Abstract
Blockade of the CD40/CD154 signal is a potential immunomodulatory strategy for T-cell–mediated diseases. As a part of a phase 1, multicenter, dose-escalating trial of humanized monoclonal antibody to CD154 (IDEC-131/E6040) in patients with refractory immune thrombocytopenic purpura (ITP), the autoimmune response to glycoprotein IIb/IIIa (GPIIb/IIIa) was evaluated at successive time points. Five patients each were given a single infusion of 1, 2, 5, or 10 mg/kg IDEC-131/E6040 and followed for 3 months. All adverse events were mild, and there were no severe infections or thromboembolic events. No increase in platelet count was observed in patients treated at 1, 2, or 5 mg/kg, but an increase was observed in 3 patients treated at 10 mg/kg. In only the patients treated at 5 or 10 mg/kg, the frequency of B cells producing anti-GPIIb/IIIa antibodies, GPIIb/IIIa-induced T-cell proliferation, and anti-GPIIb/IIIa antibody production by antigen-dependent T–B-cell collaboration were all suppressed in parallel after the treatment, with a slow return to baseline. In contrast, T-cell response to an irrelevant antigen was not affected. These findings suggest that CD40/CD154 blockade therapy is potentially effective for refractory ITP, through selective suppression of autoreactive T and B cells to platelet antigens.
Introduction
Immune thrombocytopenic purpura (ITP) is an autoimmune disease in which platelets are opsonized by antiplatelet autoantibodies and subsequently destroyed by the reticuloendothelial system.1 Approximately 70% to 80% of patients with ITP respond to either corticosteroids or splenectomy and maintain a safe platelet count with low-dose corticosteroids or even without further therapy.1 Patients with refractory ITP who fail these standard treatments require additional treatment. A variety of second-line treatments that are potentially effective for refractory ITP have been reported, but there is little evidence showing the efficacy of these therapies, because these treatment approaches are based primarily on clinical experience.1-3 Because patients with refractory ITP have significant morbidity from the disease and because of the toxicity of the treatment,4 there is a need for more effective therapeutic interventions for refractory ITP.
The major target of antiplatelet antibodies in patients with ITP is the platelet membrane glycoprotein IIb/IIIa (GPIIb/IIIa), also designated αIIbβ3 integrin or CD41/CD61.5,6 We have recently found that CD4+ T cells reactive with GPIIb/IIIa from patients with ITP have helper activity that promotes production of anti-GPIIb/IIIa antibodies capable of binding to healthy platelets, indicating that these autoreactive T cells are involved in the production of pathogenic antiplatelet antibodies.7,8 Therefore, the GPIIb/IIIa-reactive T cell is a reasonable target for a therapeutic strategy that selectively suppresses the pathogenic autoimmune response in patients with ITP. One candidate strategy is the disruption of a costimulatory signal by blocking the interaction between CD40 on antigen-presenting cells (APCs) and CD154 (also known as CD40 ligand) on activated CD4+ T cells; this interaction is essential for the T-cell priming and the T-cell–dependent humoral immune response.9-11 The efficacy of blocking this interaction therapeutically with an anti-CD154 monoclonal antibody (mAb) has been shown in animal models for various autoimmune diseases, including rheumatoid arthritis,12 systemic lupus erythematosus (SLE),13 multiple sclerosis,14,15 and myasthenia gravis.16 In these animal models, anti-CD154 mAb not only prevented disease development but also interfered with ongoing disease.13-16 Thus, disruption of CD40/CD154 signaling has been proposed as a strategy for treating human diseases mediated by T cells and/or autoantibodies11 and is currently being used in clinical studies in patients with various autoimmune diseases.17
IDEC-131/E6040 is a humanized mAb against human CD154, comprising human γ1 heavy chains and human κ light chains with complementarity-determining regions of murine mAb clone 24-31.18 It binds to the trimer of CD154 on T cells with high specificity and avidity, thereby preventing CD40 signaling. In clinical trials of IDEC-131/E6040 involving patients with SLE, this drug was well tolerated, and no serious adverse effects were observed.19,20
As a part of a phase 1, multicenter, dose-escalating trial of IDEC-131/E6040 in patients with refractory ITP, we had the opportunity to study in vivo effects of a single infusion of anti-CD154 mAb on autoreactive T- and B-cell responses to GPIIb/IIIa using human subjects.
Patients, materials, and methods
Study design
A phase 1, multicenter, dose-escalating study of IDEC-131/E6040 was conducted in Japan that involved a total of 20 patients at 5 medical centers (Keio University, Kansai Medical University, Hiroshima University, University of Tsukuba, and Toranomon Hospital) from November 1999 to August 2001. Written informed consent was obtained from all patients in accordance with the Institutional Review Boards of each of the participating institutions. All participants were hospitalized 1 day before the drug administration (day –1) and remained in the hospital for another 4 days to monitor serum drug concentration and potential short-term toxicity. Five patients each received a single 1-hour intravenous infusion of 1, 2, 5, or 10 mg/kg of IDEC-131/E6040 on day 0. The first 5 patients received 1 mg/kg of the drug, and the dosage was escalated to the next dose if no severe toxicity was reported in any of the 5 patients within 8 days of the administration. Patients were followed for the subsequent 3 months to assess pharmacokinetics, toxicity, and effects on platelets, peripheral blood lymphocytes, and serum chemistry levels. To evaluate the autoimmune response to GPIIb/IIIa, peripheral blood samples were obtained from all the patients at 3 time points: day 0 (pretreatment), and days 7 and 42 (after treatment). Additional assessments were performed on days 28 and 56 in some patients.
Patient selection
Adult patients aged between 20 and 65 years were eligible. The diagnosis of chronic ITP was based on thrombocytopenia (platelet count <100 × 109/L) persisting longer than 6 months, normal or increased bone marrow megakaryocytes without morphologic evidence for dysplasia, and no secondary immune or nonimmune diseases that could account for the thrombocytopenic state.2 Inclusion criteria were a platelet count between 10 and 50 × 109/L during the past 2 months and refractoriness to therapy with a standard dose of prednisolone.
Exclusion criteria included pregnancy, severe renal or heart disease, active psychiatric disease, chronic liver disease, recurrent or acute infection, history of malignancy in the past 5 years, and prior treatment with a monoclonal antibody or other biologic agents. Patients who had received intravenous cyclophosphamide or vincristine within the prior 6 months or patients who had received intravenous immunoglobulin within the prior 3 months were excluded. Patients taking prednisolone and/or other oral medications for ITP were allowed to continue their therapies during the study period, provided the drug dosages were maintained at a constant level until the study had been completed.
Clinical monitoring
Safety evaluations, including the monitoring of adverse events and the administration of routine laboratory tests, were performed during hospitalization and subsequent follow-up visits. Adverse events were recorded throughout the study period on the basis of the National Cancer Institute (NCI) common toxicity criteria.21 Laboratory results were recorded at regular intervals. Complete blood cell counts, serum chemistry studies, and urinalysis were performed by the local laboratories of each participating center. Lymphocyte subsets were further analyzed by 2-color flow cytometry at a central laboratory (SRL, Tokyo, Japan),22 and the subsets analyzed included cells positive for CD3, CD4, CD8, CD19, CD3/CD4, CD3/CD8, CD3/CD154, CD4/CD154, CD8/CD154, CD19/CD154, and CD19/CD40.
Response of platelet count was defined as a rise of the count to more than 50 × 109/L for at least 2 weeks and a peak platelet count more than 30 × 109/L greater than the pretreatment value. We used a platelet count of 50 × 109/L as the threshold for a response because most patients with platelet counts at or above this level do not have bleeding or require therapy.23
Cell preparations
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by Lymphoprep (Nycomed Pharma AS, Oslo, Norway) density-gradient centrifugation and resuspended in RPMI1640 supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 50 U/mL penicillin, and 50 μg/mL streptomycin.
Quantification of circulating anti-GPIIb/IIIa antibody-producing B cells
Peripheral blood B cells producing immunoglobulin G (IgG) anti-GPIIb/IIIa antibodies were quantified by using an enzyme-linked immunospot assay as described previously.24,25 Briefly, polyvinylidene diflouride–bottomed 96-well multititer plates (Millipore-Amicon, Bedford, MA) were coated with 30 μg/mL purified human GPIIb/IIIa (Enzyme Research Laboratories, South Bend, IN) and subsequently blocked with 1% bovine serum albumin. PBMCs (105 cells/well) in complete medium were incubated in the GPIIb/IIIa-coated plates at 37°C in a humidified atmosphere of 5% CO2 for 4 hours. After washing away the cells, the membranes were incubated with alkaline phosphatase–conjugated goat antihuman IgG (ICN/Cappel, Aurora, OH), and antibodies bound to the membrane were visualized as spots by incubation with nitro blue tetrazolium/5-bromo-4-chloro-indolyl phosphate. Each experiment was carried out in 5 independent wells, and the results represent the mean of the 5 values and are expressed as the number per 105 PBMCs.
T-cell proliferative response induced by GPIIb/IIIa
The autoreactive T-cell response to GPIIb/IIIa was evaluated by quantifying the proliferation of bulk PBMCs that was induced by antigenic stimulation with GPIIb/IIIa.7,8 Briefly, PBMCs were cultured in the presence or absence of 5 μg/mL trypsin-digested GPIIb/IIIa or tetanus toxoid C-fragment (List Biological Laboratories, Campbell, CA) for 7 days. After a final 16-hour incubation with 0.5 μCi/well (0.0185 MBq) of 3H-thymidine, the cells were harvested and the amount of 3H-thymidine incorporation was determined in a TopCount microplate scintillation counter (Packard, Meriden, CT). All cultures were prepared in quadruplicate, and all values represent the mean of 4 determinations. The GPIIb/IIIa-specific T-cell proliferation was expressed as a stimulation index, which was calculated as the cpm incorporated into cultures with trypsin-digested GPIIb/IIIa divided by the cpm incorporated into cultures with trypsin alone. The tetanus toxoid–specific response was also expressed as a stimulation index, which was calculated as the cpm incorporated into cultures with tetanus toxoid divided by the cpm incorporated into cultures without antigen.
Antigen-dependent anti-GPIIb/IIIa antibody production in PBMC cultures
The anti-GPIIb/IIIa antibody production mediated by GPIIb/IIIa-dependent T–B-cell collaboration was quantitatively assessed in PBMC cultures with antigenic stimulation with GPIIb/IIIa.7,8 Briefly, PBMCs were cultured with trypsin-digested GPIIb/IIIa (5 μg/mL) in the presence of pokeweed mitogen (1 μg/mL) for 10 days. The levels of IgG anti-GPIIb/IIIa antibodies in undiluted culture supernatants were measured with an enzyme-linked immunosorbent assay using GPIIb/IIIa as the antigen.7 Antibody units were calculated from the optical density (OD)450, with the results being based on the standard curve obtained from a serial concentration of mouse mAb to GPIIb/IIIa (clone HPL1; Harlan Sera Laboratory, Leicester, United Kingdom). The anti-GPIIb/IIIa antibody results represent the mean of 4 values, because all cultures were prepared in duplicate, and the antibody measurement in individual cultures was carried out in duplicate.
Statistical analysis
Differences in clinical characteristics among different dose groups were assessed by using the chi-square test or Kruskal-Wallis test. Changes in the absolute values at different time points from the baseline value taken at day 0 were compared by repeated measures analysis of variance.
Results
Patient characteristics
Twenty patients aged between 22 and 63 years were enrolled in this study. The pretreatment characteristics of the patients are shown in Table 1. Seventy-five percent were women, with a mean age of 51.6 years. The disease duration ranged from 7 to 272 months. Mean platelet count at baseline was 29 × 109/L. The number of patients with a platelet count of more than 30 × 109/L at study entry in the 1, 2, 5, and 10 mg/kg group was 1, 2, 1, and 3, respectively. Most of the patients had already received 2 or more different treatment regimens, with a mean of 2.6. Previous treatments other than prednisolone included intravenous immunoglobulin in 11 patients, splenectomy in 7, danazol in 7, azathioprine in 2, dapsone in 2, vincristine in 1, and interferon-α in 1. Of the 11 patients who underwent intravenous immunoglobulin therapy, 8 (73%) had achieved a significant response. Seventeen patients were taking prednisolone (mean, 7.6 mg/d; range, 2.5-20 mg/d) for ITP, and 2 of them also took danazol (200 mg/d) at study entry. These patients continued on stable doses of their medications throughout the trial. There was no statistical difference in these baseline characteristics among the 4 dose groups.
Summary of patients' pretreatment characteristics for patients with ITP
. | Patient groups based on the dose of IDEC-131/E6040 . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | 1 mg/kg . | 2 mg/kg . | 5 mg/kg . | 10 mg/kg . | |||
| No. of patients | 5 | 5 | 5 | 5 | |||
| Men | 0 | 1 | 1 | 3 | |||
| Women | 5 | 4 | 4 | 2 | |||
| Age, y | |||||||
| Median (range) | 55 (48-60) | 56 (29-59) | 55 (22-60) | 58 (51-63) | |||
| Mean | 55.0 | 50.4 | 43.8 | 57.0 | |||
| ITP duration at IDEC-131/E6040 treatment, mo | |||||||
| Median (range) | 89 (20-272) | 50 (7-151) | 82 (17-162) | 53 (9-226) | |||
| Mean | 118.4 | 70.9 | 84.3 | 77.1 | |||
| Baseline platelet count, × 109/L | |||||||
| Median (range) | 18 (9-77) | 31 (14-52) | 20 (9-35) | 35 (24-57) | |||
| Mean | 30 | 30 | 21 | 36 | |||
| Previous therapy, no. of patients (current therapy, no. of patients) | |||||||
| Prednisolone | 5 (3) | 5 (4) | 5 (5) | 5 (5) | |||
| Intravenous immunoglobulin | 3 | 4 | 1 | 3 | |||
| Splenectomy | 2 | 2 | 1 | 2 | |||
| Danazol | 3 | 1 | 2 (1) | 1 (1) | |||
| Azathioprine | 1 | 0 | 0 | 1 | |||
| Dapsone | 1 | 0 | 1 | 0 | |||
| Vincristine | 1 | 0 | 0 | 0 | |||
| Interferon-α | 1 | 0 | 0 | 0 | |||
. | Patient groups based on the dose of IDEC-131/E6040 . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | 1 mg/kg . | 2 mg/kg . | 5 mg/kg . | 10 mg/kg . | |||
| No. of patients | 5 | 5 | 5 | 5 | |||
| Men | 0 | 1 | 1 | 3 | |||
| Women | 5 | 4 | 4 | 2 | |||
| Age, y | |||||||
| Median (range) | 55 (48-60) | 56 (29-59) | 55 (22-60) | 58 (51-63) | |||
| Mean | 55.0 | 50.4 | 43.8 | 57.0 | |||
| ITP duration at IDEC-131/E6040 treatment, mo | |||||||
| Median (range) | 89 (20-272) | 50 (7-151) | 82 (17-162) | 53 (9-226) | |||
| Mean | 118.4 | 70.9 | 84.3 | 77.1 | |||
| Baseline platelet count, × 109/L | |||||||
| Median (range) | 18 (9-77) | 31 (14-52) | 20 (9-35) | 35 (24-57) | |||
| Mean | 30 | 30 | 21 | 36 | |||
| Previous therapy, no. of patients (current therapy, no. of patients) | |||||||
| Prednisolone | 5 (3) | 5 (4) | 5 (5) | 5 (5) | |||
| Intravenous immunoglobulin | 3 | 4 | 1 | 3 | |||
| Splenectomy | 2 | 2 | 1 | 2 | |||
| Danazol | 3 | 1 | 2 (1) | 1 (1) | |||
| Azathioprine | 1 | 0 | 0 | 1 | |||
| Dapsone | 1 | 0 | 1 | 0 | |||
| Vincristine | 1 | 0 | 0 | 0 | |||
| Interferon-α | 1 | 0 | 0 | 0 | |||
Adverse events
Nine patients experienced a total of 16 adverse events that were considered possibly or probably related or of unknown relationship to IDEC-131/E6040 treatment during the observation period (Table 2). There was no clear dose relationship in the frequency and distribution of adverse events among the study groups. All of these side effects were brief and of mild intensity (grade 1 or 2 according to the NCI criteria). Seven patients experienced these adverse events on the day of drug infusion, and these adverse events included hot flushes, nausea, vomiting, fatigue, and dizziness. One patient in the 10 mg/kg group experienced an acute allergic reaction, consisting of pruritus, rhinorrhea, urticaria (grade 2), nausea, vomiting, and dyspnea (grade 1) immediately after the drug infusion. Two patients experienced one infection each during the course of the 3-month follow-up period. These infections were an upper respiratory tract infection at day 11 (1 mg/kg group) and small blisters on the dorsum of the right hand at day 8 (2 mg/kg group). These patients recovered quickly without complication. None of the patients in the 5 or 10 mg/kg group developed an overt infection. No thromboembolic events were observed in any of the enrolled patients.
Adverse events during IDEC-131/E6040 therapy
. | . | NCI grade . | . | |
|---|---|---|---|---|
| Event . | Total . | 1 . | 2 . | |
| Any events | 16 | 11 | 5 | |
| Hot flushes | 3 | 3 | 0 | |
| Nausea | 2 | 2 | 0 | |
| Vomiting | 1 | 1 | 0 | |
| Peripheral edema | 1 | 1 | 0 | |
| Fatigue | 1 | 1 | 0 | |
| Infected blister | 1 | 1 | 0 | |
| Dizziness | 1 | 1 | 0 | |
| Dyspnea | 1 | 1 | 0 | |
| Cough | 1 | 0 | 1 | |
| Upper respiratory tract inflammation | 1 | 0 | 1 | |
| Pruritus | 1 | 0 | 1 | |
| Rhinorrhea | 1 | 0 | 1 | |
| Urticaria | 1 | 0 | 1 | |
. | . | NCI grade . | . | |
|---|---|---|---|---|
| Event . | Total . | 1 . | 2 . | |
| Any events | 16 | 11 | 5 | |
| Hot flushes | 3 | 3 | 0 | |
| Nausea | 2 | 2 | 0 | |
| Vomiting | 1 | 1 | 0 | |
| Peripheral edema | 1 | 1 | 0 | |
| Fatigue | 1 | 1 | 0 | |
| Infected blister | 1 | 1 | 0 | |
| Dizziness | 1 | 1 | 0 | |
| Dyspnea | 1 | 1 | 0 | |
| Cough | 1 | 0 | 1 | |
| Upper respiratory tract inflammation | 1 | 0 | 1 | |
| Pruritus | 1 | 0 | 1 | |
| Rhinorrhea | 1 | 0 | 1 | |
| Urticaria | 1 | 0 | 1 | |
Adverse events classified as NCI (National Cancer Institute) grades 3 and 4 were not observed.
Effects on platelet count
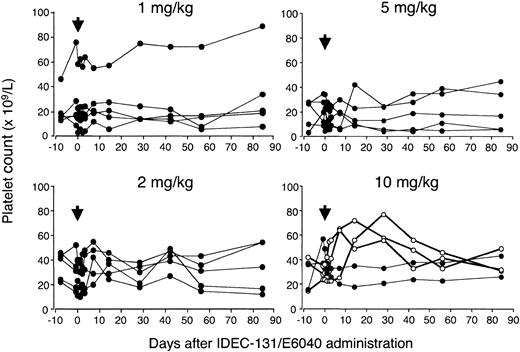
The changes in the platelet count before and after the IDEC-131/E6040 administration are illustrated in Figure 1. The effect on platelet count could not be assessed in one patient in the 1 mg/kg group, because this patient had a platelet count greater than 50 × 109/L during the entire observation period. No increase in platelet count was observed in any patient who received 1, 2, or 5 mg/kg IDEC-131/E6040, although one patient in the 5 mg/kg group experienced a transient decrease of platelet count at day 7, which quickly recovered to the baseline (28, 7, and 42 × 109/L at day 0, 7, and 14, respectively). In contrast, the platelet count of 3 of 5 patients given 10 mg/kg increased to 60 × 109/L or more following treatment; these patients (one man and 2 women) were thus classified as responders. In the responders, the platelet count started to rise within 1 week, and the effect was sustained for the subsequent 6 weeks. Of 3 responders, one had undergone splenectomy. There was no difference in pretreatment characteristics, including platelet count and previous and current therapies, between responders and nonresponders in the 10 mg/kg group, although the number of patients was very small.
Serial platelet count before and after administration of IDEC-131/E6040. Five patients each were treated with 1, 2, 5, or 10 mg/kg IDEC-131/E6040. Arrows denote the day of drug infusion (day 0). In the 10 mg/kg group, ○ indicates responder; • indicates nonresponder.
Serial platelet count before and after administration of IDEC-131/E6040. Five patients each were treated with 1, 2, 5, or 10 mg/kg IDEC-131/E6040. Arrows denote the day of drug infusion (day 0). In the 10 mg/kg group, ○ indicates responder; • indicates nonresponder.
Effects on lymphocyte count and subsets
The total lymphocyte count did not decrease in any of the patients after infusion of IDEC-131/E6040. To the contrary, a transient increase in the lymphocyte count was observed in most of the patients who received IDEC-131/E6040 (Figure 2). The lymphocyte count increased the day after the drug infusion, peaked within 3 days, and returned to the baseline level by day 7. This pattern was observed in all 4 dose groups but was most evident in the 10 mg/kg group. The increase in the lymphocyte count at days 1 and 2 compared with baseline (day 0) was statistically significant only for the 10 mg/kg group (2.2 ± 0.4 and 2.4 ± 0.4 versus 0.9 ± 0.3, P = .02 and .01, respectively). The proportions of CD3+, CD4+, CD8+, and CD19+ cells were stable during this period, indicating that these lymphocyte subsets increased uniformly. The proportion of CD154+ cells among the circulating CD3+ T cells was less than 1.5% at all time points, and this was not affected by the IDEC-131/E6040 treatment. In addition, the proportion of CD40+ cells among the CD19+ B cells was also stable throughout the observation period, although there were minor fluctuations.
Total lymphocyte count before and after administration of IDEC-131/E6040. Each data point represents the mean lymphocyte count in individual dose groups. A statistically significant change compared with pretreatment (day 0) is indicated by < (P < .05 by repeated measures analysis of variance).
Total lymphocyte count before and after administration of IDEC-131/E6040. Each data point represents the mean lymphocyte count in individual dose groups. A statistically significant change compared with pretreatment (day 0) is indicated by < (P < .05 by repeated measures analysis of variance).
Effects on autoreactive T- and B-cell responses to GPIIb/IIIa
The immunologic response induced by IDEC-131/E6040 administration was assessed at 3 different time points: pretreatment (day 0), and days 7 and 42 after treatment. The GPIIb/IIIa-specific autoimmune responses that were evaluated included the frequency of circulating B cells producing anti-GPIIb/IIIa antibodies, the degree of T-cell proliferation induced by GPIIb/IIIa, and the amount of anti-GPIIb/IIIa antibodies produced by antigen-specific T–B-cell collaboration.
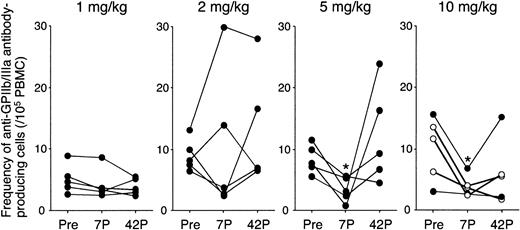
All patients showed an elevated frequency of circulating anti-GPIIb/IIIa antibody-producing B cells (>2/105 PBMCs) at baseline, further supporting the diagnosis of ITP and the autoimmune nature of the disease.25 As shown in Figure 3, there was no consistent change in the frequency of circulating anti-GPIIb/IIIa antibody-producing B cells in the 1 and 2 mg/kg groups. In contrast, the specific B-cell frequency was markedly decreased at day 7 in all patients treated at 5 or 10 mg/kg (8.4 to 3.4, P = .01 and 10.1 to 3.8, P = .03, respectively), although the frequency was restored to baseline or even increased at day 42 in most of these patients. In patients treated with 10 mg/kg IDEC-131/E6040, 2 patients maintained reduced levels at day 42, whereas 3 patients rebounded by day 42. It was of note that, in 3 responders, anti-GPIIb/IIIa antibody-producing B-cell frequency at day 42 was still lower than the baseline level (4.4 versus 10.5, P = .03).
Frequency of circulating anti-GPIIb/IIIa antibody-producing B cells before and after administration of IDEC-131/E6040. Peripheral blood B cells producing IgG anti-GPIIb/IIIa antibodies were quantified by using an enzyme-linked immunospot assay, and the results represent the mean of the 5 values (number per 105 PBMCs). Peripheral blood samples were analyzed at 3 different time points: day 0 (pretreatment; Pre), and days 7 and 42 after treatment (7P and 42P, respectively). Statistically significant change compared with pretreatment is indicated by < (P < .05 by repeated measures analysis of variance). In the 10 mg/kg group, ○ indicates responder and • indicates nonresponder.
Frequency of circulating anti-GPIIb/IIIa antibody-producing B cells before and after administration of IDEC-131/E6040. Peripheral blood B cells producing IgG anti-GPIIb/IIIa antibodies were quantified by using an enzyme-linked immunospot assay, and the results represent the mean of the 5 values (number per 105 PBMCs). Peripheral blood samples were analyzed at 3 different time points: day 0 (pretreatment; Pre), and days 7 and 42 after treatment (7P and 42P, respectively). Statistically significant change compared with pretreatment is indicated by < (P < .05 by repeated measures analysis of variance). In the 10 mg/kg group, ○ indicates responder and • indicates nonresponder.
The complete data on GPIIb/IIIa-induced T-cell proliferation were available for the patients who received IDEC-131/E6040 at 2, 5, and 10 mg/kg. As shown in Table 3, there was no consistent change in degree of GPIIb/IIIa-specific T-cell proliferation in the 2 mg/kg group, although the degree of antigen-induced T-cell proliferative responses was variable among individuals. In contrast, GPIIb/IIIa-induced T-cell proliferation tended to be suppressed at days 7 and 42 in patients treated with 5 or 10 mg/kg of the drug. There was a borderline reduction in the stimulation index following treatment in the 10 mg/kg group (P = .054 and .08 at days 7 and 42, respectively). To exclude nonspecific T-cell suppression mediated by the anti-CD154 mAb treatment, we simultaneously measured the T-cell proliferation induced by tetanus toxoid, an irrelevant foreign recall antigen. As expected, tetanus toxoid–specific T-cell proliferation was stable during the IDEC-131/E6040 treatment in all 3 dose groups.
Changes in T-cell proliferative responses to GPIIb/IIIa and tetanus toxoid after a single injection of IDEC-131/E6040
. | Stimulation index of antigen-specific T-cell proliferation . | . | . | ||
|---|---|---|---|---|---|
| IDEC-131/E6040 dose . | Day 0 pretreatment . | Day 7 after treatment . | Day 42 after treatment . | ||
| GPIIb/IIIa-specific T-cell response | |||||
| 2 mg/kg | 2.3 ± 0.8 | 2.6 ± 1.5 | 2.2 ± 1.5 | ||
| 5 mg/kg | 6.5 ± 4.9 | 4.6 ± 4.1 | 2.3 ± 1.2 | ||
| 10 mg/kg | 8.3 ± 5.9 | 4.7 ± 5.1* | 3.2 ± 1.0† | ||
| Tetanus toxoid-specific T-cell response | |||||
| 2 mg/kg | 4.9 ± 2.0 | 5.0 ± 3.3 | 3.3 ± 1.9 | ||
| 5 mg/kg | 1.9 ± 1.6 | 1.9 ± 1.5 | 2.6 ± 2.6 | ||
| 10 mg/kg | 4.5 ± 3.2 | 4.0 ± 2.6 | 3.5 ± 1.8 | ||
. | Stimulation index of antigen-specific T-cell proliferation . | . | . | ||
|---|---|---|---|---|---|
| IDEC-131/E6040 dose . | Day 0 pretreatment . | Day 7 after treatment . | Day 42 after treatment . | ||
| GPIIb/IIIa-specific T-cell response | |||||
| 2 mg/kg | 2.3 ± 0.8 | 2.6 ± 1.5 | 2.2 ± 1.5 | ||
| 5 mg/kg | 6.5 ± 4.9 | 4.6 ± 4.1 | 2.3 ± 1.2 | ||
| 10 mg/kg | 8.3 ± 5.9 | 4.7 ± 5.1* | 3.2 ± 1.0† | ||
| Tetanus toxoid-specific T-cell response | |||||
| 2 mg/kg | 4.9 ± 2.0 | 5.0 ± 3.3 | 3.3 ± 1.9 | ||
| 5 mg/kg | 1.9 ± 1.6 | 1.9 ± 1.5 | 2.6 ± 2.6 | ||
| 10 mg/kg | 4.5 ± 3.2 | 4.0 ± 2.6 | 3.5 ± 1.8 | ||
P = .054 compared with pretreatment by repeated measures analysis of variance.
P = .08 compared with pretreatment by repeated measures analysis of variance.
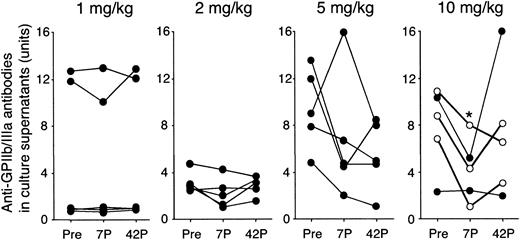
As shown in Figure 4, the amounts of anti-GPIIb/IIIa antibodies produced by antigen-specific T–B-cell collaboration were also stable in the 1 and 2 mg/kg groups. In 4 of 5 patients in the 5 mg/kg group and all 5 patients in the 10 mg/kg group, the amounts of anti-GPIIb/IIIa antibodies produced were reduced at day 7, and the changes in the 10 mg/kg group were statistically significant (P = .03).
Amounts of anti-GPIIb/IIIa antibodies produced in PBMC cultures before and after administration of IDEC-131/E6040. PBMCs were cultured with trypsin-digested GPIIb/IIIa, and the levels of IgG anti-GPIIb/IIIa antibodies in undiluted culture supernatants were measured by an enzyme-linked immunosorbent assay. The level of anti-GPIIb/IIIa antibodies is expressed as antibody units calculated on the basis of the standard curve obtained from serial concentrations of the mouse mAb to GPIIb/IIIa. Peripheral blood samples were analyzed at 3 different time points: day 0 (pretreatment; Pre), and days 7 and 42 after treatment (7P and 42P, respectively). A statistically significant change compared with pretreatment is indicated by < (P < .05 by repeated measures analysis of variance). In the 10 mg/kg group, ○ indicates responder and • indicates nonresponder.
Amounts of anti-GPIIb/IIIa antibodies produced in PBMC cultures before and after administration of IDEC-131/E6040. PBMCs were cultured with trypsin-digested GPIIb/IIIa, and the levels of IgG anti-GPIIb/IIIa antibodies in undiluted culture supernatants were measured by an enzyme-linked immunosorbent assay. The level of anti-GPIIb/IIIa antibodies is expressed as antibody units calculated on the basis of the standard curve obtained from serial concentrations of the mouse mAb to GPIIb/IIIa. Peripheral blood samples were analyzed at 3 different time points: day 0 (pretreatment; Pre), and days 7 and 42 after treatment (7P and 42P, respectively). A statistically significant change compared with pretreatment is indicated by < (P < .05 by repeated measures analysis of variance). In the 10 mg/kg group, ○ indicates responder and • indicates nonresponder.
Representative course of responders during IDEC-131/E6040 treatment
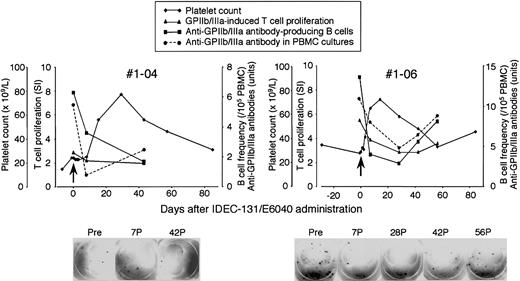
Platelet count and serial measurements of GPIIb/IIIa-specific T- and B-cell responses in representative responders are shown in Figure 5. No. 1-04 was a 51-year-old female patient who had an 18-year history of ITP, had relapsed after conventional-dose prednisolone and intravenous immunoglobulin treatment, and had not responded to splenectomy, azathioprine, or danazol. She was dependent on more than 20 mg/d oral prednisolone to keep a safe platelet count. She had prominent adverse effects related to prolonged prednisolone therapy, including severe osteoporosis, complicating compression fractures on multiple thoracic and lumbar vertebrae. After the administration of IDEC-131/E6040 at 10 mg/kg, a significant rise in platelet count was observed at day 14 and sustained until day 56, with a platelet peak of 77 × 109/L recorded on day 28. The frequency of peripheral blood B cells producing anti-GPIIb/IIIa antibodies and GPIIb/IIIa-specific T–B-cell collaboration inducing anti-GPIIb/IIIa antibody production were suppressed after the treatment. No. 1-06 was a 54-year-old female patient who had a 6-year history of ITP and had relapsed after conventional-dose prednisolone. After the administration of IDEC-131/E6040 at 10 mg/kg, a significant rise in platelet count was observed at day 7 and sustained until day 42, with a platelet peak of 72 × 109/L recorded on day 14. The frequency of B cells producing anti-GPIIb/IIIa antibodies in circulation quickly declined after the treatment but slowly climbed again after day 28. The GPIIb/IIIa-specific T-cell response and T–B-cell collaboration were also suppressed after the treatment. All 3 immunologic parameters changed in parallel and were inversely correlated with the platelet count.
Platelet count and T- and B-cell responses to GPIIb/IIIa during the course of IDEC-131/E6040 treatment in representative responders in the 10 mg/kg group. Platelet count, anti-GPIIb/IIIa antibody-producing B-cell frequency, GPIIb/IIIa-induced T-cell proliferation, and antigen-dependent anti-GPIIb/IIIa antibody production in PBMC cultures were evaluated at the indicated time points. Arrows denote the day of drug infusion (day 0). The lower panel shows representative images from the enzyme-linked immunospot assay for the detection of anti-GPIIb/IIIa antibody-producing B cells. Pre, pretreatment (day 0); 7P, 28P, 42P, and 56P, days 7, 28, 42, and 56 after treatment.
Platelet count and T- and B-cell responses to GPIIb/IIIa during the course of IDEC-131/E6040 treatment in representative responders in the 10 mg/kg group. Platelet count, anti-GPIIb/IIIa antibody-producing B-cell frequency, GPIIb/IIIa-induced T-cell proliferation, and antigen-dependent anti-GPIIb/IIIa antibody production in PBMC cultures were evaluated at the indicated time points. Arrows denote the day of drug infusion (day 0). The lower panel shows representative images from the enzyme-linked immunospot assay for the detection of anti-GPIIb/IIIa antibody-producing B cells. Pre, pretreatment (day 0); 7P, 28P, 42P, and 56P, days 7, 28, 42, and 56 after treatment.
Discussion
We have evaluated the effects of a single dose of IDEC-131/E6040 on the GPIIb/IIIa-specific autoimmune response in patients with refractory ITP. No increase in platelet count was observed in any patient who received the mAb at 1, 2, or 5 mg/kg, but an increase was observed in 3 of 5 patients given IDEC-131/E6040 at 10 mg/kg. We believe that the platelet count response observed in the 10 mg/kg group was induced by the IDEC-131/E6040 administration, because this change was temporary, observed exclusively in the high-dose group, and accompanied by suppression of GPIIb/IIIa-specific T- and B-cell responses. Therefore, a single injection of IDEC-131/E6040, when given at a sufficient dosage, induced temporary suppression of the antiplatelet autoimmune response and a subsequent increase in the platelet count in some patients with refractory ITP.
GPIIb/IIIa-specific T- and B-cell responses were analyzed ex vivo using peripheral blood samples. The activation of GPIIb/IIIa-reactive T and B cells occurs primarily in the spleen or some other reticuloendothelial system, and a significant number of these activated T and B cells are released into circulation as memory cells.24 It is presumed that the interruption of GPIIb/IIIa-specific T–B-cell interactions in the reticuloendothelial system results in the suppression of their activation and release into circulation. Therefore, ongoing antiplatelet autoimmune responses in patients with ITP can be adequately monitored by using immune parameters that mainly reflect the frequencies of GPIIb/IIIa-specific T and B cells in circulation.
Theoretically, CD40/CD154 blockade during antigen-specific T–B-cell interaction results in the suppression of a specific humoral immune response, by disrupting a helper signal mediated by cognate cell-cell interactions.9,10 In the present study, patients who received IDEC-131/E6040 at doses of 5 and 10 mg/kg had decreased GPIIb/IIIa-specific T- and B-cell responses on day 7 and substantiate this effect statistically. This effect is likely to be the result of a CD40/CD154 blockade in vivo, based on the following findings: (1) a suppressive effect was detected solely in the high-dose group; (2) the effect was temporary, and the T- and B-cell responses returned to baseline by 6 weeks after the treatment in most patients; and (3) all 3 immunologic parameters changed in parallel. Our finding is consistent with an in vitro study using PBMCs from patients with ITP, in which an anti-CD154 mAb inhibited antigen-induced anti-GPIIb/IIIa antibody production in a dose-dependent manner.26 Analogous antigen-specific immune suppression was reported in an experimental model using severe combined immunodeficient mice reconstituted with human peripheral blood lymphocytes, in which the IgG antitetanus toxoid antibody response that was induced by the immunization with tetanus toxoid was completely suppressed by treatment with murine anti-CD154 mAb 24-31.27 Moreover, in a clinical trial of another humanized anti-CD154 mAb hu5c8/BG-9588/ruplizumab/Antova (Biogen, Cambridge, MA) in patients with SLE complicating active nephritis, the frequency of circulating antidouble-stranded DNA (dsDNA) antibody-secreting B cells as well as the anti-dsDNA antibody titer were markedly reduced after the treatment.28,29
It has been proposed that the transient nature of CD154 expression on T cells, which coincides with antigen triggering, offers the possibility that blocking CD154 action would preferentially target T cells being activated at the time of blockade.9,10 In this study, the selective effect of anti-CD154 mAb treatment was demonstrated by the lack of an apparent suppressive effect on the T-cell response to tetanus toxoid, which would not be exposed to the immune system during the study period. Such selectivity is not achievable with most immunosuppressive strategies currently under investigation as a treatment for refractory ITP, such as cyclosporin A,30 anti-CD52 humanized mAb (alemtuzumab [Campath-1; ILEX Pharmaceuticals, San Antonio, TX]),31 and anti-CD20 chimeric mAb (rituximab).32
There was a discrepancy between the effect on the platelet count and the response of the GPIIb/IIIa-specific immune parameters in patients with ITP who received IDEC-131/E6040. Namely, in the 5 mg/kg group, T- and B-cell responses to GPIIb/IIIa were suppressed after the treatment in all patients, although no increase in platelet count was observed in any of them. In this regard, it was noted that a low frequency of anti-GPIIb/IIIa antibody-producing B cells was sustained until day 42 in responders in the 10 mg/kg group but had been restored to baseline at day 42 in most of the nonresponders in the 5 and 10 mg/kg groups. Therefore, short-term suppression of GPIIb/IIIa-specific immune repertoires might be insufficient to reduce the level of pathogenic antiplatelet autoantibodies.
In animal models, it has been shown that failure to deliver the essential CD40/CD154 signal during the T-cell–APC interaction results in a long-term suppression of specific T-cell responses.15,33 Several mechanisms for the long-lasting suppressive effects of CD40/CD154 blockade on specific T-cell response have been proposed, and these mechanisms include a shift in the T-helper (Th) 1/Th2-type immune response,14,16 up-regulation of cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) on T cells,16 and the induction of regulatory cells sharing the properties of natural killer and dendritic cells.34 Moreover, using an in vitro culture system with GPIIb/IIIa-specific CD4+ T-cell lines generated from patients with ITP, some of us (M.K. and Y.I.) demonstrated that T-cell–dependent anti-GPIIb/IIIa antibody production was suppressed through the induction of GPIIb/IIIa-specific anergic T cells with potential regulatory function.26 The effect observed in this single-dose study was temporary, but repeated doses of IDEC-131/E6040 treatment may induce tolerance to platelet autoantigens.
To date, 2 different humanized anti-CD154 mAbs (IDEC-131/E6040 and hu5c8) have been used in clinical trials, but it is difficult to compare the findings, because the trials were conducted using different protocols and in cohorts of patients with different disease severity. In a phase 2 clinical trial of IDEC-131/E6040 in patients with relatively mild SLE, several different disease activity indices and anti-dsDNA antibody titers were improved in both treatment and placebo groups without statistically significant differences.20 In another open-label study using hu5c8 in patients with SLE with active nephritis, the patients who received the humanized mAb showed statistically significant reductions in disease activity indices and anti-dsDNA antibody titers, as well as a rise in serum complement levels.28,29 In addition, the disappearance of hematuria and proteinuria after the treatment in some patients suggested favorable effects of the hu5c8 treatment, although there was no placebo-treated control group.29 Several clinical trials using hu5c8 in patients with refractory ITP have been reported.35-37 In a phase 1, multicenter, dose-escalating trial of hu5c8, 2 of 6 patients with ITP given the antibody at 10 mg/kg had an increased platelet count.35 Moreover, an elevated platelet count during repeated treatment with hu5c8 at 10 or 20 mg/kg was detected in 5 of 15 patients in one study36 and in 5 of 17 patients in another.37 Because these reports on clinical trials of hu5c8 in patients with ITP were abstracts, detailed findings are not available. Taking these observations together with the results of the present study, anti-CD154 mAb treatment has the potential to be effective for treating human autoimmune diseases, including refractory ITP.
The depletion of CD154+ lymphocytes was not observed after the administration of IDEC-131/E6040, consistent with the lack of lysis of CD154+ cells following IDEC-131/E6040 treatment in vitro and in vivo.18 A stable lymphocyte count was also observed in clinical trials of IDEC-131/E6040 in patients with SLE.19,20 In this study, we found for the first time that the circulating lymphocyte count was increased immediately after the anti-CD154 mAb treatment and returned to baseline within a week. The mechanism for this effect is uncertain, but a quick response after the treatment suggests the recruitment of lymphocytes into circulation rather than enhanced production of lymphocytes.
An overall favorable safety profile of IDEC-131/E6040 has been demonstrated at all dose levels examined, similar to previous clinical trials of single- or multiple-dose treatment with this humanized mAb.19,20 The adverse events experienced by patients were similar among different dose groups. Most reactions were related to the infusion of biologic agents, which has been observed with many other therapeutic antibodies. Although the risk of infection is potentially increased by blocking the CD40/CD154 interaction, only mild infections were seen and only in the low-dose groups. Taking these results together with those of previous clinical trials of hu5c828,29 and IDEC-131/E6040,19,20 there seems to be no increased risk of infection during CD40/CD154 blockade, at least in the short term.
However, previous clinical studies of hu5c8 have raised serious concerns that thromboembolic events could be a complication of this form of treatment.38 Kawai et al39 treated monkeys with murine mAb 5c8 and observed an unusually high incidence of thromboembolic complications. Currently, the precise mechanism for this adverse effect is not understood. In this respect, it is intriguing that CD154 preexists within the intracellular stores of platelets and is rapidly expressed on their surface after their activation and during thrombus formation.40 The CD154 on activated platelets binds the CD40 expressed on platelets and endothelial cells and facilitates their inflammatory and thrombotic properties.41 It is conceivable that the binding of the anti-CD154 mAb to activated platelets might enhance their aggregation, perhaps through the additional interaction of anti-CD154 mAb with Fcγ receptors on platelets and endothelial cells. However, CD154 was recently shown to be involved in the stabilization of thrombus in a GPIIIa-dependent manner.42 The inhibition of this interaction by anti-CD154 mAb might render platelet plugs unstable and thus ready to embolize. In contrast to hu5c8, no definite thromboembolic events were observed in this study or in the previous clinical trials of IDEC-131/E6040 in patients with SLE patients.19,20 However, a recent report of thromboembolic events during IDEC-131/E6040 therapy led to a halt in all clinical trials of IDEC-131/E6040.43 Nevertheless, it is currently believed that thromboembolic events are more prevalent during hu5c8 treatment than IDEC-131/E6040 treatment. It is necessary to determine whether thromboembolic events are generally associated with anti-CD154 mAb treatment or if they are specific to the hu5c8 treatment. In this regard, a detailed structural analysis of the CD154–anti-CD154 mAb complex revealed that hu5c8 participated in the formation of large aggregates of immune complexes by bivalent mAb binding to a trivalent ligand on the cell surface, which may provoke biologic effects such as thrombus formation. These aggregates were not formed by an another murine anti-CD154 mAb, TRAP1.44 The question of whether there is an increased risk of thromboembolic complications may haunt the future development of anti-CD154 mAb therapy and should be carefully investigated in future clinical studies.
In summary, the CD40/CD154 interaction provides an attractive target for immunotherapy in patients with refractory ITP. Although the results of this phase 1 trial are encouraging, further studies are necessary to confirm the clinical benefit of anti-CD154 mAb treatment in patients with ITP.
Prepublished online as Blood First Edition Paper October 9, 2003; DOI 10.1182/blood-2003-06-2167.
Supported by research grants from Japanese Ministry of Health, Labour and Welfare and from Eisai Company Ltd, Tokyo, Japan.
One of the authors (S.T.) was employed by a company (Eisai Company Ltd) whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Yuka Okazaki, Yoshiyuki Murata, and Hideyuki Samma for expert technical assistance, and Fuminori Ohba and Keiko Yanai for coordinating the clinical trial.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal