Abstract
There is a strong graft-versus-leukemia (GVL) effect of allogeneic stem cell transplantation (SCT) due to elimination of tumor cells by alloimmune effector lymphocytes. When leukemia relapses after allogeneic SCT, donor lymphocyte transfusions (DLTs) can induce sustained remissions in some patients. This review summarizes the current status on clinical use of DLT, the basis of GVL reactions, problems associated with this therapy, and new strategies to improve DLT. Several multicenter surveys demonstrated that the GVL effect of DLT is most effective in chronic myelogenous leukemia (CML), whereas it is less pronounced in acute leukemia and myeloma. Cytokine stimulation to induce differentiation of myeloid progenitor cells or to up-regulate costimulatory molecules on tumor cells may improve the efficacy of DLT. Infections and graft-versus-host disease (GVHD) are major complications of DLT. Control of GVHD may be improved using suicide gene–modified T cells for DLT, allowing T-cell elimination if severe GVHD develops. Hopefully, in the future, GVL effect can be separated from GVHD through adoptive transfer of selected T cells that recognize leukemia-specific antigens or minor histocompatibility antigens, which are expressed predominantly on hematopoietic cells, thereby precluding attack of normal tissues. In patients with leukemia and lymphomas with fast progression, tumor growth may outpace development of effector T cells. Here it may be preferable to select stem cell transplant donors with HLA-mismatches that allow alloreactive natural killer cells, which appear early after transplantation, to retain their cytolytic function. New approaches for adoptive immune therapy of leukemia, which promise a better prognosis for these patients, are being developed.
Introduction
Allogeneic stem cell transplantation (SCT) is an established form of treatment for leukemia and is now being explored as a treatment for a variety of other hematologic and nonhematologic malignancies. In the last decade, the paradigm for treatment of leukemia by SCT has changed. The initial focus was to use myeloablative doses of radiation and chemotherapy to eliminate the leukemia, and SCT was performed to prevent death from marrow failure. Increasingly today, the emphasis has shifted to eradicating leukemia with alloimmune effector cells, while limiting radiation and chemotherapy to those doses sufficient to permit donor stem cell engraftment as a platform for adoptive immune therapy and reducing the conditioning toxicity for SCT.
The antileukemia effect of the graft-versus-host (GVH) reaction was recognized early in murine models1 and soon applied to human patients.2 A systematic analysis by the Seattle team showed that patients surviving acute graft-versus-host disease (GVHD) benefited from a reduced tumor relapse rate.3 The important role of T cells in eliminating chronic myelogenous leukemia (CML) was suggested by a retrospective study of the International Bone Marrow Transplant Registry (IBMTR),4 which found an increased leukemia relapse rate when the stem cell transplant was T-cell–depleted to prevent GVHD.5 Unfortunately, the transfusion of donor lymphocytes early after transplantation produced severe GVHD and was not successful in reducing the relapse rate in advanced leukemia.6
The therapeutic impasse in leukemia control, bought only at the expense of severe GVHD and its attendant risks, could be circumvented however by temporally separating the SCT from the subsequent donor lymphocyte transfusions (DLTs). In dog experiments, DLTs did not produce GVHD if the adoptively transferred lymphocytes were transfused at least 2 months after transplantation of T-cell–depleted marrow.7 Dogs that were mixed chimeras after SCT became complete donor chimeras after DLT, without appearance of clinical GVHD. Similarly, the delay of DLT prevented GVHD in a murine model of adoptive immune therapy.8 The absence of GVHD after DLT in stable chimeras9 and the evidence for the elimination of residual host hematopoiesis provided the experimental basis for the first attempts to treat relapse of CML with DLT.10 In 1988, DLT therapy was given to 3 allogeneic SCT patients with CML whose tumors relapsed in chronic phase and who failed to achieve remission after interferon-α therapy.10 Now after more than 13 years, these patients still remain in cytogenetic and molecular remission. GVHD, which developed initially in 2 individuals, ceased over time. The graft-versus-leukemia (GVL) effect of DLT in CML has been subsequently confirmed by transplantation centers worldwide.11,12
Survey cohorts of patients receiving DLT following allogeneic SCT
Based on the first encouraging examples of DLT in CML, a number of centers implemented studies of DLT in conjunction with allogeneic SCT for treatment of a variety of hematologic malignancies. Complete remissions were observed in a minority of patients with other forms of leukemia or lymphoma. This review summarizes the cumulative experience using DLT based on several retrospective multicenter surveys (Table 1). Additional relevant information from individual centers, as well as some unpublished data from the Munich Working Group on Bone Marrow Transplantation (AG-KMT Munich), is also included.
Survey of patient cohorts receiving DLT after allogeneic SCT
Patient cohort/survey designation . | Author and reference . |
|---|---|
| EBMT registry | |
| EBMT-1995 | Kolb et al13,14 |
| EBMT-2000 | Schmidt et al15 |
| EBMT-2002 | Guglielmi et al16 |
| North American multicenter | |
| USA-1997 | Collins et al17 |
| USA-2000 | Salama et al18 |
| USA-2002 | Levine et al19 |
| European multicenter | |
| Myeloma-2000 | Lokhorst et al20 |
| England multicenter | |
| Lymphoma-2002 | Marks et al21 |
| Munich Bone Marrow Transplant Group | |
| AG-KMT | Kolb et al* |
Patient cohort/survey designation . | Author and reference . |
|---|---|
| EBMT registry | |
| EBMT-1995 | Kolb et al13,14 |
| EBMT-2000 | Schmidt et al15 |
| EBMT-2002 | Guglielmi et al16 |
| North American multicenter | |
| USA-1997 | Collins et al17 |
| USA-2000 | Salama et al18 |
| USA-2002 | Levine et al19 |
| European multicenter | |
| Myeloma-2000 | Lokhorst et al20 |
| England multicenter | |
| Lymphoma-2002 | Marks et al21 |
| Munich Bone Marrow Transplant Group | |
| AG-KMT | Kolb et al* |
Published results of 8 surveys on the use of DLT following allogeneic SCT are reviewed here, as well as results from local Munich transplantation studies.
EBMT indicates European Bone Marrow Transplant.
See “Survey cohorts of patients receiving DLT following allogeneic SCT.”
The retrospective survey (EBMT-1995) of DLT given after SCT for treatment of various hematologic malignancies, based on an analysis of more than 400 EBMT patients, is summarized in Table 2.14
Graft-versus-leukemia effect of DLT: EBMT-95 survey
. | No. of patients . | . | . | ||
|---|---|---|---|---|---|
| Diagnosis . | Studied . | Evaluable* . | Complete remission (%) . | ||
| CML | |||||
| Cytogenetic relapse | 57 | 50 | 40 (80) | ||
| Hematologic relapse | 124 | 114 | 88 (77) | ||
| Transformed phase | 42 | 36 | 13 (36) | ||
| Polycythemia vera/MPS | 2 | 1 | 1 | ||
| AML/MDS | 97 | 58 | 15 (26) | ||
| ALL | 55 | 20 | 3 (15) | ||
| MMY | 25 | 17 | 5 (29) | ||
. | No. of patients . | . | . | ||
|---|---|---|---|---|---|
| Diagnosis . | Studied . | Evaluable* . | Complete remission (%) . | ||
| CML | |||||
| Cytogenetic relapse | 57 | 50 | 40 (80) | ||
| Hematologic relapse | 124 | 114 | 88 (77) | ||
| Transformed phase | 42 | 36 | 13 (36) | ||
| Polycythemia vera/MPS | 2 | 1 | 1 | ||
| AML/MDS | 97 | 58 | 15 (26) | ||
| ALL | 55 | 20 | 3 (15) | ||
| MMY | 25 | 17 | 5 (29) | ||
The EBMT-95 cohort included 402 patients who received DLT following allogeneic SCT. Treated were 223 patients with chronic myelogenous leukemia (CML) in 3 different phases of relapse. There were 97 patients with acute myelogenous leukemia (AML) or myelodysplastic syndromes (MDS) who received DLT. Smaller numbers of patients with acute lymphocytic leukemia (ALL) and multiple myeloma (MMY) were studied. Single patients with polycythemia vera and myeloproliferative syndrome (MPS) were also evaluated.
Patients surviving less than 30 days after DLT were excluded from analysis, leaving a cohort of 296 patients that was included in the evaluation of the frequencies of tumor responses, as defined by complete remission. Patients with chemotherapy responses were excluded; 14 patients with recurrent CML were excluded because of unknown relapse phase, and 7 patients with other diagnoses were also excluded (2 NHL, 1 juvenile CML, and 4 unknown).
Graft-versus-leukemia effects in chronic myelogenous leukemia
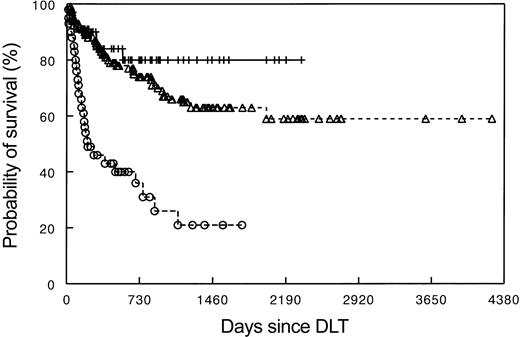
The EBMT-95 survey13 and a North American survey (USA-97)17 both found that the best DLT responses occurred in CML patients having only cytogenetic or molecular evidence of disease. In patients with hematologic relapse, DLT responses were more frequent in patients with chronic-phase disease than in those with recurrent disease in accelerated or blastic transformation. Patients who received T-cell–depleted SCT for prophylaxis of GVHD and who showed no GVHD at the time of DLT experienced more favorable outcomes. The presence of donor chimerism was necessary for a GVL response. In a single center study, the absence of donor T cells in the host at the time of DLT was associated with failure to achieve a GVL response.22 The EBMT-95 survey included patients with HLA-identical sibling donors, HLA-compatible unrelated donors, HLA-mismatched family donors, and identical twin donors. A GVL effect was observed in all groups, with the exception of the identical twin transplants.13 Most responses were durable and were associated with improved survival23,24 (Figure 1).
Survival after DLT for recurrent CML based on type of relapse. Data as reported by the EBMT-1995 survey. Upper line (++) indicates molecular and cytogenetic relapse (n = 62); middle line (▵), hematologic relapse (n = 133); and lower line (○), relapse in transformed phase/blastic phase (n = 42).
Survival after DLT for recurrent CML based on type of relapse. Data as reported by the EBMT-1995 survey. Upper line (++) indicates molecular and cytogenetic relapse (n = 62); middle line (▵), hematologic relapse (n = 133); and lower line (○), relapse in transformed phase/blastic phase (n = 42).
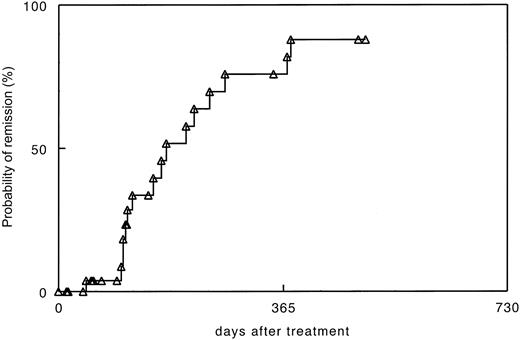
In early studies, antileukemia responses were measured by elimination of tumor cells as determined by cytogenetic analysis and fluorescent in situ hybridization (FISH) for BCR/ABL. More recently, molecular analysis using quantitative reverse transcriptase–polymerase chain reaction (RT-PCR), has become the standard to detect minimal residual disease. Typically, antileukemia responses occurring after DLT were delayed by weeks or months, with an average time of 4 to 6 months required before molecular remission was achieved (Figure 2). Occasionally more than a year elapsed before a leukemia clone was suppressed to undetectable levels.
Time to molecular remission after donor lymphocyte transfusions for recurrent CML after allogeneic stem cell transplantation in 30 patients treated at University of Munich. RT-PCR was performed in blood at monthly intervals and in marrow aspirates in the case of negative blood samples.
Time to molecular remission after donor lymphocyte transfusions for recurrent CML after allogeneic stem cell transplantation in 30 patients treated at University of Munich. RT-PCR was performed in blood at monthly intervals and in marrow aspirates in the case of negative blood samples.
Complications of DLT were myelosuppression and GVHD. Myelosuppression with thrombocytopenia, leukopenia, or reticulocytopenia occurred in 34% of the EBMT-95 cohort.13 Cytopenias were more frequent in patients with hematologic relapse than in those with cytogenetic relapse, but they were transient in most cases. Persistent cytopenia could be treated with recombinant human growth factors, such as granulocyte colony-stimulating factor (G-CSF) and erythropoietin. The lack of donor hematopoiesis was shown to predict myelosuppression after DLT,19,25 and transfusion of donor stem cells or bone marrow could lead to complete hematologic reconstitution. However, transfusion of G-CSF–mobilized donor blood cells instead of DLT was not found to decrease the risk of myelosuppression.26 In patients with chronic GVHD, application of hematopoietic growth factors or transfusion of stem cells was not helpful, whereas treatment with corticosteroids was often useful (Kolbe et al, unpublished observations, August 1993). GVHD of grades II and higher was observed in 41% of patients treated with DLT in the EBMT-95 survey13 (Table 3).
GVL effect of DLT: EBMT-95 survey
Grade of GVHD . | No. of patients studied . | Responders (%) . |
|---|---|---|
| 0 | 115 | 58 (50) |
| I | 40 | 30 (75) |
| II or higher | 85 | 72 (85) |
Grade of GVHD . | No. of patients studied . | Responders (%) . |
|---|---|---|
| 0 | 115 | 58 (50) |
| I | 40 | 30 (75) |
| II or higher | 85 | 72 (85) |
In the EBMT-95 cohort the frequencies of allogeneic SCT patients showing GVL responses after DLT increased with the severity of GVHD. While 75% of patients with grade I GVHD showed tumor regression, this increased to 85% in patients with GVHD of grade II or greater. Nevertheless, 50% of patients showing no clinical signs of GVHD also showed complete tumor remissions following DLT.
In the USA-97 survey, 60% of patients treated with DLT developed GVHD.17 In both the EBMT-95 and the USA-97 cohorts, the GVL effects correlated with the severity of GVHD. Importantly, however, complete responses were seen in a significant proportion of patients lacking any signs of GVHD. This observation provided further evidence that GVL effects may be separable from GVHD.
The clinical picture of GVHD after DLT has some distinct features that are attributable to the absence of the acute inflammatory response induced by conditioning treatment. When compared with GVHD following SCT, skin rash and diarrhea were often less severe and delayed in onset, whereas fungal and viral infections posed a greater threat (Kolbe et al, unpublished observations, April 1994).
Several strategies have been developed to prevent and control GVHD associated with DLT.27-29 A reasonable approach is to initiate DLT using a dose of 1 × 107 T cells/kg body weight and then to escalate the dose of donor lymphocytes if no GVL effect occurs.27 Doses less than 107 T cells/kg did not show GVL effects in patients with HLA-identical family donors. In a recent analysis of 298 patients treated in 51 European centers (EBMT-02), a first dose of donor lymphocytes of 2 × 107/kg or less of body weight led to reduced DLT-related toxicity and improved survival, while retaining response rates comparable with those achieved with higher initial doses.16 In the EBMT-02 survey, doses were escalated once in 61% of patients, twice in 27% of patients, and 3 times in 16% of patients, with an average interval of 48 days between doses. The treatment-related mortality at 3 years after initiation of treatment was 5% when an initial low dose was used compared with 20% mortality when higher initial doses were applied. Currently, the preferred approach is to follow patients at 3- to 6-month intervals using quantitative RT-PCR analysis to identify residual leukemia and to apply DLT (1 × 107 T cells/kg body weight) if molecular or cytogenetic evidence of disease persists after immune suppression has been discontinued for at least 3 months. An escalating dose strategy may not be applicable for patients with advanced phase relapses with rapid progression. In patients with more advanced relapses or in patients with GVHD, DLT can be combined with selective tyrosine kinase inhibitor (imatinib) treatment, which has shown activity in patients not responding to DLT alone.30
Graft-versus-leukemia effects in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDSs)
In contrast to CML, depletion of T cells in allogeneic SCT had no significant effect on the relapse rates of AML treated in first remission.4 Nevertheless, an allogeneic immune effect may operate in AML since syngeneic transplants carried a 2.5-fold higher risk of leukemia relapse when compared with allogeneic transplants. Furthermore, acute or chronic GVHD was associated with a significantly lower relapse risk.4 In the EBMT-95 survey of SCT patients with recurrent AML and MDS, DLT induced complete remissions in 26% of patients not given chemotherapy or not responding to chemotherapy (Tables 2, 4; Figure 3).
Response to DLT for recurrent AML/MDS after BMT: EBMT-95 Survey
Treatment . | No. patients in CR/patients studied . | Survival time, d . |
|---|---|---|
| No chemotherapy | 9/36 | 112+, 155+, 291, 439, 503, 598+, 1007, 1014+, 2374 |
| Chemotherapy with CR | 14/15 | 60, 96, 159, 312+, 372+, 438+, 527, 646, 1173, 1245, 1416+, 1453+, 1463+, 1563+ |
| Chemotherapy without CR | 6/22 | 152, 617+, 800, 977, 1234+, 1263+ |
Treatment . | No. patients in CR/patients studied . | Survival time, d . |
|---|---|---|
| No chemotherapy | 9/36 | 112+, 155+, 291, 439, 503, 598+, 1007, 1014+, 2374 |
| Chemotherapy with CR | 14/15 | 60, 96, 159, 312+, 372+, 438+, 527, 646, 1173, 1245, 1416+, 1453+, 1463+, 1563+ |
| Chemotherapy without CR | 6/22 | 152, 617+, 800, 977, 1234+, 1263+ |
Following allogeneic SCT, 73 patients with acute myelogenous leukemia (AML) or myelodysplastic syndromes (MDSs) in the EBMT-95 cohort were treated with DLT. One group of 36 patients received no chemotherapy prior to DLT; 9 individuals showed complete remissions with survival times beyond 100 days. Following chemotherapy 15 patients entered complete remission; their survival times after DLT revealed a broad time span, with most remissions lasting more than 150 days. Of 22 patients who failed to achieve complete remission with chemotherapy, 6 benefited from DLT, achieving survival times of more than 150 days.
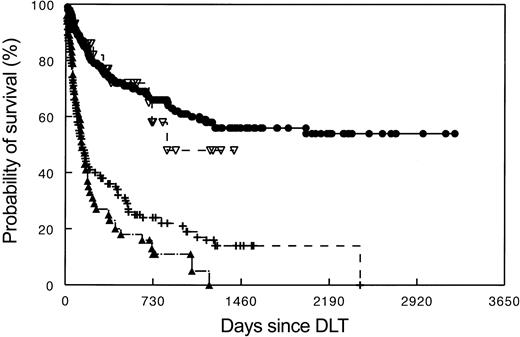
Survival of patients treated for recurrent leukemia and multiple myeloma after allogeneic stem cell transplantation with DLT. • indicates CML (N = 257); ▿, myeloma (N = 30); –, AML/MDS; ▴, ALL, DLT, donor lymphocyte transfusions; and N, number of patients studied.
Survival of patients treated for recurrent leukemia and multiple myeloma after allogeneic stem cell transplantation with DLT. • indicates CML (N = 257); ▿, myeloma (N = 30); –, AML/MDS; ▴, ALL, DLT, donor lymphocyte transfusions; and N, number of patients studied.
The EBMT-95 survey13,14 analyzed 3 patient groups that received DLT (Table 4): 36 patients received no prior chemotherapy, 15 patients received chemotherapy and entered complete remission, and 22 patients received chemotherapy but failed to achieve complete remission. The duration of responses following DLT was not influenced by the outcome of chemotherapy.
In the USA-97 survey of chemotherapy and DLT, response rates between 20% and 37% were observed, whereby better responses were seen in patients who received chemotherapy.17 More recently, the outcome of DLT for relapsed AML and MDS was evaluated in 108 of 120 patients reported to the EBMT (EBMT-02).15 Here the overall response rate was 41%, including patients given chemotherapy. Unfavorable factors were a short remission after SCT and the absence of GVHD after DLT. Notably, the French-American-British subtype and the karyotype did not influence response or survival. The use of chemotherapy and G-CSF–mobilized blood cells was studied by a group of 34 centers in North America (USA-02).19 Again, the time from transplantation until relapse was important for response and survival. The 2-year survival rate of all patients treated was approximately 20%, whereby patients showing a complete response had a 2-year survival rate of 40%.
Graft-versus-leukemia effects in acute lymphoid leukemia (ALL)
The allogeneic GVL effect in ALL was found to be weaker than that in myeloid malignancies.4,13 Acute GVHD, however, may lower the relapse rate in ALL in first remission,4 in particular in patients with minimal residual disease.31 Although the first patient successfully treated with DLT was a child with persistent ALL one month after transplantation,32 DLT showed only limited benefit in the treatment of recurrent ALL after allogeneic SCT in both the USA-9715,17 and EBMT-95 surveys13 (Table 5). Most patients in the EBMT cohort were treated with chemotherapy and received DLT for consolidation. Only 13% of patients survived 2 years beyond treatment.33 It is not known whether the fast pace of the disease or an intrinsic resistance to immune attack is responsible for the poor outcome of DLT in ALL.
Response to DLT for recurrent ALL after BMT: EBMT-95 survey
Treatment . | No. of patients in CR/patients studied . | Survival time, d . |
|---|---|---|
| No chemotherapy | 1/8 | 417 |
| Chemotherapy with CR | 15/20 | 55, 59, 110, 122+, 132, 158, 185, 192, 241, 361, 464, 701+, 721, 1053, 1197 |
| Chemotherapy without CR | 2/12 | 367, 371+ |
Treatment . | No. of patients in CR/patients studied . | Survival time, d . |
|---|---|---|
| No chemotherapy | 1/8 | 417 |
| Chemotherapy with CR | 15/20 | 55, 59, 110, 122+, 132, 158, 185, 192, 241, 361, 464, 701+, 721, 1053, 1197 |
| Chemotherapy without CR | 2/12 | 367, 371+ |
Following allogeneic SCT, 40 patients in the EBMT-95 cohort were treated with DLT. One group of 8 patients received no chemotherapy prior to DLT; only one of these individuals achieved complete remission. Following chemotherapy, 20 patients entered complete remission; in 5 patients ALL had relapsed prior to DLT. Their survival times after DLT revealed a broad time span, with most responses lasting more than 100 days. Of 12 patients who failed to achieve complete remission with chemotherapy, 2 benefited from DLT, achieving survival times of more than one year.
Transplantation of ALL patients using bone marrow from HLA-haploidentical family members on day 0 and CD6-depleted, mobilized blood cells on day 634 appears more promising.35 CD6– marrow and mobilized blood cells contain natural killer (NK) cells and CD8+ NK-T cells that have strong suppressor effects on activated lymphocytes and leukemia cells.35
Graft-versus-leukemia effects in multiple myeloma (MMY)
The success of allogeneic SCT for multiple myeloma has been limited by a high rate of complications.36 Survival has improved with earlier transplantation37 and with the use of reduced-intensity conditioning regimens,38 taking advantage of a graft-versusmyeloma effect. This effect has been demonstrated after DLT for recurrent or persistent myeloma.13,39-41 Recent surveys of European (Myeloma-2000) and North American (USA-2000) cohorts indicate that complete and partial responses were induced in patients given DLT in 52%20 and 36%18 of cases, respectively. Chemotherapy was administered to 13 of 27 patients in the European study and to 2 of 22 patients in the North American study. However, responses were seen only after transfusion of more than 108 T cells/kg body weight and most responses were short-lived. Responses correlated with the occurrence of acute or chronic GVHD. Transplantation of T-cell–depleted marrow with subsequent transfusion of CD8-depleted donor lymphocytes may increase progression-free survival, but GVHD remains a problem.42 The combination of high-dose chemotherapy and autologous transplantation to reduce tumor burdens, followed by allogeneic transplantation after a nonmyeloablative conditioning treatment (“tandem protocol”) to take advantage of a graft-versus-myeloma effect, appears most promising.43
Graft-versus-leukemia effects in lymphoma (NHL), Hodgkin disease, and chronic lymphocytic leukemia (CLL)
The experience of treating recurrent lymphoma, Hodgkin disease, and chronic lymphocytic leukemia with DLT is limited. A retrospective survey (Lymphoma-02) of DLT in allogeneic SCT after reduced-intensity conditioning showed complete responses in follicular lymphoma in more than 60% of patients, but only partial remissions were observed in single patients with CLL and Hodgkin disease.21 There was a trend however toward a lower relapse rate in patients with chemotherapy-sensitive Hodgkin disease and GVHD.44 In lymphoma, the relapse rate was lower after allogeneic transplantation than after autologous transplantation.45 Relapses were rare after allogeneic transplantation in low malignant lymphoma.46,47 Transplants from HLA-mismatched family members were found to have activity against high-grade malignant lymphoma.35,48
Treatment of virus-induced malignancy
Epstein-Barr virus–associated posttransplantation lymphoproliferative disease (EBV-PTLPD) is a serious complication of allogeneic transplantation in children and in patients receiving HLA-mismatched or T-cell–depleted transplants. Adoptive transfer of very small numbers of donor lymphocytes has been used successfully to treat PTLPD, demonstrating the great sensitivity of this tumor to immune therapy.49-52 However, severe inflammatory reactions may ensue after DLT. Prophylactic transfusion of EBV-reactive donor T-cell lines has protected high-risk patients from PTLPD. Recently, adoptive cellular therapy has been replaced by treatment with CD20 monoclonal antibody, which is highly effective in controlling EBV-PTLPD.53 Treatment of EBV-positive Hodgkin disease with EBV-specific cytotoxic T lymphocytes (CTLs) has been used with some success.54
Graft-versus-tumor for nonhematologic malignancies
The use of allogeneic SCT for the treatment of nonhematologic malignancies arose from the hypothesis that a graft-versus-tumor (GVT) effect, analogous to GVL effect, could be generated either by alloresponses directed against commonly expressed minor histocompatibility antigens (minor H antigens) or against tumor-specific antigens (TSAs). Indeed, GVT effects with occasional complete remissions were observed in metastatic renal, breast, ovarian, and possibly some gastrointestinal cancers. Both minor H antigens and TSAs were implicated in these GVT responses.55,56
Mechanisms of GVL effect after DLT
In CML, the delayed response to DLT may be due to the time required for the elimination of all progeny of a primitive leukemia stem cell. The late induction of cytogenetic and molecular remission and the durability of remission most likely reflect the expansion of tumor-reactive T-cell clones. It is unclear whether GVL effects are due to CD8+ or CD4+ T cells (Table 6).
The graft-versus-leukemia effect
Effectors . | Targets . | |
|---|---|---|
| CD4+ T cells | HLA class II—restricted | |
| CD8+ T cells | HLA class I—restricted | |
| Leukemia-specific antigens | ||
| Minor histocompatability antigens | ||
| NK cells | Alloreactive group | |
| Dendritic cells, macrophages, cytokines | ||
Effectors . | Targets . | |
|---|---|---|
| CD4+ T cells | HLA class II—restricted | |
| CD8+ T cells | HLA class I—restricted | |
| Leukemia-specific antigens | ||
| Minor histocompatability antigens | ||
| NK cells | Alloreactive group | |
| Dendritic cells, macrophages, cytokines | ||
Different components of the immune system target leukemia cells for elimination. In the GVL effect, T cells and NK cells play a major role in eliminating leukemia cells. The target structures for immune attack by T cells may be leukemia-specific antigens or minor histocompatibility antigens, which are expressed on all cells or limited to expression on hematopoietic cells. NK cells attack allogeneic target cells which do not express class I ligands that interact with their KIR (alloreactive groups). Dendritic cells, macrophages, and cytokines play roles in modulating the GVL effect.
Cytotoxic CD4+ T-cell clones with an ability to suppress leukemia colony formation have been described in vitro.55,57 CD8+ cells could be depleted from the DLT without jeopardizing the GVL effect,28 while concurrently diminishing GVHD. However, CD4+ T cells may have exerted their greatest benefit through a helper effect by recruiting leukemia-reactive, minor H-antigen–specific CD8+ T cells that were present in the recipient.58 A limited number of T-cell clones may be responsible for the elimination of CML. Analysis of the T-cell repertoire after DLT using T-cell receptor (TCR) Vβ typing showed a clonal imbalance in all patients, lasting for about one year. The emergence of single T-cell clones coincided with a cytogenetic response.59,60 Falkenburg et al generated cytotoxic T lymphocytes (CTLs) in vitro from marrow donors. Transfusion of pooled CTL lines specific for CML induced remission in a post-SCT patient with a relapse in accelerated phase who failed to achieve remission after conventional DLT.61 More recently, the response to DLT could be correlated with the presence of CTLs directed against the minor H antigens, HA1 and HA2, which are restricted in their expression to cells of the hematopoietic system.62
Role of dendritic cells in GVL effect
Development of effective T-cell immunity to malignancy requires efficient antigen presentation to T cells, alongside delivery of appropriate costimulatory signals. We suggested that the better response of myeloid leukemia to DLT, compared with lymphoid leukemia (ALL and CLL), is due to the direct presentation of leukemia antigens to donor T cells by myeloid leukemia–derived dendritic cells.13 This contention has been supported by the finding that dendritic cells in CML display the bcr/abl translocation, as shown by FISH analysis.63-66 Dendritic cells of leukemia origin are stimulatory for allogeneic T cells63 and induce CTLs.65,66 In AML, dendritic-like cells may be differentiated from tumor cell blasts by culture with granulo-monocyte colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4).67-69 In early relapse after allogeneic SCT, spontaneous differentiation of blasts to mature cells may also occur in patients.70 Currently, however, the precise role of leukemia-derived dendritic cells in inducing GVL effect is unclear.
Role of NK cells in GVL effect
In HLA-mismatched SCT, NK cells were found to exert strong alloimmune responses71 and contributed to the eradication of acute leukemia72,73 and high-grade lymphomas.48 NK cells are inhibited by signals delivered through their surface killer immunoglobulin-like receptors (KIRs) following interactions with autologous HLA class I molecules expressed by target cells. This KIR–major histocompatibility complex (MHC) interaction inhibits the cytotoxic activity of NK cells.74 In the absence of self-HLA class I molecules, autologous NK cells are not inhibited and they can mediate target cell lysis (“missing self hypothesis”75 ) given proper activation. In HLA-mismatched SCT, the lack of appropriate HLA class I ligands in the recipient that were able to inhibit all the KIRs of donor NK cells facilitated a strong GVL effect.76 Costimulatory molecules, such as the lymphocyte function antigen 1 (LFA-1), may direct NK cells to leukemia cells. AML and CML may be better target cells for NK cells than ALL because of their higher LFA-1 expression, and they may be better able to provide activation signals to the NK cells. Alloreactive NK cells not only contributed to an antileukemia effect in AML, but they also improved engraftment through elimination of recipient hematopoietic cells. In addition, they decreased the incidence of GVHD, probably through elimination of recipient dendritic cells that serve to activate T-cell responses.73 Although these multiple benefits of NK alloreactivity were found in HLA-haploidentical SCT, similar benefits were not seen for KIR ligand incompatibility in the outcome of unrelated bone marrow transplantation.74 Differences in transplantation procedures and complexity with respect to genetic diversity in NK activating and inhibitory receptors may account for the differences obtained in these 2 studies. Future studies will help to elucidate whether NK alloreactivity can be beneficial in allogeneic transplantation settings other than HLA-haploidentical SCT.
Unlike NK cells, NK-T cells are CD3+ cells that express a distinct Vα24+ T-cell receptor (TCR) together with an NK cell marker (CD161). A subpopulation of marrow-derived CD8+ NK-T cells was shown to kill leukemia cells via FAS-ligand and perforin, without causing GVHD.77 After stimulation with interferon-γ (IFN-γ) and anti-CD3 monoclonal antibody, and expansion with interleukin-2 (IL-2), CD8+ NK-T cells were cytolytic via their IFN-γ secretion and did not cause GVHD.78 In a study of HLA-haploidentical SCT, using marrow on day 0 and G-CSF–mobilized blood cells depleted of CD6+ cells on day 6, we observed a strong GVL effect with only limited GVHD. Here, CD8+ cells present in the CD6-depleted grafts were responsible for suppression of alloimmune responses.32,35 The ligand of CD6 is the activated leukocyte cell adhesion molecule (ALCAM), which is present on activated lymphocytes and some leukemia cells. Presumably this molecule directs CD8+, CD6– T cells toward activated lymphocytes and leukemia cells.
Immune escape mechanisms of leukemia
A number of different mechanisms can contribute to the failure of DLT to successfully eradicate leukemia. In some types of leukemia, cells may escape elimination by DLT because their rapid proliferation outpaces the development of immune responses. Effector cells may be unable to reach tumor cells localized in privileged sites. In addition, tumor cells may acquire altered expression of surface or intracellular molecules, which are essential for recognition or elimination by effector lymphocytes. Furthermore, tumor cells may directly down-regulate effector cells through secretion of inhibitory factors (Table 7).
Mechanisms of immune escape of leukemia
1. Invasion of immunologically privileged sites: CNS, gonads |
| 2. Down-regulation of HLA class I and class II expressions |
| 3. Deficient processing and presentation of peptides |
| 4. Deficient expression of costimulatory molecules: CD80, CD83, CD86, CD40, LFA-1, ICAM |
| 5. Secretion of inhibitory cytokines by leukemia cells: IL-10, TGF-β |
| 6. Abnormal secretion of and resistance to proinflammatory cytokines: TNF-α, IFN-γ |
| 7. Nonfunctional FAS on leukemia cells |
| 8. FAS-L expression by leukemia cells |
1. Invasion of immunologically privileged sites: CNS, gonads |
| 2. Down-regulation of HLA class I and class II expressions |
| 3. Deficient processing and presentation of peptides |
| 4. Deficient expression of costimulatory molecules: CD80, CD83, CD86, CD40, LFA-1, ICAM |
| 5. Secretion of inhibitory cytokines by leukemia cells: IL-10, TGF-β |
| 6. Abnormal secretion of and resistance to proinflammatory cytokines: TNF-α, IFN-γ |
| 7. Nonfunctional FAS on leukemia cells |
| 8. FAS-L expression by leukemia cells |
Different mechanisms can contribute to the failure of DLT to eradicate leukemia. Leukemia cells may persist in immunologically privileged sites, such as the central nervous system (CNS) and gonads or in extramedullary sites like the skin or the kidney, while still showing a continued remission in marrow. Malignant cells may escape immune detection and elimination if they have an altered expression of target antigens or costimulatory molecules required for efficient recognition. They may also directly down-regulate effector cells through secretion of inhibitory cytokines. Modulation of expression of FAS or FAS-ligand can also contribute to tumor escape from immune control.
Down-regulation of HLA class I and class II molecules occurred in only a minority of patients with acute leukemia79 but alterations in antigenic peptide sequences may occur. Studies in the mouse have demonstrated that single amino acid exchanges in a target peptide could prevent presentation by a murine leukemia.80 Low or missing expression of costimulatory molecules, such as CD80, CD83, CD86, CD40, and intercellular adhesion molecule (ICAM), on acute myeloid leukemia cells81-84 can prohibit proper development of T-cell responses. Down-regulation of HLA-DR and ICAM was observed on CD34+ progenitors of untreated CML.84 Consequently, cells of untreated CML patients were poorly stimulatory in mixed leukocyte reactions and poor targets of cell-mediated cytotoxicity (H.J.K. et al, unpublished observations, March 1998). Leukemia cells produce variable amounts of cytokines, such as interleukin-10 (IL-10) and tumor necrosis factor-α (TNF-α).85 IL-10 was shown to inhibit GVL reactions,86 whereas TNF-α stimulated allogeneic GVL responses. However, leukemia cells producing increased amounts of TNF-α may themselves become resistant to the effects of TNF-α. Tumor cells were also found to secrete TGF-β, which is a potent inhibitor of activated lymphocytes.87
Cytotoxicity of leukemia cells could be mediated through FAS or TNF receptors or through direct binding of perforin and activation of granzyme B.88 Resistance of AML blasts to NK cell killing was associated with a reduced perforin binding.89 The FAS receptor was shown to be present on leukemia blast cells but often it was not active due to lack of a transmembrane domain. Its soluble form could bind to FAS-ligand on effector cells, thereby preventing their cytotoxicity.90 Reciprocally, FAS-expressing effector cells were shown to be inactivated by leukemia cells expressing FAS-ligand.91,92 Moreover, TRAIL (TNF receptor apoptosis-inducing ligand) induced apoptosis in most Philadelphia chromosome–positive cell lines,93 whereas B-CLL cells were resistant due to their low expression of TRAIL receptors.94
Defective effector functions of T cells, which were associated with diminished expression of the CD3-ζ and CD3-ϵ chains, have also been described.95,96 Decreased expression of CD3-ζ and CD3-ϵ was more likely on lymphocytes of patients with advanced disease. Diminished expression of CD3-ζ and CD28 was also observed in patients with advanced malignancies, chronic viral infections, or autoimmune diseases, occurring as a consequence of chronic lymphocyte stimulation.97 T cells with these phenotypes were prone to apoptosis but they could be rescued with interferon-α and IL-2.96
Future developments
The clinical experience with DLT to date has revealed the great potential of this therapy for achieving eradication of some hematologic malignancies, such as CML. Nevertheless, broader success in the use of DLT for other hematologic and nonhematologic tumors will require further advances to enhance GVL effects. Furthermore, approaches to separate GVHD and GVL effect merit extensive effort in order to overcome the major complication of DLT.
Enhancement of GVL effects
Relatively straightforward ways to enhance GVL effects include measures to reduce leukemia burden, the suspension of immune suppression, and the use of cytokines to modulate the tumor cells, enabling better effector cell recognition. Reduction of tumor cell mass by radiation, chemotherapy, or antibody therapy may improve the efficiency of DLT to eradicate residual leukemia. In patients with recurrent AML after allogeneic SCT, chemotherapy induced remission in about 40% of patients compared with 25% of patients treated with DLT alone.15 Interestingly, 27% of AML patients failing to respond to chemotherapy went into remission after DLT. There are numerous reports of remission or loss of minimal residual disease following suspension of immune suppression, which is not necessarily accompanied by GVHD. There are 2 recent studies from Italy98,99 in adult and pediatric recipients with leukemia that found a significant reduction in the relapse rate when cyclosporine was used at lower doses in the early posttransplantation period, suggesting that much of the GVL effect due to the SCT alone is routinely obscured by the immune suppression used to control GVHD.
IL-2 has been used widely to treat malignant diseases.100,101 In the context of SCT, Slavin et al32,102 pioneered the use of DLT combined with IL-2. While low doses of IL-2 only supplemented the IL-2 deficiency occurring after allogeneic SCT, intermediate doses could activate NK cells and high doses could induce lymphokine activated killer (LAK) cells.
In CML, IFN-α has been used regularly to treat tumor relapse after allogeneic SCT,103 however most remissions were not durable. The combination of IFN-α and DLT has not been studied prospectively. In a retrospective analysis, IFN-α did not improve the outcome of DLT13 ; although in combination with DLT, IFN-α induced remission in isolated cases of DLT failure. IFN-α may improve GVL effects by up-regulating HLA and costimulatory molecules on tumor cells, making them more susceptible to immune attack. The immune phenotype of CML progenitor cells changed from DR-negative to DR-positive after treatment with IFN-α.84 In CML, the combination of IFN-α and GM-CSF achieved the best enhancement of the GVL effect.65 The triple combination of DLT, IFN-α, and GM-CSF induced remission in patients refractory to treatment with DLT alone or to DLT plus IFN-α. This triple combination also functioned in patients who relapsed in advanced phase.
Most AML blasts do not express CD80 and only a minority of blasts express CD86 costimulatory molecules, both of which are necessary for optimal activation of T cells.81 In 70% of cases of AML, the leukemia blasts could be differentiated into dendritic-like cells in short-term cultures using GM-CSF, IL-4, and TNF-α, with or without stem cell factor (SCF) and FLT-3 ligand.67-69 The conversion of AML cells into efficient antigen-presenting cells by certain cytokines may explain the high remission rate of 67% described in patients with AML, relapsing after allogeneic SCT, who received low-dose cytosine arabinoside followed by G-CSF–mobilized donor cells and GM-CSF.104 The survival probability of responders was 37.5% at 2 years and favorable factors for a durable remission included response to low-dose cytosine arabinoside prior to DLT, use of an HLA-identical family donor, and occurrence of chronic GVHD. Major complications were acute GVHD and infections, and the nonrelapse mortality was 25% at 2 years.
Strategies to avoid GVHD while conserving GVL effect
Despite improvements in treatment with new immune suppressive drugs and antibodies, GVHD remains the most serious complication of adoptive immune therapy with donor lymphocytes. As previously mentioned, both animal experiments and clinical results suggest that DLT can conserve GVL effects while reducing the potential for GVHD.7,8 Several investigators have therefore adopted an approach using a T-cell–depletion step in SCT followed by a delayed T-cell add-back transplant for patients with evidence of residual disease around 3 months after transplantation. This approach, which aims to minimize the GVHD component of the allogeneic SCT, resulted in favorable disease control, at least in CML.105
Depletion of subsets of T cells has been explored with some success. Based on the possibility that the GVL effect in CML is caused by CD4+ cells, whereas CD8+ cells are prime effectors of GVHD, some investigators have used CD8-depleted SCT or DLT. These approaches reduced GVHD after DLT.28,57 Several approaches have also been developed to selectively deplete alloreactive T cells from DLT, based on evidence that some T cells recognize antigens uniquely expressed on leukemic cells or hematopoietic lineages. Animal models have validated this selective depletion method in MHC-mismatched transplant models.106,107 In this approach, T cells are activated by exposure to nonleukemic host cells and eliminated with antibody against the IL-2 receptor, CD69, and the FAS receptor or by the differential sensitivity of activated T cells to photosensitive dyes. Clinical trials are in progress but at present it is not known whether the DLT will contain adequate numbers of leukemia-specific T cells to provide an effective GVL response108 following elimination of alloreactive T cells that recognize immunodominant minor H antigens.
Another promising method to control GVHD is to introduce a suicide gene into the T cells prior to DLT.29 Suicide genes can encode proteins that lead either to the death of cells following activation by a drug that is otherwise nontoxic to mammalian cells29 or by activating the death program in cells.109 The suicide gene technology has been introduced into clinical practice with initial success,29 but a number of problems remain to be solved. The function of T cells may be impaired by the selection and expansion procedures required to obtain sufficient numbers of suicide gene–expressing T cells.110 After DLT, patients may become immunized against the foreign suicide protein and reject the modified T cells.111 Alternatively, splice variants of the suicide gene may produce only inactive protein.112 Furthermore, recent gene therapy trials using retrovirally transduced stem cells revealed the risk for secondary malignancies that develop from transduced cells through insertional mutagenesis.113
Leukemia-specific immune therapy
There are 2 major categories of leukemia-specific, or at least hematopoietic-restricted, antigens that are capable of inducing CTL-recognizing tumor cells bearing these antigens: nonpolymorphic TSA and polymorphic minor H antigens. A growing number of nonpolymorphic leukemia-specific antigens (LSAs) are now being investigated. These include BCR/ABL fusion peptide,114 RARA fusion peptide,115 immunoglobulin idiotype,116 and peptides derived from proteinase 3,117,118 myeloperoxidase,119 and the Wilms tumor zinc finger transcription factor (WT1).120 Whether these LSAs can be used to develop leukemia-specific T-cell reactions has not yet been established. In contrast, minor H antigens selectively expressed by hematopoietic cells serve as potent targets of GVH reactions against hematopoietic cells, including leukemia. It is not yet clear whether T-cell responses to these immunodominant antigens will provide GVL responses without GVHD.121,122 To enhance responses to these antigens, T cells with higher affinity TCRs can be selected when peptides presented via foreign HLA molecules are used for stimulation. This strategy has been evaluated both for minor H antigens and for WT1.123
Separation of GVL effects from GVHD may also be achieved through the powerful alloresponses mediated by NK cells reacting to mismatched MHC class I molecules. NK cells do not induce GVHD and their cytotoxicity is mainly directed against hematopoietic cells and tumor cells. One study linked NK alloreactivity (as measured by posttransplantation NK cytotoxicity) with the chance of remaining in remission.124 More recently NK alloresponses to leukemia have been identified in both haploidentical transplants and in unrelated HLA-matched donor-recipient pairs.74,125
Outlook
In the past decade the paradigm of SCT has changed. Nowadays confidence in the power of the GVL effect has encouraged the use of transplants following low-intensity, nonmyeloablative preparative regimens. The results of these studies will determine how much we can rely upon the GVL effects of the engrafted donor immune system to cure leukemia rather than on the traditional use of high-dose chemotherapy and irradiation to eradicate tumor cells. To achieve maximum GVL effects it will also be necessary to perform SCT without immune suppression after transplantation. This requires perfection of strategies currently under development to selectively eliminate the GVHD alloresponse. Meanwhile, in transplantations between HLA-mismatched individuals, the deliberate selection of NK alloincompatible donors to obtain maximum GVL effects seems worthwhile. While DLT can induce remission in chimeric patients with indolent malignancies, the rapid disease progression seen in acute leukemia will require the transfusion of donor lymphocytes presensitized to leukemia and expanded in vitro to achieve a more rapid GVL effect. For this purpose, the discovery and characterization of antigens able to elicit highly cytotoxic leukemia-reactive T cells will be critical.
Prepublished online as Blood First Edition Paper, September 4, 2003; DOI 10.1182/blood-2003-02-0342.
Supported by the Deutsche José Carreras Leukaemie Stiftung eV, and European Union (EU) contracts Suicide gene therapy in stem cell transplantation (SCT) QLRT-2000-01265, Eurobank QLRT-2000-00010, Transeurope QLRT-2001-01936, Eurocord QLK3-CT-2002-01918, and Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 455.
Special thanks are expressed to Dr Elizabeth Simpson for critically reading the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal