Abstract
CD45 is a key protein tyrosine phosphatase regulating Src-family protein tyrosine kinases (Src-PTKs) in lymphocytes; precisely how it exerts its effect remains controversial, however. We previously demonstrated that CD45 negatively regulates Lyn in the WEHI-231 B-cell line. Here we show that negative regulation by CD45 is physiologically significant in B cells and that some CD45 is constitutively associated with glycolipid-enriched microdomains (GEMs), where it inhibits Src-PTKs by dephosphorylating both the negative and the positive regulatory sites. Upon B-cell receptor (BCR) ligation, however, CD45 dissociates from GEMs within 30 seconds, inducing phosphorylation of 2 regulatory sites and activation of Src-PTKs, but subsequently reassociates with the GEMs within 15 minutes. Disruption of GEMs with methyl-β-cyclodextrin results in abrogation of BCR-induced apoptosis in WEHI-231 cells, suggesting GEMs are critical to signals leading to the fate determination. We propose that the primary function of CD45 is inhibition of Src-PTKs and that the level of Src-PTK activation and the B-cell fate are determined in part by dynamic behavior of CD45 with respect to GEMs.
Introduction
Upon B-cell receptor (BCR) ligation, signals are transmitted downstream through multiple pathways, ultimately leading to cell activation, proliferation, death, or anergy.1-4 Initiation of such immune responses is driven primarily by activation of Src-family protein tyrosine kinases (Src-PTKs) whose activity is regulated, in part, by phosphorylation of 2 tyrosine residues: the autophosphorylation site, located in the activation loop of the catalytic domain, and the COOH-terminal negative regulatory site.5 Phosphorylation of the former is a prerequisite for Src-PTK activation. Phosphorylation of the latter by COOH-terminal Src kinase (Csk) results in the intramolecular binding of the phosphotyrosine (PY) in the COOH-terminal tail to the Src homology 2 (SH2) domain, yielding a closed conformation in which Src-PTK activity is attenuated. Thus, phosphorylation of the respective regulatory tyrosine residues has opposing effects on Src-PTK activity.
CD45, a receptor-type protein tyrosine phosphatase (PTP) exclusively expressed in nucleated hematopoietic cells, is known to be a major regulator of Src-PTK activation and of lymphocyte fate,6-8 though its precise role in the regulation of Src-PTKs remains controversial.9-13 One conventional model holds that when Csk phosphorylates the negative regulatory COOH-terminal tyrosine residue, leading to the aforementioned closed and inactive conformation, the dominant role of CD45 is to dephosphorylate this site, setting the stage for antigen receptor signaling to activate Src-PTKs by autophosphorylation.14,15 This scenario is based on the findings that the COOH-terminal tyrosine residues of Lck and Fyn are hyperphosphorylated and T-cell receptor (TCR) signaling is attenuated in CD45-deficient T-cell lines and in thymocytes from Ptprc-targeted mice.16-20 Moreover, similar results have also been obtained in B cells.21-23 However, CD45 is also shown to dephosphorylate both of Src-PTK's regulatory tyrosine residues in both T cells24-26 and B cells,27,28 thereby inactivating it.
We previously showed that CD45 exerts a negative regulatory effect on BCR-induced Ca2+ mobilization, activation of c-Jun NH2-terminal kinase (JNK) and p38, and growth arrest in immature WEHI-231 cells,29,30 but exerts a positive regulatory effect on these processes in mature BAL-17 cells.30,31 We further showed that, in WEHI-231 cells, CD45 constitutively inhibits Lyn by dephosphorylating both the COOH-terminal and autophosphorylation sites, but that BCR ligation induces phosphorylation of the 2 sites.27,28 We therefore suggested that the inhibitory activity of CD45 was somehow diminished upon BCR ligation.28 One possible explanation might be that the phosphorylation status of Src-PTKs is dictated by their physical proximity to CD45, which is modulated by BCR ligation. Accumulating evidence suggests that sphingolipid- and cholesterol-rich microdomains (GEMs or lipid rafts) play a key role in signal transduction through a broad array of cell surface receptors in different cell types.32-34 GEMs are enriched in a variety of signaling molecules, including doubly acylated Src-PTKs, glycosylphosphatidylinositol-anchored proteins, phosphatidylinositol 4, 5-bisphosphate, heterotrimeric G proteins, and transmembrane docking proteins such as linker of activated T cells (LAT)35 and phosphoprotein associated with GEMs (PAG),36 also known as COOH-terminal Src kinase-binding protein (Cbp).37
In the present study, we examined the generality of negative regulation of Src-PTKs by CD45 and the possible dynamic nature of CD45 localization with respect to the GEMs in which Src-PTKs reside. We show that, as in WEHI-231 cells, CD45 constitutively dephosphorylates and inactivates Fyn in mature BAL-17 cells and Lyn in splenic B cells, underscoring the physiologic significance of the negative regulation of Src-PTKs by CD45 in B cells. We further show that some CD45 is constitutively associated with GEMs, where it inhibits Src-PTKs by dephosphorylating both regulatory sites, and that BCR ligation induces transient sequestration of CD45 from GEMs. Given that GEMs are critical to generating signals for apoptosis, our findings corroborate the importance of the dynamic behavior of CD45 with respect to GEMs in the “fine-tuning” of Src-PTK activation and the determination of cell fate.
Materials and methods
Cell culture
WEHI-231 and BAL-17 cells and their CD45-deficient clones are described elsewhere.27,29,31 These cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 50 μM 2-mercaptoethanol (2-ME), 100 μg/mL streptomycin, and 100 U/mL penicillin. CD45 exon 9 knock-out (KO)38 and C57BL/6 (B6) mice were obtained from Jackson Laboratory (Bar Harbor, ME) and Japan SLC (Hamamatsu, Japan), respectively, and used at 6 to 12 weeks of age. B cells were prepared from these mice by treating spleen cells with anti–Thy-1.2 monoclonal Ab (mAb) and rabbit complement. The resulting population was more than 94% surface IgM–positive.
Antibodies and reagents
Goat F(ab′)2 fragments of antimouse IgM Ab (Cappel, West Chester, PA) were used for BCR ligation. Rat antimouse CD45 (RA3-3A1/6.1; IgM),39 anti-PY (4G10), and antipaxillin mAbs were purchased from American Type Culture Collection (Rockville, MD), Upstate Biotechnology (Lake Placid, NY), and BD Transduction Laboratories (Lexington, KY), respectively. Abs against CD71 (transferrin receptor), Lyn, Fyn, Blk, Lck, Syk, and Csk were from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase (HRP)–conjugated antirabbit IgG and antigoat IgG were from Biosource International (Camarillo, CA). HRP-conjugated antimouse IgG and antirat IgG and alkaline phosphatase–conjugated goat antimouse IgG were from Jackson ImmunoResearch Laboratories (West Grove, PA). Anti–Ig-α Ab was a gift from Dr Marcus Clark (University of Chicago). HRP-conjugated cholera toxin B (CTB) subunit and methyl-β-cyclodextrin (MCD) were from Sigma (St Louis, MO).
Preparation of GEMs
Cells (2 × 108) were preincubated for 3 hours at 37°C and then stimulated with 20 μg/mL anti-IgM Ab for the indicated times. They were then washed with ice-cold phosphate-buffered saline (PBS) containing 2 mM Na3VO4 and 2 mM EDTA (ethylenediaminetetraacetic acid), and lysed for 30 minutes on ice in 1 mL of 0.2% to 0.5% Triton X-100 in TNE (25 mM Tris [tris(hydroxymethyl)aminomethane, pH 6.5], 150 mM NaCl, 5 mM EDTA) containing 5 mM Na3VO4, 10 μg/mL aprotinin, and 10 μg/mL leupeptin. The lysis solution was homogenized in a Wheaton loose-fitting dounce homogenizer (Millville, NJ), gently mixed with an equal volume of 85% sucrose (wt/vol) in TNE, and placed in the bottom of an SW40 Ti centrifuge tube. The sample was then overlaid with 6 mL of 35% sucrose and 3.5 mL of 5% sucrose in TNE with 1 mM Na3VO4 and spun for 17 to 19 hours at 200 000 × g at 4°C, after which 1-mL fractions were collected from the top of the gradient. In some experiments, before sucrose gradient ultracentrifugation, cells were incubated for 25 minutes with 5 mM MCD at 37°C in lipid-free media and then washed 3 times with PBS to remove drugcholesterol complexes. Some of these cells were then placed in cholesterol-containing media for 4 hours to allow GEM recovery.
Western blotting and in vitro kinase assays
Western blotting and in vitro kinase (IVK) assays were carried out as previously described.27 For kinase assays, Lyn and Fyn immunoprecipitates were washed with TNE lysis buffer and kinase buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 10 mM magnesium acetate, 20 mM MnCl2), after which 0.37 MBq [γ-33P] adenosine triphosphate (ATP, 37-110 TBq/mmol; Amersham, Buckinghamshire, United Kingdom) with 10 mM cold ATP in kinase buffer was added. After terminating the reactions, the samples were subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The resultant gels were treated for 90 minutes with 1 N KOH at 60°C to hydrolyze phosphoserine and phosphothreonine, dried, and analyzed using a BAS 2000 Bio-Imaging Analyzer (Fuji Photo Film, Tokyo, Japan).
Metabolic labeling, cyanogens bromide digestion, and phosphoamino acid analysis
As previously described,28 cells were cultured for 3 hours at 37°C in phosphate-free medium supplemented with 10% dialyzed FBS, and then labeled for 1 hour at room temperature with 370 MBq 32P-orthophosphate in 3 mL of medium. After then adding 8 mL phosphate-free medium, the cells were incubated for 3 hours at 37°C. Lyn immunoprecipitates were subjected to 10% SDS-PAGE and transferred onto a nitrocellulose membrane, after which the Lyn (p53 and p56) bands were excised and treated for 2 hours at room temperature with 250 μL of 150 μg/mL cyanogens bromide (CNBr) in 70% formic acid. The eluted proteins were subjected to SDS-PAGE in a 15% to 25% gradient gel, after which the separated proteins were transferred to a polyvinylidene fluoride (PVDF) membrane and analyzed with a BAS 2000.
For phosphoamino acid analysis, proteins were separated by SDS-PAGE, transferred to a PVDF membrane, and the excised Lyn bands were hydrolyzed by incubation for 2 hours with 6 N HCl at 100°C. The samples were then dried, dissolved in 5 μL distilled water, and spotted onto thin-layer chromatography (TLC) plates. Electrophoresis was performed in pH 1.9 buffer for 20 minutes at 1.5 kV. After drying, the plates were subjected to second-dimension electrophoresis in pH 3.5 buffer for 16 minutes at 1.3 kV. Separated phosphoamino acids were analyzed with a BAS 2000.
Apoptosis assays
DNA fragmentation was assessed as previously described.29 Briefly, cells (2 × 105/mL) were either left untreated, treated with 5 mM MCD, or treated and allowed to recover, after which they were either left unstimulated or were stimulated for 24 hours with anti-IgM Ab (20 μg/mL) and then lysed for 10 minutes at 4°C in buffer containing 10 mM Tris-HCl, pH 8.0, 10 mM EDTA, and 0.5% Triton X-100. After centrifugation, the supernatant was treated first with 100 μg/mL RNase for 1 hour at 37°C, and then with 200 μg/mL proteinase K in 1% SDS for 2 hours at 50°C. The DNA was subjected to electrophoresis in 1% agarose gel containing 80 mM Trisphosphate, pH 8.0, 2 mM EDTA, and 0.1 μg/mL ethidium bromide. Apoptosis was also assayed using an annexin V–fluorescein isothiocyanate (FITC) kit (Bender MedSystems, Vienna, Austria) according to the manufacturer's protocol. In this case, cells cultured as in the DNA fragmentation assay were incubated with annexin V–FITC for 10 minutes at room temperature and then with propidium iodide, after which they were subjected to fluorescence-activated cell-sorter (FACS) analysis. Cells positively labeled for both annexin V and propidium iodide were considered apoptotic.
Results
CD45 negatively regulates Src-PTK activity in B cells
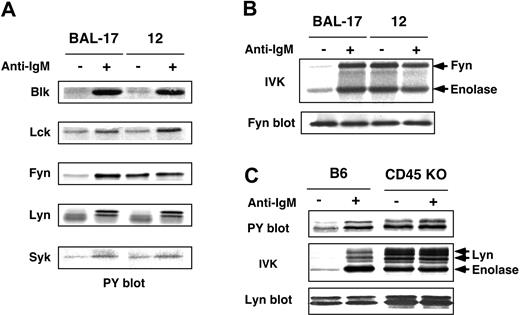
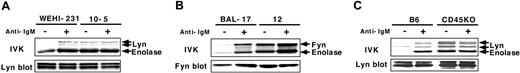
Our previous studies demonstrated that CD45 inhibits Lyn activity in WEHI-231 cells by dephosphorylating the 2 regulatory tyrosine residues, and that BCR ligation activates Lyn by inducing their phosphorylation.27,28 To examine the generality of the effects of CD45, we set out to determine its Src-PTK substrates and its regulatory effects in other B cells. To accomplish this we examined mature BAL-17 B cells and the CD45-deficient clone 12 cells. The results demonstrated that tyrosine phosphorylation of Fyn, but not other Src-PTKs (Blk, Lck, and Lyn) or Syk, was constitutively high in clone 12 cells, suggesting Fyn is a selective CD45 substrate in BAL-17 cells (Figure 1A). IVK assays showed Fyn activity to be constitutively enhanced in clone 12 cells and not significantly augmented upon BCR ligation (Figure 1B).
Negative regulation of Src-PTKs by CD45 in B cells. (A) Phosphorylation of Src-PTKs and Syk in BAL-17 cells. BAL-17 and CD45-deficient clone 12 cells were incubated for 5 minutes (–) without or (+) with 20 μg/mL (Fab′)2 fragments of anti-IgM Ab (+); PTKs were then subjected to Western blotting with anti-PY Ab. (B) Fyn activation in BAL-17 and CD45-deficient clone 12 cells. Cells were treated as in panel A; Fyn was then subjected to IVK assays with enolase as a substrate and to blotting with anti-Fyn Ab. (C) Phosphorylation and activation of Lyn in splenic B cells. B cells from B6 and CD45 KO mice were treated as in panel A, after which Lyn was subjected to IVK assays using enolase and to Western blotting with anti-PY and anti-Lyn Abs.
Negative regulation of Src-PTKs by CD45 in B cells. (A) Phosphorylation of Src-PTKs and Syk in BAL-17 cells. BAL-17 and CD45-deficient clone 12 cells were incubated for 5 minutes (–) without or (+) with 20 μg/mL (Fab′)2 fragments of anti-IgM Ab (+); PTKs were then subjected to Western blotting with anti-PY Ab. (B) Fyn activation in BAL-17 and CD45-deficient clone 12 cells. Cells were treated as in panel A; Fyn was then subjected to IVK assays with enolase as a substrate and to blotting with anti-Fyn Ab. (C) Phosphorylation and activation of Lyn in splenic B cells. B cells from B6 and CD45 KO mice were treated as in panel A, after which Lyn was subjected to IVK assays using enolase and to Western blotting with anti-PY and anti-Lyn Abs.
Significantly, such negative regulation by CD45 was likewise observed in ex vivo B cells. Splenic B cells isolated from CD45 exon 9 KO38 and control C57BL/6 (B6) mice were either left unstimulated or stimulated for 5 minutes with F(ab′)2 fragments of anti-IgM Ab (20 μg/mL), after which Lyn was subjected to IVK assays using enolase as a substrate.As shown in Figure 1C, Lyn was constitutively hyperphosphorylated and activated in CD45-deficient B cells, and BCR ligation did not significantly enhance its activity. Together, these results suggest that CD45 normally dephosphorylates and inactivates Src-PTKs in B cells, but BCR signals somehow reduce CD45's negative regulatory effects.
Effect of CD45 on the dynamics of Src-PTKs, BCR, and Csk
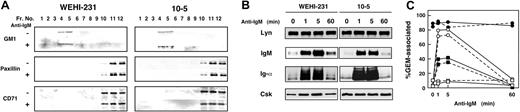
What then is the mechanism by which BCR attenuates CD45 activity? One possibility is that BCR signaling alters the physical distance between CD45 and its substrates. To test this possibility, we focused on GEMs as Src-PTK platform markers and examined the spatiotemporal relationship between Src-PTKs and CD45. GEMs were isolated based on their insolubility in Triton X-100 and their buoyancy on sucrose gradients. As shown in Figure 2A, ganglioside GM1-enriched GEMs from WEHI-231 and CD45-deficient clone 10-5 cells were detected in fractions 4 and 5 by blotting with CTB subunit. The GEMs were not contaminated with soluble proteins, as evidenced by the absence of paxillin and CD71 (transferrin receptor), and BCR ligation did not significantly affect the solubility or purity of the GEM fractions.
Dynamics of GEMs in WEHI-231 and CD45-deficient clone 10-5 cells. (A) Characterization of GEMs in WEHI-231 and 10-5 cells. Cells were either left unstimulated (–) or were stimulated for 5 minutes at 37°C with 20 μg/mL (Fab′)2 fragments of antimouse IgM Ab (+), after which they were lysed in 0.5% Triton X-100 and subjected to sucrose gradient ultracentrifugation. Equal volumes of gradient fraction were separated by SDS-PAGE and blotted with HRP-conjugated CTB subunit to detect the ganglioside GM1, or with Abs against paxillin and CD71. Fr indicates fraction. (B) Association of Lyn, IgM, Ig-α, and Csk with GEMs. WEHI-231 and 10-5 cells were either left unstimulated or were stimulated with 20 μg/mL antimouse IgM Ab for 1, 5, or 60 minutes, and then treated up to the ultracentrifugation step as in panel A. Equal volumes of GEMs (sucrose gradient fractions 4 and 5 combined) were separated by SDS-PAGE and blotted with Abs against Lyn, IgM, Ig-α, and Csk. (C) Percentages of GEM-associated Lyn (•), IgM (○), Ig-α (▪), and Csk (□) in WEHI-231 (solid line) and 10-5 (broken line) cells.
Dynamics of GEMs in WEHI-231 and CD45-deficient clone 10-5 cells. (A) Characterization of GEMs in WEHI-231 and 10-5 cells. Cells were either left unstimulated (–) or were stimulated for 5 minutes at 37°C with 20 μg/mL (Fab′)2 fragments of antimouse IgM Ab (+), after which they were lysed in 0.5% Triton X-100 and subjected to sucrose gradient ultracentrifugation. Equal volumes of gradient fraction were separated by SDS-PAGE and blotted with HRP-conjugated CTB subunit to detect the ganglioside GM1, or with Abs against paxillin and CD71. Fr indicates fraction. (B) Association of Lyn, IgM, Ig-α, and Csk with GEMs. WEHI-231 and 10-5 cells were either left unstimulated or were stimulated with 20 μg/mL antimouse IgM Ab for 1, 5, or 60 minutes, and then treated up to the ultracentrifugation step as in panel A. Equal volumes of GEMs (sucrose gradient fractions 4 and 5 combined) were separated by SDS-PAGE and blotted with Abs against Lyn, IgM, Ig-α, and Csk. (C) Percentages of GEM-associated Lyn (•), IgM (○), Ig-α (▪), and Csk (□) in WEHI-231 (solid line) and 10-5 (broken line) cells.
Using fractions 4 and 5 as a GEM preparation, localization of Lyn, BCR components IgM and Ig-α, and Csk, and the effect of CD45 on their dynamics were examined. WEHI-231 and 10-5 cells were incubated without or with anti-IgM Ab for 1, 5, and 60 minutes, after which the GEMs were analyzed by Western blotting with Abs against Lyn, IgM, Ig-α, and Csk. As shown in Figure 2B-C, IgM and Ig-α were strongly recruited to GEMs within 1 minute of BCR ligation, after which their levels returned to baseline over a period of 60 minutes. However, recruitment of Syk PTK to GEMs was not detected in our hands (data not shown). Association between Syk and GEMs may be of low affinity, such that it is easily disrupted under our experimental conditions. No significant difference was observed between the kinetics of the BCR components in the parental and 10-5 cells. As compared with the BCR components, recruitment of Csk to GEMs was only marginal in the parental cells; recruitment of Csk to GEMs from the CD45-deficient clone was slightly higher and was maintained throughout the BCR signaling.
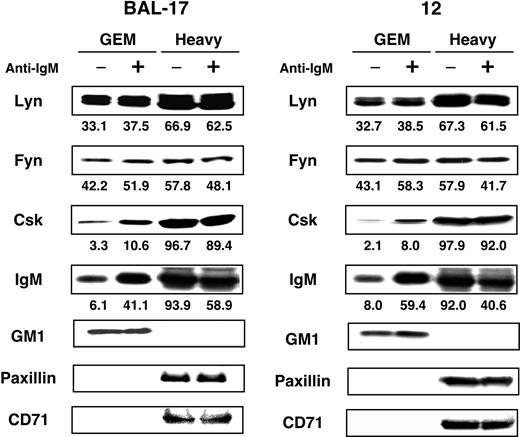
GEMs (fractions 4 and 5) were also isolated from BAL-17 and clone 12 cells, and the dynamics of the movements of Fyn, Lyn, IgM, and Csk were examined (Figure 3). As in WEHI-231 cells, IgM was strongly recruited to GEMs upon BCR ligation, and recruitment of Csk was greater than in WEHI-231 cells. CD45 made no apparent contribution to these processes, however (Figure 3). It is thus unlikely that CD45 is involved in the regulation of the dynamics of Src-PTKs and BCR. On the other hand, recruitment of Csk does not appear to be regulated by CD45 in BAL-17 cells, though the contribution of CD45 to this process has not been completely ruled out in WEHI-231 cells.
Association of IgM, Src-PTKs, and Csk with GEMs in BAL-17 cells. BAL-17 and CD45-deficient clone 12 cells were either left unstimulated (–) or stimulated (+) for 5 minutes at 37°C with 20 μg/mL (Fab′)2 fragments of antimouse IgM Ab, after which they were lysed in 0.5% Triton X-100 and subjected to sucrose gradient ultracentrifugation. GEMs (fractions 4 and 5) and heavy fractions (fractions 10-12) were separated by SDS-PAGE and blotted with Abs against Lyn, Fyn, Csk, and IgM. Control blots were performed with HRP-conjugated CTB subunit to detect the ganglioside GM1, or with Abs against paxillin and CD71. Numbers at the bottom are percentages of total molecules present in each fraction.
Association of IgM, Src-PTKs, and Csk with GEMs in BAL-17 cells. BAL-17 and CD45-deficient clone 12 cells were either left unstimulated (–) or stimulated (+) for 5 minutes at 37°C with 20 μg/mL (Fab′)2 fragments of antimouse IgM Ab, after which they were lysed in 0.5% Triton X-100 and subjected to sucrose gradient ultracentrifugation. GEMs (fractions 4 and 5) and heavy fractions (fractions 10-12) were separated by SDS-PAGE and blotted with Abs against Lyn, Fyn, Csk, and IgM. Control blots were performed with HRP-conjugated CTB subunit to detect the ganglioside GM1, or with Abs against paxillin and CD71. Numbers at the bottom are percentages of total molecules present in each fraction.
Some CD45 is localized at GEMs and is transiently sequestered upon BCR ligation
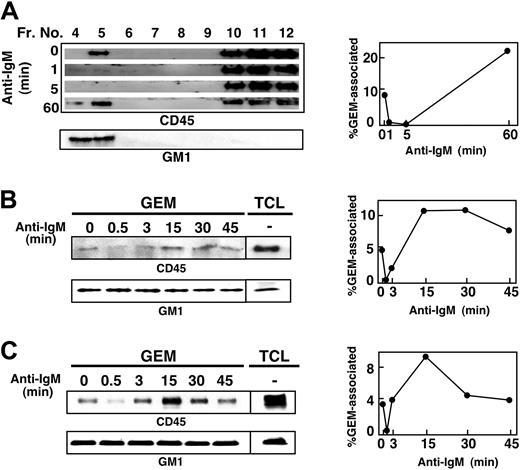
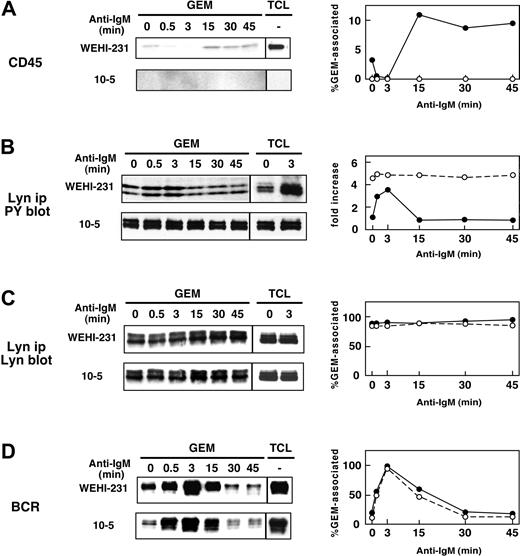
We next examined the localization of CD45 with respect to GEMs in WEHI-231, BAL-17, and splenic B cells. Cells were stimulated with anti-IgM Ab for 1 to 60 minutes, and then fractionated samples were blotted with anti-CD45 mAb and CTB subunit. Figure 4A shows that in WEHI-231 cells, about 8% of the total cellular CD45 was constitutively associated with GEMs, but within one minute after BCR ligation, it had dissociated from the GEMs. BCR ligation elicited a 6.8-fold reduction of GEM-associated CD45 within 1 minute, and an 82-fold reduction at 5 minutes. Significantly, CD45 reassociated with the GEMs within 60 minutes. Similarly, 4.8% and 4.2% of total CD45 were constitutively associated with GEMs in BAL-17 cells (Figure 4B) and splenic B cells (Figure 4C). In both cases, within 30 seconds after BCR ligation, it had dissociated from the GEMs, only to be reassociated within 3 to 15 minutes. Detailed time-course experiments in WEHI-231 cells also showed that CD45 dissociated from GEMs within 30 seconds and returned to GEMs between 3 and 15 minutes (Figure 5). As previously shown, tyrosine phosphorylation of Lyn was constitutively enhanced and maintained during BCR stimulation in CD45-deficient 10-5 cells. It is of note that Lyn phosphorylation in the parental cells was inversely correlated with the presence of CD45 in the GEMs, strongly suggesting that CD45 may directly dephosphorylate Lyn at the GEMs.
Dynamic association of CD45 with GEMs in B cells. WEHI-231 (A), BAL-17 (B), and splenic B (C) cells were left unstimulated or were stimulated with 20 μg/mL anti-IgM Ab for the times indicated. After lysis in 0.5% Triton X-100 and sucrose gradient ultracentrifugation, each fraction (A) or GEM fraction (fractions 4 and 5 combined) and total cell lysates (TCL; B-C), was separated by SDS-PAGE and blotted with anti-CD45 mAb and HRP-conjugated CTB subunit to detect GM-1. In the upper panel of panel C, GEM fractions and TCL were immunoprecipitated with anti-CD45 mAb and then subjected to blotting with anti-CD45 mAb. Right panels indicate the time courses of the percentages of total CD45 that was GEM-associated, as determined by densitometric analysis.
Dynamic association of CD45 with GEMs in B cells. WEHI-231 (A), BAL-17 (B), and splenic B (C) cells were left unstimulated or were stimulated with 20 μg/mL anti-IgM Ab for the times indicated. After lysis in 0.5% Triton X-100 and sucrose gradient ultracentrifugation, each fraction (A) or GEM fraction (fractions 4 and 5 combined) and total cell lysates (TCL; B-C), was separated by SDS-PAGE and blotted with anti-CD45 mAb and HRP-conjugated CTB subunit to detect GM-1. In the upper panel of panel C, GEM fractions and TCL were immunoprecipitated with anti-CD45 mAb and then subjected to blotting with anti-CD45 mAb. Right panels indicate the time courses of the percentages of total CD45 that was GEM-associated, as determined by densitometric analysis.
Inverse correlation between association of CD45 with GEMs and tyrosine phosphorylation of Lyn. WEHI-231 (•) and 10-5 (○) cells were stimulated with 20 μg/mL anti-IgM Ab for 0.5, 3, 15, 30, and 45 minutes, and then lysed in 0.5% Triton X-100. GEMs (fractions 4 and 5 combined) and TCL were subjected to SDS-PAGE and blotting with anti-CD45 mAb (A). Tyrosine phosphorylation of Lyn was examined by blotting with anti-PY mAb (B). Association of Lyn and IgM with GEMs was also examined by blotting with anti-Lyn and anti-IgM Abs (C, D). The panels on the right show percentages of GEM-associated CD45, Lyn, and IgM, and fold increases in tyrosine phosphorylation of Lyn. Fold increases of Lyn phosphorylation were calculated by setting the level of unstimulated WEHI-231 cells as 1. ip indicates immunoprecipitated.
Inverse correlation between association of CD45 with GEMs and tyrosine phosphorylation of Lyn. WEHI-231 (•) and 10-5 (○) cells were stimulated with 20 μg/mL anti-IgM Ab for 0.5, 3, 15, 30, and 45 minutes, and then lysed in 0.5% Triton X-100. GEMs (fractions 4 and 5 combined) and TCL were subjected to SDS-PAGE and blotting with anti-CD45 mAb (A). Tyrosine phosphorylation of Lyn was examined by blotting with anti-PY mAb (B). Association of Lyn and IgM with GEMs was also examined by blotting with anti-Lyn and anti-IgM Abs (C, D). The panels on the right show percentages of GEM-associated CD45, Lyn, and IgM, and fold increases in tyrosine phosphorylation of Lyn. Fold increases of Lyn phosphorylation were calculated by setting the level of unstimulated WEHI-231 cells as 1. ip indicates immunoprecipitated.
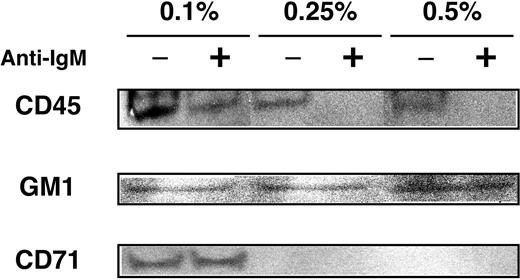
To exclude the possibility that the observed movements were due to biased solubility of GEM preparation, we titrated concentration of Triton X-100. As shown in Figure 6, when treated with 0.1% Triton X-100, the combined fractions 4 and 5 were contaminated with non-GEM components, as evidenced by the presence of CD71. However, in the same fraction from samples solubilized with 0.25% Triton X-100, the constitutive presence of CD45 and dynamic behavior of CD45 were consistently observed, suggesting that our findings were not influenced by particular experimental conditions.
Effect of detergent concentration on GEM preparation. WEHI-231 cells were left unstimulated (–) or were stimulated (+) with 20 μg/mL anti-IgM Ab for 5 minutes. Cells were lysed in 0.5%, 0.25%, and 0.1% Triton X-100 and were subjected to sucrose gradient ultracentrifugation. Fractions 4 and 5 combined were then separated by SDS-PAGE and blotted with anti-CD45 mAb, anti-CD71 mAb, and HRP-conjugated CTB subunit to detect GM-1.
Effect of detergent concentration on GEM preparation. WEHI-231 cells were left unstimulated (–) or were stimulated (+) with 20 μg/mL anti-IgM Ab for 5 minutes. Cells were lysed in 0.5%, 0.25%, and 0.1% Triton X-100 and were subjected to sucrose gradient ultracentrifugation. Fractions 4 and 5 combined were then separated by SDS-PAGE and blotted with anti-CD45 mAb, anti-CD71 mAb, and HRP-conjugated CTB subunit to detect GM-1.
GEM-associated Src-PTKs are negatively regulated by CD45
So how are GEM-associated Src-PTKs regulated by the dynamic behavior of CD45? To address this question, WEHI-231, BAL-17, and splenic B cells, and their respective CD45-deficient cells were incubated for 5 minutes without or with anti-IgM Ab, after which Lyn in WEHI-231 cells (Figure 7A) and splenic B cells (Figure 7C) and Fyn in BAL-17 cells (Figure 7B) were immunoprecipitated from GEMs and subjected to IVK assays. The results clearly showed that the activities of GEM-associated Src-PTKs from CD45-deficient cells were all constitutively enhanced with respect to levels of autophosphorylation and phosphorylation of an exogenous substrate (enolase), further confirming that GEM-associated Src-PTKs are indeed negatively regulated by CD45.
Negative regulation of GEM-associated Src-PTKs by CD45. WEHI-231 (A), BAL-17 (B), and splenic B (C) cells, and their CD45-deficient clones were left unstimulated (–) or were stimulated (+) for 5 minutes with 20 μg/mL anti-IgM Ab. Lyn in WEHI-231 and splenic B cells and Fyn in BAL-17 cells were then immunoprecipitated from GEMs (fractions 4 and 5) and subjected to IVK assays with enolase and Western blotting with Abs against Lyn and Fyn.
Negative regulation of GEM-associated Src-PTKs by CD45. WEHI-231 (A), BAL-17 (B), and splenic B (C) cells, and their CD45-deficient clones were left unstimulated (–) or were stimulated (+) for 5 minutes with 20 μg/mL anti-IgM Ab. Lyn in WEHI-231 and splenic B cells and Fyn in BAL-17 cells were then immunoprecipitated from GEMs (fractions 4 and 5) and subjected to IVK assays with enolase and Western blotting with Abs against Lyn and Fyn.
CD45 dephosphorylates both regulatory tyrosine residues of GEM-associated Lyn
We then more precisely defined the Src-PTK sites dephosphorylated by CD45 using WEHI-231 cells as a model. WEHI-231 and 10-5 cells were metabolically labeled with 32P-orthophosphate and then incubated for 5 minutes with or without anti-IgM Ab. Lyn isolated from GEMs (fractions 4 and 5) was then subjected to CNBr cleavage followed by SDS-PAGE analysis. Phosphoamino acid analysis (Figure 8A) confirmed that phosphorylation of GEM-associated Lyn occurred exclusively on tyrosine residues. Moreover, examination of its CNBr cleavage fragments showed that, in the parental cells, Lyn was minimally phosphorylated before BCR ligation, but was strongly phosphorylated afterward, not only at the autophosphorylation site (Y397), but also at the negative regulatory site (Y508) (Figure 8B). It is important to note that BCR ligation does not induce dephosphorylation of Y508, but rather enhances its phosphorylation. By contrast, both sites were highly phosphorylated in 10-5 cells even before stimulation (Figure 8B). It would thus seem reasonable to conclude that CD45 constitutively dephosphorylates both of Lyn's regulatory sites, thereby inhibiting its kinase activity.
Dephosphorylation of Lyn's regulatory sites by CD45 and induction of their phosphorylation by BCR ligation. (A) 32P-orthophosphate–labeled WEHI-231 and CD45-deficient clone 10-5 cells were left unstimulated (–) or were stimulated (+) for 5 minutes with 20 μg/mL anti-IgM Ab, after which Lyn was immunoprecipitated from GEMs and subjected to SDS-PAGE analysis. 32P-labeled Lyn bands were excised from the PVDF membrane, hydrolyzed, and subjected to phosphoamino acid analysis. S indicates serine; T, threonine; and Y, tyrosine. (B) Cells were treated as in panel A, and the excised Lyn bands were treated with CNBr. The eluted proteins were subjected to SDS-PAGE in a 15% to 25% gradient gel, transferred to a PVDF membrane, and analyzed with a BAS 2000. The Lyn protein applied was assessed by Western blotting with anti-Lyn Ab. Y397 indicates the autophosphorylation site; Y508, negative regulatory site; and MW, molecular weight.
Dephosphorylation of Lyn's regulatory sites by CD45 and induction of their phosphorylation by BCR ligation. (A) 32P-orthophosphate–labeled WEHI-231 and CD45-deficient clone 10-5 cells were left unstimulated (–) or were stimulated (+) for 5 minutes with 20 μg/mL anti-IgM Ab, after which Lyn was immunoprecipitated from GEMs and subjected to SDS-PAGE analysis. 32P-labeled Lyn bands were excised from the PVDF membrane, hydrolyzed, and subjected to phosphoamino acid analysis. S indicates serine; T, threonine; and Y, tyrosine. (B) Cells were treated as in panel A, and the excised Lyn bands were treated with CNBr. The eluted proteins were subjected to SDS-PAGE in a 15% to 25% gradient gel, transferred to a PVDF membrane, and analyzed with a BAS 2000. The Lyn protein applied was assessed by Western blotting with anti-Lyn Ab. Y397 indicates the autophosphorylation site; Y508, negative regulatory site; and MW, molecular weight.
GEMs are critical to BCR-induced apoptosis
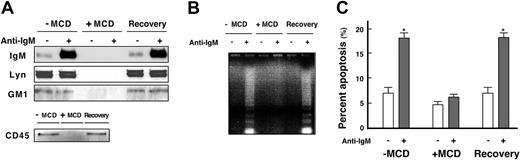
It was recently suggested that GEMs do not serve as a platform for BCR-initiated signaling in immature B cells, including WEHI-231 cells.40 We therefore re-evaluated GEM function in WEHI-231 cells using MCD, which preferentially extracts plasma membrane cholesterol, disrupting GEMs.41 Pretreatment of WEHI-231 cells with a nontoxic dose of MCD (5 mM) for 25 minutes at 37°C in lipid-free medium led to almost complete disruption of GEMs, as indicated by the loss of GM1, IgM, Lyn, and CD45 (Figure 9A). Subsequent incubation of MCD-treated cells in cholesterol-containing medium for 4 hours induced recovery of GEM fractions (Figure 9A), indicating that GEMs are effectively and reversibly disrupted by MCD treatment under these conditions.
Effect of MCD on BCR-induced apoptosis. (A) WEHI-231 cells were left untreated, treated with 5 mM MCD for 25 minutes in lipid-free medium, or treated with 5 mM MCD for 25 minutes and allowed to recover for 4 hours. Cells were then left unstimulated (–) or were stimulated (+) for 5 minutes with 20 μg/mL anti-IgM Ab. Following lysis in 0.5% Triton X-100 and sucrose gradient ultracentrifugation, GEM fractions were blotted with CTB subunit and Abs against Lyn, IgM, and CD45. (B) WEHI-231 cells were incubated as in panel A and then either left unstimulated (–) or stimulated (+) for 24 hours with 20 μg/mL anti-IgM Ab and subjected to DNA fragmentation assays. (C) Effect of MCD on BCR-induced apoptosis. WEHI-231 cells treated as in panel B were labeled with FITC–annexin V and propidium iodide and subjected to flow cytometry. Cells that labeled positively for both were considered to be apoptotic. The results are expressed as means ± SEM of 3 independent experiments. *P < .005.
Effect of MCD on BCR-induced apoptosis. (A) WEHI-231 cells were left untreated, treated with 5 mM MCD for 25 minutes in lipid-free medium, or treated with 5 mM MCD for 25 minutes and allowed to recover for 4 hours. Cells were then left unstimulated (–) or were stimulated (+) for 5 minutes with 20 μg/mL anti-IgM Ab. Following lysis in 0.5% Triton X-100 and sucrose gradient ultracentrifugation, GEM fractions were blotted with CTB subunit and Abs against Lyn, IgM, and CD45. (B) WEHI-231 cells were incubated as in panel A and then either left unstimulated (–) or stimulated (+) for 24 hours with 20 μg/mL anti-IgM Ab and subjected to DNA fragmentation assays. (C) Effect of MCD on BCR-induced apoptosis. WEHI-231 cells treated as in panel B were labeled with FITC–annexin V and propidium iodide and subjected to flow cytometry. Cells that labeled positively for both were considered to be apoptotic. The results are expressed as means ± SEM of 3 independent experiments. *P < .005.
To evaluate the effects of GEM disruption on BCR-induced final outcome, WEHI-231 cells, untreated or treated with 5 mM MCD, were left unstimulated or were stimulated with anti-IgM Ab for 24 hours and then subjected to DNA fragmentation assays (Figure 9B). We found BCR-induced DNA fragmentation to be strongly inhibited by MCD, an effect totally reversed by restoration of GEM cholesterol (Figure 9B). Evaluation of the percentage of apoptotic cells using annexin V staining showed that GEM disruption reduced BCR-induced apoptosis to background levels, but that apoptosis was restored by GEM recovery (Figure 9C). Thus, GEMs apparently constitute obligatory platforms for BCR signaling leading to the determination of B-cell fate, even in immature B cells.
Discussion
The experiments reported here demonstrate that, in the absence of CD45, Src-PTKs are constitutively activated and hyperphosphorylated at both the COOH-terminal negative regulatory site and the autophosphorylation site, suggesting a negative regulatory role for CD45 in B cells. It is notable that, in parental cells, BCR ligation induces phosphorylation of not only the autophosphorylation site but also the negative regulatory site, yet activating Src-PTKs. This is consistent with our findings in WEHI-231 cells27,28 and suggests to us that BCR signaling somehow reduces the negative regulatory effect of CD45.28 To better understand the inhibitory effect of CD45 on Src-PTKs and its reversal by BCR ligation, we tested the possibility that the spatiotemporal positioning of CD45 with respect to GEMs may dictate the activity of Src-PTKs. Our findings demonstrate that some CD45 is constitutively associated with GEMs, inhibiting Src-PTKs by dephosphorylating the 2 regulatory sites, but that it transiently dissociates from them shortly after BCR ligation. It is this movement of CD45 away from the GEMs that releases its inhibitory effect, enabling phosphorylation of the regulatory tyrosine residues and Src-PTK activation. Thus, the function of CD45 is not to activate Src-PTKs but to keep them inactive, with its dynamic behavior serving to fine-tune this regulation of Src-PTK activity.
These results are not necessarily compatible with the consensus view14,15 in which the postulated role of CD45 is to dephosphorylate predominantly the negative regulatory site, setting the stage for antigen receptor signaling to activate Src-PTKs by inducing autophosphorylation of the positive regulatory site.16-23 Nevertheless, several studies have shown that CD45 also dephosphorylates the autophosphorylation site, exerting an inhibitory effect. For example, Lck and Fyn are constitutively hyperphosphorylated and activated in 3 CD45-deficient T-cell lines,24 in which CNBr cleavage mapping revealed both of Lck's regulatory sites to be hyperphosphorylated.25 Lck is likewise constitutively hyperphosphorylated and activated in thymocytes from CD45 gene-targeted mice.26,42 It also appears that CD45 suppresses the tumorigenic potential of Lck by dephosphorylating the autophosphorylation site in transgenic mice expressing an active form of Lck, in which the negative regulatory tyrosine is mutated to phenylalanine (Lck Y505F).43 Another study showed that expression of Lck Y505F rescues thymocyte development in CD45-deficient mice, indicating the importance of dephosphorylation of the negative regulatory site by CD45 in T-cell development.42 However, the phosphorylation status of the positive regulatory site was not addressed in their study. The negative regulatory function of CD45 also affects integrin-mediated adhesion of macrophages.44 For instance, Lyn and Hck are more active in bone marrow–derived macrophages from CD45 KO mice than in wild-type macrophages, and addition of CD45 leads to dephosphorylation of the autophosphorylation site, decreasing Src-PTK activity. Finally, Lyn is constitutively hyperphosphorylated at both regulatory sites in CD45-deficient chicken DT40 B cells, whereas neither site was phosphorylated in the parental cells. In contrast to our findings, the hyperphosphorylated Lyn is inactive and is not activated by BCR ligation, presumably due to phosphorylation of the negative regulatory site, suggesting a positive role for CD45 in that case.22
Recent analyses of the crystal structure of Src-PTKs, as well as functional studies, have shown that the binding of the phosphorylated COOH-terminal site to the SH2 domain does not block the catalytic center; the inactive state is effectively conferred by the binding of the SH3 domain and the polyproline stretch in the linker between the catalytic domain and the SH2 domain.45-47 One recent study further suggests that the SH2 and SH3 domains of inactive Src-PTKs are tightly coupled, and that dephosphorylation of the negative site reduces this coupling between the SH2 and SH3 domains.48 If there are indeed doubly phosphorylated pools of Src-PTKs, it is likely that SH2 and SH3 ligands capable of disrupting the inhibitory intramolecular binding of Src-PTKs are incorporated, a priori, into the BCR signaling machinery and are functional in GEMs from CD45-deficient cells and BCR-stimulated parental cells.
Another issue is the localization of CD45 with respect to GEMs. Some studies indicate that CD45 is excluded from GEMs in both T cells49-51 and B cells.40,52,53 By contrast, others show that a small fraction of total CD45 is constitutively present in GEMs of T cells54-56 and that CD45 is at the T-cell-antigen–presenting cell contact site57 or is recruited to the central region of the mature immunologic synapse.58 One of the reasons for the discrepancy between those earlier findings and the findings presented here is that perhaps because only a small fraction of the total cellular CD45 is associated with GEMs, it escaped detection due to experimental limitations. It is also possible there may be cell type–dependent differences in the molecular organization of critical components regulating CD45 localization. Given the BCR-dependent nature of the dynamic behavior of CD45, it may be that interactions of CD45 with transmembrane proteins present within GEMs or with cytoskeletal elements may dictate the movement of CD45. The fact that GEM-associated CD45 and its dynamic behavior during BCR signaling are found not only in B cell lines but also in splenic B cells underscores the physiologic relevance of our findings. Interestingly, a recent study using deconvolution microscopy showed that a fraction of CD45 is associated with lipid rafts and BCR ligation enhances its association and aggregate formation during 5 to 30 minutes in BAL-17 cells.59 Our results also demonstrated that a significantly greater amount of CD45 was recovered within 15 minutes from GEMs not only in BAL-17 but also WEHI-231 and splenic B cells, although there was a dissociation of CD45 from GEMs within 30 seconds of stimulation. We do not have a satisfactory explanation for this discrepancy, but it may be due to differences in the techniques involved: biochemical versus microscopic assays. The present study also has important clinical implications pertaining to the regulation of abnormal B-cell activation in that effective inhibition of initiation of BCR signaling can be accomplished not by inhibition of CD45 activity, per se, but by blocking the movement of CD45 from GEMs.
It has been postulated that dimerization of receptor-type PTPs, including CD45, induces their inactivation through symmetrical interactions between the inhibitory wedge and the catalytic site.60,61 The importance of negative regulation of CD45 by dimerization is emphasized by the observation that substitution of an arginine for a critical glutamate in the inhibitory wedge, which resulted in attenuation of the inhibition of CD45 caused by dimerization, induced polyclonal T- and B-cell activation and severe autoimmune nephritis with autoantibody production within a few months time.61,62 Although the role of CD45 dimerization in the regulation of an early phase of BCR signaling is not clear at present, it is tempting to speculate that whereas GEM-associated CD45 exists as a monomer, retaining its phosphatase activity, CD45 exists in an inactive dimerized form outside of GEMs, thus coordinately regulating the effects of CD45 on Src-PTK activity.
It is notable that the issue of the selectivity of CD45 action on Src-PTKs in B cells has emerged from this and earlier studies. Among Src-PTKs, all of which are presumably present in GEMs, CD45 selectively regulates Lyn in WEHI-231 and splenic B cells and Fyn in BAL-17 cells. There have been reports that CD45 selectively associates with Lyn but not Blk or Fyn in splenic B cells,63 and that CD45 does not act on Lck when the 2 are coexpressed in Hela cells.44 This may indicate that CD45 has a preferential affinity for particular Src-PTKs or that its binding is influenced by other cellular components. Alternatively or in addition, Src-PTKs and CD45 may be differentially distributed within GEMs, and the physical distance between CD45 and particular Src-PTKs may determine substrate specificity.
It has been suggested that differences in the signaling molecules recruited to GEMs may explain the differing responses of immature versus mature B cells or normal versus tolerant B cells.40,53,64 One study has shown that BCR is not recruited to GEMs after ligation in WEHI-231 cells, whereas it is successfully recruited in mature CH27 cells, suggesting BCR signals are generated from outside GEMs in immature B cells.40 The present study showed BCR to be strongly recruited to GEMs even in WEHI-231 cells. There are several differences between the 2 experimental conditions, including detergent concentrations and detection systems. It may be that association between GEM and BCR is sensitive to the detergent concentration in a maturational stage-dependent manner such that 1%, but not 0.5%, of Triton X-100 disrupts GEM/BCR association of immature B cells. Specific conditions of solubilization were also shown to be required for detecting association between T-cell receptor and GEMs.65 In addition to these experimental conditions, phenotypic and functional differences observed in WEHI-231 cell lines may contribute to these discrepant results.
GEMs are considered to be obligatory platforms for initiating signaling cascades. This premise is based primarily on the approaches in which GEMs are disrupted by cholesterol-extracting agents. For example, treatment of A20 B cells with filipin led to impaired BCR-induced tyrosine phosphorylation of phospholipase γ2 (PLCγ2) and calcium mobilization.66 The present study also demonstrated that disruption of GEMs using MCD almost completely abrogates BCR-induced apoptosis. In experiments using drugs, their nonspecific effects should always be taken into consideration. However, given that dynamic association of signaling molecules with GEMs corresponds well with the regulation of initial events such as Src-PTK activation and its functional consequences, our results may support the importance of GEMs in initiating BCR signals leading to apoptosis.
In summary, we showed that the primary function of CD45 is inhibition of Src-PTKs by dephosphorylating the 2 regulatory tyrosine residues and that dynamic localization of CD45 with respect to GEMs and the corresponding changes in the activity of the Src-PTKs therein have a physiologically significant impact on BCR signal transduction and B-cell outcome.
Prepublished online as Blood First Edition Paper, October 16, 2003; DOI 10.1182/blood-2003-03-0716.
Supported in part by grants-in-aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology. P.S. received a fellowship from the Science and Technology Agency of Japan.
P.S. and T.K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Marcus Clark for anti–Ig-α Ab and Dr Atsushi Kosugi (Osaka University) for valuable advice on the preparation of GEMs.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal