscreened transfusion before organ procurement. Before transplantation the recipient was repeatedly CMV antigen-negative and had no history of CMV disease, and his positive CMV antibody status was attributed to IVIg infusions. All blood products the recipient received were CMV antigen-negative and irradiated. At procurement the donor received intravenous OKT3 and thymoglobulin, whereas the recipient's immunosuppression was methylprednisolone (20 mg/kg bolus) followed by a corticosteroid taper, basiliximab (10 mg at transplantation and on POD 4), and tacrolimus from day 1. Between POD 6 and POD 10, tacrolimus was withheld because of acute renal dysfunction, and lymphocyte immune globulin (ATGAM) was substituted during this period. Polyclonal IVIg, CMV IVIg, and intravenous ganciclovir (days 1-14) were administered after transplantation, per protocol. On POD 12, radiographs demonstrated diffuse pulmonary infiltrates, and the child was intubated for respiratory distress probably secondary to aspiration. On POD 28, leukopenia and rash developed. Bone marrow examination was normocellular with trilineage hematopoiesis, and a skin biopsy was consistent with grade 1 GVHD. The rash and cytopenias responded rapidly to methylprednisolone and granulocyte-colony-stimulating factor. Full enteral tolerance was established on POD 30. On POD 58, a CMV illness characterized by fever, worsening respiratory status, and leukopenia with positive viral cultures developed. He responded to intravenous ganciclovir, CMV IVIg, and reduced immunosuppression. The child was discharged after a total inpatient stay of 5 months. Protocol small bowel biopsies have never demonstrated allograft rejection. Since discharge he has had chronic lung disease related to multiple pneumonias (including parainfluenza), tracheomalacia, and possible pulmonary GVHD.10 Recurrent CMV disease of the gastrointestinal tract, mild to moderate skin GVHD, and iatrogenic Cushing syndrome have also developed.
Developmental milestones other than walking and speech (tracheostomy) are normal. With the exception of hypoalbuminemia, liver and intestinal functions have been normal. He has not developed antibody responses to immunizations. Two years after transplantation the child is enterally fed, free of CMV disease, and given 30 mg prednisone every other day.
Methods
HLA typing of class 1 and 2 antigens used a standardized complement-dependent cytotoxicity assay. Results were confirmed using short-tandem repeat (STR) analysis, as previously described.11 Three- and 4-color flow cytometric analyses were performed on peripheral blood samples collected at multiple time points using techniques previously reported.12 All data were acquired on a FACStarPlus (Becton Dickinson, San Jose, CA), and detailed analysis was performed using the Attractors software from Becton Dickinson. HLA A2 (recipient) and B8 (donor) were selected as informative markers for determinations of cellular origin. Immunoglobulin serum levels (IgG, IgM, and IgA) were measured on a Dade Behring (Deerfield, IL) nephelometer using Dade Behring reagents. Immunofixation electrophoresis (IFE) was performed using the Beckman Paragon (Fullerton, CA) IFE system. Anti-CMV IgG was measured using Abbott Diagnostics (Abbott Park, IL) reagents on the Abbott Axym, and anti-CMV IgM levels were measured by the Abbott IMx assay. CMV culture was performed with shell vial amplification and backup culture on MRC5 cells from Viral Medical Laboratories (Minneapolis, MN). Parainfluenza serology to isotype 3 was performed using complement fixation (ARUP Laboratories, University of Utah).
Results and discussion
Origin of hematopoietic cells
From POD 30, repeated phenotypic and STR analyses of peripheral blood (PB) and bone marrow (BM) revealed that granulocytes and monocytes were of recipient origin. Lymphocytes have been predominantly of donor origin, with more than 99% of T lymphocytes (CD3+) and approximately 50% of B lymphocytes (CD19+) expressing donor markers from POD 30 to 26 months after LSBT.
In the PB on POD 118, relative and absolute numbers of CD4+ T cells, CD19+ B cells, and CD56+ lymphocytes were significantly reduced, whereas relative CD8+ T cell numbers were within reference intervals for age (Table 1). Within the CD3+ T-cell population, which accounted for 90% of lymphocytes, 61.2% expressed a CD4-CD8- phenotype, and 28.3% were CD4-CD8+. CD8 receptor analysis revealed that 65% of the CD4-CD8+ T cells expressed CD8αα homodimers. TCR studies revealed that 77% of all CD3+ T cells were TCR γδ+. Of the CD3+CD8αβ T cells, 88% were TCR γδ+, and 15% of the CD3+CD8αα T cells were TCR γδ+ (Table 1). These studies were repeated at 6, 13, 19, and 26 months after LSBT, and relative lymphocyte subset distributions remained essentially unchanged. Repeated mitogen response assays after LSBT have revealed low to absent responses to all mitogens tested.
Patient's peripheral blood lymphocyte subpopulations 4 months after liver-small bowel transplantation*
. | Total lymphocytes, % . | . | TCRαβ, % . | . | TCRγδ, % . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Marker . | Patient . | Control . | Patient . | Control . | Patient . | Control . | |||
| CD3+ | 90.5 ± 2.4 | 73.0 ± 2.5 | 23.0 ± 4.7 | 93.0 ± 2.1 | 77.0 ± 4.7 | 6.5 ± 0.7 | |||
| CD3+ CD4− CD8− | 61.2 ± 6.2 | 3.1 ± 0.6 | 3.1 ± 2.7 | 0.3 ± 0.0 | 85.7 ± 5.1 | 1.1 ± 0.3 | |||
| CD3+ CD4+ CD8− | 1.8 ± 0.8 | 43.0 ± 2.6 | 97.1 ± 1.0 | 99.0 ± 1.6 | 6.3 ± 3.1 | 0.1 ± 0.0 | |||
| CD3+ CD4− CD8+ | 28.3 ± 2.5 | 27.0 ± 1.6 | 33.3 ± 2.1 | 93.0 ± 2.3 | 60.0 ± 5.6 | 6.3 ± 1.3 | |||
| CD3+ CD4− CD8αβ† | 10.6 ± 1.8 | — | 2.7 ± 0.6 | — | 87.5 ± 5 | — | |||
| CD3+ CD4− CD8αα† | 19.6 ± 1.1 | — | 87.7 ± 1.4 | — | 14.6 ± 4.1 | — | |||
| CD19+ | 2.2 ± 0.8 | 12.5 ± 1.4 | — | — | — | — | |||
| CD56+ | 1.9 ± 1.2 | 13.3 ± 1.6 | — | — | — | — | |||
. | Total lymphocytes, % . | . | TCRαβ, % . | . | TCRγδ, % . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Marker . | Patient . | Control . | Patient . | Control . | Patient . | Control . | |||
| CD3+ | 90.5 ± 2.4 | 73.0 ± 2.5 | 23.0 ± 4.7 | 93.0 ± 2.1 | 77.0 ± 4.7 | 6.5 ± 0.7 | |||
| CD3+ CD4− CD8− | 61.2 ± 6.2 | 3.1 ± 0.6 | 3.1 ± 2.7 | 0.3 ± 0.0 | 85.7 ± 5.1 | 1.1 ± 0.3 | |||
| CD3+ CD4+ CD8− | 1.8 ± 0.8 | 43.0 ± 2.6 | 97.1 ± 1.0 | 99.0 ± 1.6 | 6.3 ± 3.1 | 0.1 ± 0.0 | |||
| CD3+ CD4− CD8+ | 28.3 ± 2.5 | 27.0 ± 1.6 | 33.3 ± 2.1 | 93.0 ± 2.3 | 60.0 ± 5.6 | 6.3 ± 1.3 | |||
| CD3+ CD4− CD8αβ† | 10.6 ± 1.8 | — | 2.7 ± 0.6 | — | 87.5 ± 5 | — | |||
| CD3+ CD4− CD8αα† | 19.6 ± 1.1 | — | 87.7 ± 1.4 | — | 14.6 ± 4.1 | — | |||
| CD19+ | 2.2 ± 0.8 | 12.5 ± 1.4 | — | — | — | — | |||
| CD56+ | 1.9 ± 1.2 | 13.3 ± 1.6 | — | — | — | — | |||
Values presented as mean ± standard error of the mean (SEM). CD indicates cluster of differentiation; TCR, T-cell receptor; and —, not assessed.
Absolute lymphocyte number 1.65 × 109/L. Ninety-nine percent of CD3+ cells and 45% of CD19+ cells were of donor origin at the time of this analysis.
CD8αβ and CD8αα frequencies were not assessed on the control population.
On POD 163, the frequency of hematopoietic progenitor cells in the PB, defined by CD34 and CD38 expression, was 0.4% CD34+CD38- and 0.9% CD34+CD38+ of total nucleated cells. Of these cells, 51% of the CD34+CD38- and 46% of the CD34+CD38+ cells were of donor origin. In addition, 65.6% of the lymphocyte progenitors (CD34+CD7+) were of donor origin. Concurrent BM samples had frequencies of 0.1% CD34+CD38- cells and 1.31% CD34+CD38+ cells, and less than 5% of each population was of donor origin.
Immunoglobulin analysis
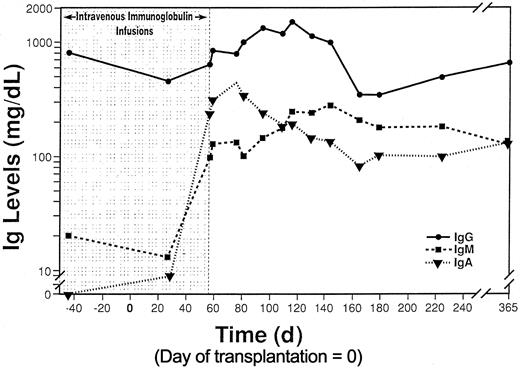
No serum IgA was measurable before transplantation. Production of IgA was first detected on POD 28, at 0.08 g/L. IgA levels peaked on POD 75, at 4.39 g/L, and measured 1.25 g/L at 1 year (Figure 1). IgM levels were 0.15 g/L before transplantation and were attributed to IVIg infusions. IgM levels increased to 0.98 g/L on POD 60, when IVIg was discontinued, and were 2.81 g/L by POD 90. At 1 year, a normal IgM value of 1.25 mg/dL was observed. Serum IgG followed a pattern similar to that for IgM and was measured at 7.82 g/L at 1 year (Figure 1). IFE demonstrated an oligoclonal pattern of immunoglobulins early after transplantation, with 4 to 5 clonal immunoglobulins accounting for most measurable immunoglobulin. The patient progressively converted to a polyclonal pattern, and IFE demonstrated normal-appearing IgG, IgM, and IgA profiles with 4 small monoclonal bands remaining at 1 year (results not displayed).
Profile of immunoglobulin levels. Periodic intravenous immunoglobulin was administered until day 57 after transplantation (shaded).
Profile of immunoglobulin levels. Periodic intravenous immunoglobulin was administered until day 57 after transplantation (shaded).
Antibody responses
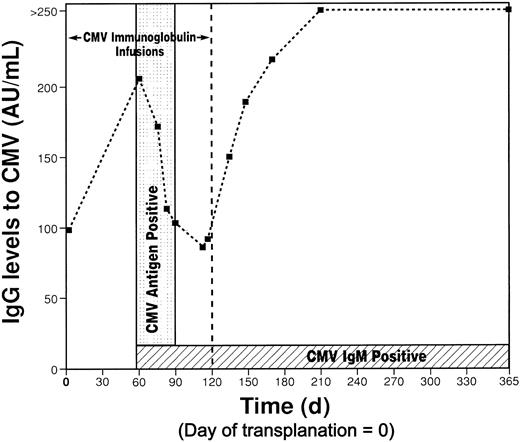
CMV IgM has been detected since CMV disease developed on POD 58. Quantitative CMV IgG levels increased in the absence of any change in dose or frequency of anti-CMV immunoglobulin therapy and continued to increase after anti-CMV immunoglobulin was discontinued (Figure 2). CMV antigenemia was detected on POD 58 and was undetectable after POD 90. At 13, 19, and 26 months, CMV IgM levels remain positive and CMV IgG levels are higher than 250 AU/mL. Eight months after transplantation a parainfluenza infection developed and was accompanied by antibody production to parainfluenza 3. Before transplantation, isoagglutinin titers were weakly positive at 1:1, and 13 months after transplantation they were 1:16 for anti-A and 1:8 for anti-B.
Time course of CMV immunoglobulin. The period of detectable circulating CMV antigenemia is highlighted. CMV immunoglobulin infusions were given for the first 120 days only.
Time course of CMV immunoglobulin. The period of detectable circulating CMV antigenemia is highlighted. CMV immunoglobulin infusions were given for the first 120 days only.
At 2 years after LSBT, the patient remains lymphopenic and has decreased mitogen responses. He receives alternate-day corticosteroids to manage GVHD of the skin and recipient upper gastrointestinal tract. Episodes of acute GVHD are less severe and less frequent, suggesting the development of tolerance. Evidence for immune reconstitution includes recovery from CMV and parainfluenza infections with viral clearance and development of appropriate IgM and IgG antibodies. It remains unclear whether donor or host B cells are responsible for antibody production. The marked production of IgA early after LSBT (Figure 1) suggests donor B cells are functional. Immunoglobulin production with few circulating B cells (Table 1) suggests the presence of other populations of differentiated B cells, probably in the allograft.
We postulate that this child, with congenital immunodeficiency, who received a liver and small bowel allograft from a 1-of-6 HLA-matched cadaveric donor, reconstituted his immune system with T-lymphocyte precursors from the engrafted intestine. With absent host immunity and engraftment of the donor immune system, he is incapable of rejecting his allograft. The engrafted organs were extensively T-cell depleted. OKT3 and thymoglobulin removed most of the mature, thymic-derived T cells, and the allograft-derived T cells are incapable of mediating severe or fatal GVHD.9,13 The predominance of TCR γδ expression on the engrafted T cells might also have contributed to the reduced GVHD response.14
Phenotyping of peripheral blood lymphocytes supports the idea that essentially all the circulating T cells are of donor origin and are derived from the intestine. These studies demonstrate that the T lymphocytes are predominantly CD3+CD4-CD8- T cells, have a high frequency of TCR γδ heterodimers, and include CD3+CD4-CD8+ T cells expressing CD8αα homodimers (Table 1), consistent with the phenotype of IEL.15 IELs are found predominantly between the basement membrane and the epithelium of the intestine, lung, skin, and urogenital systems. These lymphocytes represent the largest single population of lymphocytes in the intestines and are responsible for immune surveillance and mucosal integrity.15 In rodent models, a putative stem cell for extramedullary lymphopoiesis has been demonstrated in “cryptopatches.”16
Children with severe combined immunodeficiency who receive transplanted bone marrow from HLA-identical sibling donors without immunosuppression routinely demonstrate complete immune reconstitution.17 In the present report, we show that an immunodeficient child undergoing LSBT from a 1-of-6 HLA-matched donor engrafted some donor hematopoietic and lymphoid progenitors but that after 2 years he has generated few donor CD3+ CD4+ and CD3+ CD8+ TCR αβ T cells. Instead, most of the donor T cells are TCR γδ, have an IEL phenotype, and provide only partial immune correction. The mechanism of the sustained TCR γδ T-cell production remains unknown. Possibilities include the engraftment of bowel-resident lymphoid progenitor cells that continue to generate new IELs, peripheral expansion of mature IELS transferred from the allograft, or provision of an appropriate microenvironment in the allograft for selective differentiation of donor stem cells to mature IELs. Whatever the mechanism, the lack of a functional host immune system prevents destruction of the allograft-derived IELs. These issues should be addressed in future patients with MIAI or in animal models.
The functional potentials of passenger lymphocytes and lymphoid stem cells that accompany solid organ allografts certainly deserve further study. In recipients of immunocompetent solid organ transplants, passenger lymphocytes occasionally mediate GVHD reactions but do not reconstitute functional immune compartments.4,8,9,18,19 Passenger lymphocytes may persist in the host for long periods and may promote allograft tolerance.20 Careful donor-recipient chimerism studies of lymphoid populations and measures of immune function after solid organ transplantation should be performed to better understand allograft tolerance and immunosuppression requirements to improve transplantation outcomes. Potentially, advances in cellular engineering may allow donor immune reconstitution without GVHD in patients after solid organ transplantation and may obviate the need for lifelong immunosuppression.
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-04-1187.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal