Abstract
Data on the application of donor lymphocyte infusions (DLIs) following reduced-intensity transplantation (RIT) remain limited. Persistence of host antigen-presenting cells might increase the efficacy or toxicity of cellular immunotherapies. We report the results of dose-escalating DLIs in 46 patients undergoing RIT, who received a total of 109 infusions to treat mixed chimerism or residual or progressive disease. Diagnoses were myeloma (n = 19), Hodgkin lymphoma (n = 13), non-Hodgkin lymphoma (n = 10), and other (n = 4). Thirty-two had an HLA-matched family donor and 14 an unrelated donor. Grades II to IV graft-versus-host disease (GVHD) occurred in 5 sibling and 7 unrelated donor recipients. GVHD was more common (P = .002), occurred at lower T-cell doses, and was more severe in the unrelated donor cohort. Conversion from mixed to multilineage full donor chimerism occurred in 30 of 35 evaluable patients. Presence of mixed chimerism in the granulocyte lineage at the time of DLI did not predict for chimerism response or GVHD. Disease responses occurred in 63% of patients with myeloma and 70% of those with Hodgkin lymphoma and were not predicted by changes in chimerism. These data support the presence of clinically relevant graft-versus-Hodgkin activity and indicate that DLI may be associated with a significantly increased toxicity in unrelated compared to sibling donor transplant recipients receiving identical treatment protocols.
Introduction
The most efficient method for prevention of graft-versus-host disease (GVHD) following allogeneic hematopoietic stem cell transplantation (HSCT) is T-cell depletion of the graft. Concurrent T-cell depletion of the recipient facilitates engraftment and limits the requirement for escalation of the intensity of conditioning that is otherwise often required to prevent an increased incidence of graft rejection. The incorporation of T-cell depletion strategies also results in more frequent cases of mixed chimerism, a state that was observed to be associated with a reduced incidence of GVHD in some early clinical series of HSCT and in murine models. This state of mixed chimerism therefore became the initial aim of transplantation in many nonmyeloablative and reduced-intensity conditioning regimens. However, the application of reduced-intensity transplantation (RIT) approaches to humans has been associated with a relatively high incidence of GVHD (both acute and chronic), and often a more rapid conversion to donor-type chimerism. Incorporation of alemtuzumab (anti-CD52 monoclonal antibody) effectively reduces GVHD in both the HLA-matched sibling and unrelated donor setting.1,2 However, the reduced antitumor activity of such protocols (resultant on both the decreased cytotoxicity of conditioning chemotherapy and the delayed reconstitution of immunity secondary to T-cell depletion) may require the early use of adjuvant donor lymphocyte infusions (DLIs) to promote graft-versus-malignancy activity.
The ability of DLI to effect antitumor responses was initially described in patients with chronic myeloid leukemia (CML) suffering hematologic relapse following allogeneic stem cell transplantation.3 Although the list of malignancies that are sensitive to the effects of DLI has since been expanded to include myeloma, lymphomas, acute myeloid leukemia/myelodysplasia, and some solid tumors, CML remains the most sensitive and best studied disorder.4,5 Early experience established that treatment following hematologic relapse resulted in a significant incidence of marrow aplasia, resultant on an immune-mediated destruction of the reconstituted host hematopoeisis.6 Treatment earlier in the course of relapse (at the cytogenetic or molecular level) largely avoided this complication because donor hematopoiesis remained intact. Further evidence that the immune-mediated effect may not be truly tumor specific but is rather aimed at antigenic determinants of the host lymphohematopoietic system came from studies of lineage-specific chimerism. Mixed chimerism in T cells, a compartment that would not be predicted to be involved in the neoplastic process in CML, was shown to be an independent marker for subsequent disease relapse/progression.7 Administration of DLI could target this compartment leading to conversion to full donor chimerism.
Although T-cell depletion regimens carry an increased risk of disease relapse in CML, the remarkable sensitivity of CML to DLI has led to the successful use of protocols incorporating T-cell depletion with salvage DLI early in the course of disease relapse.8 It remains unclear whether this type of approach is suitable for patients with other malignancies. The timing and dosage that can be administered with relative safety remain poorly defined, particularly in the reduced-intensity setting where the early administration of DLI in combination with the persistence of host antigen-presenting cells (APCs) could have an impact on the development of GVHD. This remains the most common and serious adverse effect of DLI and continues to be a limiting factor in its application. Although there is currently little evidence that DLI is more toxic in the unrelated donor setting,9-11 as might be predicted to be the case from the greater genomic disparity between donor and recipient, interpretation of published data is complicated by a number of factors. In the majority of previous studies the unrelated donor cohort has received different transplantation protocols from the sibling cohort. In addition there has generally been a lack of demonstration of equivalence in terms of dosing and timing of DLI. Numerous strategies have been used to try to dissociate this harmful effect from the beneficial graft-versus-malignancy activity. One approach has been the use of escalating doses of DLI, given in the hope that graft-versus-leukemia (GVL) and GVHD will demonstrate differential dose-response relationships and that the 2 effects may therefore be separable. This has been shown to be the case in some patients with CML who received DLI more than 1 year after transplantation.12 However, whereas it may be possible to induce GVL at a T-cell dose below the threshold necessary to cause GVHD, the dose of DLI required to induce a graft-versus-malignancy effect is likely to differ among malignancies and according to the tumor mass at the time of infusion. In addition, it also remains unclear whether limiting T-cell dose will compromise the durability of remission.
To address these issues we have systematically examined the toxicity and efficacy of escalating doses of unmanipulated DLIs following an alemtuzumab-based reduced-intensity regimen. Because the risk of a given dose of DLI inducing GVHD appears to be modulated by the length of time since transplantation, the protocol was designed to delay administration of prophylactic or adjuvant DLI until 6 months following transplantation in patients with residual disease or mixed chimerism. DLI could be given at any time point for disease progression.
Patients, materials, and methods
Study design
The study protocol was approved by the University College London Hospitals Ethics Committee. Patients and donors gave written informed consent. The conditioning regimen consisted of intravenous infusion of alemtuzumab (humanized anti-CD52) monoclonal antibody 20 mg/d from day –8 to –4 (total 5 doses), fludarabine 30 mg/m2/d from day –7 to –3 (total 5 doses), and melphalan 140 mg/m2 on day –2. Cyclosporine A (CsA) was administered as an intravenous infusion over 6 hours at 3 mg/kg/d starting on day –1, with a target level of 300 ng/mL. On day 0, patients received granulocyte colony-stimulating factor (G-CSF)–mobilized unmanipulated allogeneic peripheral blood stem cell (PBSC) grafts from their HLA-matched siblings/cousin (n = 30/1), or unmanipulated bone marrow grafts from an unrelated donor (n = 14). One sibling graft recipient received unmanipulated donor marrow. Targeted stem cell dose was 4 × 106 CD34+ cells/kg.
In the absence of GVHD, CsA was tapered from 3 months following transplantation. Patients who had residual disease or mixed chimerism at 6 months after transplantation were eligible to receive DLI if there was no evidence of active GVHD. Escalating doses of CD3+ lymphocytes were administered at starting a dose of 1 × 106 T cells/kg. Increasing doses were administered at 3-month intervals (3 × 106, 1 × 107, 3 × 107, 1 × 108 T cells/kg) in the absence of development of GVHD if mixed chimerism persisted or if there was no evidence of disease response. The doses administered for disease progression depended on the disease type and the time since transplantation. Although every effort was made to adhere to the 3-month escalation protocol, moderate delays were incurred in some cases due to delays in restaging investigations, chimerism analyses, and accessing donors. In addition, to limit the potential risk for GVHD, DLI was delayed in those patients with disease responses until restaging investigations suggested a lack of continuing response. Lineage-specific chimerism (in T-cell and myeloid lineages) was assessed by means of polymerase chain reaction (PCR) analysis of informative minisatellite regions (short tandem repeat [STR] loci) as previously described.1 In some cases lymphopenia precluded T-cell chimerism analysis.
Patients
Forty-six patients with hematologic malignancy entered the study. This group was derived from a total of 106 patients receiving RIT with this protocol at our institution. Patient characteristics are shown in Table 1. Thirty-two had a matched related and 14 had a matched unrelated donor. Five of the unrelated donors had some degree of mismatch at HLA class I or class II (or both) alleles (molecular typing). These included 3 cases of bidirectional one antigen class C mismatch, one case of bidirectional one antigen DQB1 mismatch, and one case of bidirectional one antigen DRB1 mismatch. Eleven of 28 male recipients had a female donor. Eight patients had developed acute GVHD following transplantation (grades I-II), although this was quiescent at the time of DLI. None had developed chronic GVHD and none were on immunosuppression at the time of DLI.
Patient characteristics
Characteristic . | Patients . |
|---|---|
| Median age, y (range) | 46 (22-59) |
| No. male/female | 28/18 |
| No. donors, matched family/unrelated | 32/14 |
| No. female donor/male recipient sex mismatches | 11 |
| Disease | |
| Myeloma | 19 |
| Hodgkin lymphoma | 13 |
| LG-NHL/CLL/PLL | 7/2/1 |
| HG-NHL | 3 |
| CML | 1 |
| GVHD prior to DLI | |
| Related donor, grade I/II | 3/1 |
| Unrelated donor, grade I/II | 3/1 |
Characteristic . | Patients . |
|---|---|
| Median age, y (range) | 46 (22-59) |
| No. male/female | 28/18 |
| No. donors, matched family/unrelated | 32/14 |
| No. female donor/male recipient sex mismatches | 11 |
| Disease | |
| Myeloma | 19 |
| Hodgkin lymphoma | 13 |
| LG-NHL/CLL/PLL | 7/2/1 |
| HG-NHL | 3 |
| CML | 1 |
| GVHD prior to DLI | |
| Related donor, grade I/II | 3/1 |
| Unrelated donor, grade I/II | 3/1 |
LG-NHL indicates low-grade non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; PLL, prolymphocytic leukemia, HG-NHL, high-grade non-Hodgkin lymphoma; CML, chronic myeloid leukemia.
Thirteen patients received DLI for residual disease and 19 for disease progression/relapse (Table 2). Eleven of the former and 13 of the latter were mixed chimeras at the time of DLI. Four were not evaluated for chimerism status and 4 were full donor chimeras. Fourteen patients were treated for mixed chimerism alone. Four of these received subsequent doses for disease progression (2 with low-grade non-Hodgkin lymphoma [LG-NHL], 1 with chronic lymphocytic leukemia [CLL] demonstrated to have minimal residual disease following the introduction of a flow cytometric technique for monitoring, and 1 with Hodgkin lymphoma).
DLI indication and disease type
. | No. with indication for DLI . | . | . | ||
|---|---|---|---|---|---|
| Disease . | Residual disease . | Progression/relapse . | Mixed chimerism . | ||
| Myeloma | 11 | 8 | 0 | ||
| Hodgkin lymphoma | 1 | 8 | 4 | ||
| LG-NHL/CLL/PLL | 1/0/0 | 0/0/1 | 6/2/0 | ||
| HG-NHL | 0 | 1 | 2 | ||
| CML | 0 | 1 | 0 | ||
. | No. with indication for DLI . | . | . | ||
|---|---|---|---|---|---|
| Disease . | Residual disease . | Progression/relapse . | Mixed chimerism . | ||
| Myeloma | 11 | 8 | 0 | ||
| Hodgkin lymphoma | 1 | 8 | 4 | ||
| LG-NHL/CLL/PLL | 1/0/0 | 0/0/1 | 6/2/0 | ||
| HG-NHL | 0 | 1 | 2 | ||
| CML | 0 | 1 | 0 | ||
To attain further cytoreduction prior to immunotherapy, 7 patients received specific antitumoral therapy prior to DLI: 3 of these patients had Hodgkin lymphoma, 2 had myeloma, and 1 each had LG-NHL and PLL.
Definitions
Acute and chronic GVHD were graded according to the consensus criteria. Patients were considered evaluable for GVHD if they survived 100 days or longer following the infusion or if they developed clinical signs of GVHD before this time. Pre-DLI restaging investigations were used as the reference point for subsequent evaluations of disease responses. Responses were determined by standard disease-specific criteria as defined by the International Bone Marrow Transplant Registry. Complete donor chimerism was defined by lack of detection of a previously determined recipient-specific peak on STR PCR, and mixed chimerism by detection of both donor- and recipient-specific peaks.
Statistical methods
The Fisher exact test for categorical data was used to compare variables in 2-by-2 tables as outlined in the text. Unless otherwise stated, the 2-tail test was performed.
Results
Toxicity
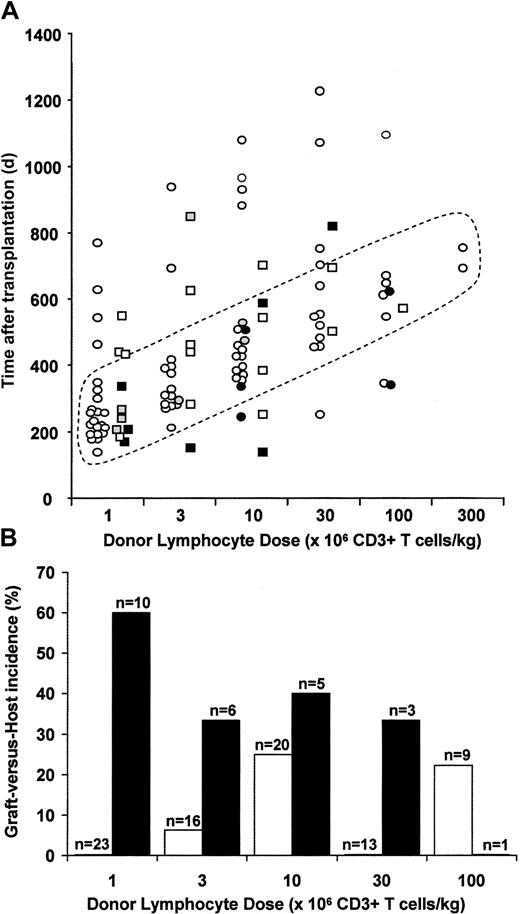
Forty-six patients received a total of 109 infusions of donor lymphocytes (median, 2; range, 1-6 per patient; Figure 1). Median follow-up from the time of the initial DLI is 768 days (range, 209-1173 days). Forty-five patients are evaluable for GVHD. There were no episodes of graft hypoplasia. Thirty-three started with a T-cell dose of 1 × 106/kg administered at a median of 254 days after transplantation (range, 144-780 days; Figure 1; Table 3). None of the 23 with a sibling donor receiving this dose developed GVHD, compared with 6 of 10 with an unrelated donor (Figure 1A-B; P = .001, Fisher exact test). Twenty-two patients received a dose of 3 × 106/kg at a median of 360 days after transplantation (range, 155-1413 days). One of the 16 with a sibling donor developed limited chronic GVHD. Two of 6 with an unrelated donor developed GVHD. Twenty-six patients received a dose of 1 × 107/kg at a median of 452 days after transplantation (range, 144-1570 days). Five of 20 with a sibling donor developed GVHD. One patient with an unrelated donor died of disease progression and was not evaluable for GVHD. Two of the remaining 5 developed GVHD. Sixteen patients received a dose of 3 × 107/kg at a median of 606 days after transplantation (range, 258-1695 days). None of the 13 with a sibling donor and one of 3 with an unrelated donor developed GVHD. Ten patients received a dose of 1 × 108/kg at a median of 631 days after transplantation (range, 347-1805). In total, 10 of 13 evaluable patients with an unrelated donor developed GVHD compared to 8 of 32 with a matched family donor (P = .002, Fisher exact test). GVHD also occurred at lower T-cell doses in the unrelated donor cohort (Figure 1). One of 39 doses of sibling donor DLI of less than 1 × 107/kg (a dose previously demonstrated in sibling donor dose-escalation studies to be associated with a low risk of GVHD when administered at 1 year following transplantation) was associated with the development of GVHD, compared to 8 of 16 unrelated donor DLI (P < .0001, Fisher exact test). DLI doses of 1 × 107/kg or more were associated with an increased risk of GVHD in sibling recipients compared with doses below this level (7 of 44 versus 1 of 39, P = .04, 1-tail Fisher exact test).
Development and incidence of GVHD. (A) Relationship between donor lymphocyte dose, timing after transplantation, and development of GVHD. Sibling donor DLIs are represented as circles and unrelated donor DLIs as squares. Open symbols represent cases without GVHD. Filled symbols represent those with GVHD: gray with grade I GVHD, black with grades II-IV. Four doses of DLI were given beyond the maximum time point illustrated. (B) Incidence of GVHD according to DLI dose and donor type. □ indicates sibling DLI; ▪, unrelated donor DLI.
Development and incidence of GVHD. (A) Relationship between donor lymphocyte dose, timing after transplantation, and development of GVHD. Sibling donor DLIs are represented as circles and unrelated donor DLIs as squares. Open symbols represent cases without GVHD. Filled symbols represent those with GVHD: gray with grade I GVHD, black with grades II-IV. Four doses of DLI were given beyond the maximum time point illustrated. (B) Incidence of GVHD according to DLI dose and donor type. □ indicates sibling DLI; ▪, unrelated donor DLI.
DLI doses, timing, and GVHD incidence
. | Median time after transplantation, d (range) . | No. with GVHD . | . | |
|---|---|---|---|---|
| T-cell dose . | . | Sibling . | MUD . | |
| 1 × 106/kg | 254 (144-780) | 0/23 | 6/10 (3 grades I-II, 2 grade IV, 1 limited chronic) | |
| 3 × 106/kg | 360 (155-1413) | 1/16 (limited chronic) | 2/6 (1 grade I, 1 grade IV) | |
| 1 × 107/kg | 452 (144-1570) | 5/20 (2 grade I, 3 grades III-IV) | 2/5* (2 grades III-IV) | |
| 3 × 107/kg | 606 (258-1695) | 0/13 | 1/3 (1 grade IV) | |
| 1 × 108/kg | 631 (347-1805) | 2/9 (2 grade IV) | 0/1 | |
| 3 × 108/kg | (705-764) | 0/2 | — | |
. | Median time after transplantation, d (range) . | No. with GVHD . | . | |
|---|---|---|---|---|
| T-cell dose . | . | Sibling . | MUD . | |
| 1 × 106/kg | 254 (144-780) | 0/23 | 6/10 (3 grades I-II, 2 grade IV, 1 limited chronic) | |
| 3 × 106/kg | 360 (155-1413) | 1/16 (limited chronic) | 2/6 (1 grade I, 1 grade IV) | |
| 1 × 107/kg | 452 (144-1570) | 5/20 (2 grade I, 3 grades III-IV) | 2/5* (2 grades III-IV) | |
| 3 × 107/kg | 606 (258-1695) | 0/13 | 1/3 (1 grade IV) | |
| 1 × 108/kg | 631 (347-1805) | 2/9 (2 grade IV) | 0/1 | |
| 3 × 108/kg | (705-764) | 0/2 | — | |
MUD indicates matched unrelated donor. — indicates that no doses were given at this level to MUD recipients.
One additional patient could not be evaluated, due to rapid disease progression.
Nine of the 10 cases of GVHD in unrelated donor transplant recipients occurred following the initial DLI dose, compared to 4 of the 8 cases in matched family donor transplant recipients. This included 3 of 3 unrelated donor recipients given a starting T-cell dose of more than 1 × 106/kg and 4 of 9 matched related donor recipients given a starting T-cell dose of more than 1 × 106/kg. Thus in the matched related donor cohort GVHD was not significantly more likely to occur with the initial dose as compared with subsequent escalating doses, although there was a trend in this direction (4 of 8 versus 4 of 24; P = .08, 1-tail Fisher exact test). GVHD was more common in the matched related donor cohort following the initial dose if it was more than 1 × 106/kg (4 of 9 versus 0 of 23; P = .004, Fisher exact test), although this is obviously modulated by the administration of initial high doses at relatively early time points in some cases (Figure 1A). However, GVHD was not significantly more common in the group receiving an initial dose of more than 1 × 106/kg if onset following subsequent dose escalation was included (4 of 9 versus 4 of 23; P = .13, 1-tail Fisher exact test). The GVHD occurring following initial doses of more than 1 × 106/kg was more severe (2 grade III, 2 grade IV) than that developing during dose escalation (2 grade I, 1 limited chronic, 1 grade IV), supporting the principle of dose escalation in this group.
Chronic GVHD occurred as a de novo presentation in one sibling and one unrelated donor DLI recipient (Table 3). Three of 4 evaluable sibling donor recipients developed chronic GVHD following acute GVHD (1 limited, 2 extensive; Table 4). Five of 8 evaluable unrelated donor recipients developed chronic GVHD following acute GVHD (1 limited, 4 extensive).
GVHD grade and treatment
Patient no. . | Donor . | Acute GVHD grade . | Chronic GVHD . | Treatment . |
|---|---|---|---|---|
| 1 | Sib | No | Limited | Topical steroids |
| 2 | MUD | III, hepatic/cutaneous | Limited* | Systemic steroids, CsA, infliximab, daclizumab |
| 3 | MUD | II, cutaneous/gut | Limited | Topical steroids |
| 4 | MUD | IV, hepatic/cutaneous | Extensive | Systemic steroids, CsA, ATG |
| 8 | MUD | I, cutaneous | No | Topical steroids |
| 11 | Sib | IV, hepatic/cutaneous/gut | NA | Systemic steroids, CsA, ATG |
| 12 | MUD | IV, hepatic/cutaneous | Extensive | Systemic steroids, CsA, thalidomide |
| 13 | MUD | IV, hepatic/cutaneous/gut | Extensive | Systemic steroids, MMF, ATG |
| 14 | Sib | IV, hepatic/cutaneous/gut | Extensive | Systemic steroids, CsA |
| 21 | MUD | III, hepatic/cutaneous | Extensive | Systemic steroids, CsA |
| 23 | Sib | III, cutaneous/gut | Extensive | Systemic steroids, CsA, azathioprine, thalidomide |
| 24 | Sib | IV, hepatic/cutaneous | NA | Systemic steroids, CsA, ATG |
| 25 | Sib | III, gut | NA | Systemic steroids, CsA, ATG |
| 29 | Sib | I, cutaneous | Limited | Topical steroids |
| 33 | Sib | I, cutaneous | No | Topical steroids |
| 38 | MUD | I, cutaneous | No | Topical steroids |
| 39 | MUD | I, cutaneous | No | Topical steroids |
| 46 | MUD | IV, hepatic/cutaneous/gut | NA | Systemic steroids, CsA, ATG |
Patient no. . | Donor . | Acute GVHD grade . | Chronic GVHD . | Treatment . |
|---|---|---|---|---|
| 1 | Sib | No | Limited | Topical steroids |
| 2 | MUD | III, hepatic/cutaneous | Limited* | Systemic steroids, CsA, infliximab, daclizumab |
| 3 | MUD | II, cutaneous/gut | Limited | Topical steroids |
| 4 | MUD | IV, hepatic/cutaneous | Extensive | Systemic steroids, CsA, ATG |
| 8 | MUD | I, cutaneous | No | Topical steroids |
| 11 | Sib | IV, hepatic/cutaneous/gut | NA | Systemic steroids, CsA, ATG |
| 12 | MUD | IV, hepatic/cutaneous | Extensive | Systemic steroids, CsA, thalidomide |
| 13 | MUD | IV, hepatic/cutaneous/gut | Extensive | Systemic steroids, MMF, ATG |
| 14 | Sib | IV, hepatic/cutaneous/gut | Extensive | Systemic steroids, CsA |
| 21 | MUD | III, hepatic/cutaneous | Extensive | Systemic steroids, CsA |
| 23 | Sib | III, cutaneous/gut | Extensive | Systemic steroids, CsA, azathioprine, thalidomide |
| 24 | Sib | IV, hepatic/cutaneous | NA | Systemic steroids, CsA, ATG |
| 25 | Sib | III, gut | NA | Systemic steroids, CsA, ATG |
| 29 | Sib | I, cutaneous | Limited | Topical steroids |
| 33 | Sib | I, cutaneous | No | Topical steroids |
| 38 | MUD | I, cutaneous | No | Topical steroids |
| 39 | MUD | I, cutaneous | No | Topical steroids |
| 46 | MUD | IV, hepatic/cutaneous/gut | NA | Systemic steroids, CsA, ATG |
Sib indicates matched sibling donor; NA, not applicable; MMF, mycophenolate mofetil.
Patient no. 2 developed limited chronic GVHD after the initial DLI; following resolution, further dose escalation (3 doses) was associated with development of more severe GVHD.
GVHD treatment
Treatment of GVHD followed standard approaches (Table 4). Six patients received antithymocyte globulin (ATG) for steroid-refractory GVHD. Two subsequently developed fatal Epstein-Barr virus (EBV)–associated posttransplantation lymphoproliferative disorders (PTLDs). Three further patients died as a consequence of GVHD (including 1 treated for mixed chimerism), and in 3 with an underlying diagnosis of multiple myeloma both GVHD and disease progression were felt to be significant contributory factors to death (Table 5).
Disease responses
Patient no. . | Donor . | Indication . | Best response . | GVHD . | Maximum DLI dose, T cells/kg . | Most recent response . | Status* . |
|---|---|---|---|---|---|---|---|
| 1 | Sib | MM residual disease | CR | Yes | 1 × 107 | Relapse | A |
| 2 | MUD | MM residual disease | PR | Yes | 3 × 107 | PR | A |
| 3 | MUD | MM residual disease | PR | Yes | 3 × 107 | PR | A |
| 4 | MUD | MM residual disease | PR | Yes | 1 × 106 | PR | D-GVHD |
| 5 | MUD | MM residual disease | PR | No | 1 × 108 | Progression | D-dis |
| 6 | Sib | MM residual disease | MR | No | 1 × 107 | Progression | D-dis |
| 7 | Sib | MM residual disease | NC | No | 1 × 106 | NC | A |
| 8 | MUD | MM residual disease | NC† | Yes | 3 × 106 | Progression | A |
| 9 | Sib | MM residual disease | NC | No | 1 × 108 | Progression | A |
| 10 | Sib | MM residual disease | NC | No | 3 × 106 | Progression | A |
| 11 | Sib | MM residual disease | NC | Yes | 1 × 107 | Progression | D-PTLD |
| 12 | MUD | MM progression | PR | Yes | 1 × 107 | Progression | D-GVH/dis |
| 13 | MUD | MM progression | PR | Yes | 3 × 106 | Progression | D-GVH/dis |
| 14 | Sib | MM progression | PR† | Yes | 1 × 107 | Progression | D-GVH/dis |
| 15 | Sib | MM progression | PR | No | 3 × 108 | Progression | A |
| 16 | Sib | MM progression | NC | No | 1 × 108 | NC | A |
| 17 | Sib | MM progression | NC | No | 1 × 108 | Progression | A |
| 18 | Sib | MM progression | NR | No | 3 × 107 | Progression | D-dis |
| 19 | Sib | MM progression | NR | No | 3 × 106 | Progression | D-dis |
| 20 | MUD | HL residual disease | NR | No | 1 × 106 | Progression | A |
| 21 | MUD | HL progression | CR | Yes | 1 × 107 | CR | A |
| 22 | Sib | HL progression | CR | No | 1 × 107 | CR | A |
| 23 | Sib | HL progression | CR† | Yes | 1 × 108 | Relapse | A |
| 24 | Sib | HL progression | CR† | Yes | 1 × 108 | CR | D-GVH |
| 25 | Sib | HL progression | CR | Yes | 1 × 107 | CR | D-PTLD |
| 26 | Sib | HL progression | PR | No | 1 × 108 | PR | A |
| 27 | Sib | HL progression | PR | No | 3 × 108 | Progression | A |
| 28 | Sib | HL progression | NR | No | 1 × 108 | Progression | A |
| 29 | Sib | MC progression | NR† | Yes | 3 × 107 | Progression | D-dis |
| 30 | Sib | LG-NHL residual disease | PR† | No | 1 × 108 | PR | A |
| 31 | Sib | LG-NHL MC progression | CR | No | 3 × 107 | CR | A |
| 32 | Sib | LG-NHL MC progression | NE | No | 3 × 107 | Progression | A |
| 33 | Sib | CLL MC-MRD | CR | Yes | 3 × 107 | CR | A |
| 34 | Sib | HG-NHL progression | NE | No | 1 × 107 | CR | A |
| 35 | MUD | PLL progression | NR† | No | 1 × 107 | Progression | D-dis |
| 36 | Sib | CML progression | Cyt CR | No | 3 × 106 | Cyt CR | A |
Patient no. . | Donor . | Indication . | Best response . | GVHD . | Maximum DLI dose, T cells/kg . | Most recent response . | Status* . |
|---|---|---|---|---|---|---|---|
| 1 | Sib | MM residual disease | CR | Yes | 1 × 107 | Relapse | A |
| 2 | MUD | MM residual disease | PR | Yes | 3 × 107 | PR | A |
| 3 | MUD | MM residual disease | PR | Yes | 3 × 107 | PR | A |
| 4 | MUD | MM residual disease | PR | Yes | 1 × 106 | PR | D-GVHD |
| 5 | MUD | MM residual disease | PR | No | 1 × 108 | Progression | D-dis |
| 6 | Sib | MM residual disease | MR | No | 1 × 107 | Progression | D-dis |
| 7 | Sib | MM residual disease | NC | No | 1 × 106 | NC | A |
| 8 | MUD | MM residual disease | NC† | Yes | 3 × 106 | Progression | A |
| 9 | Sib | MM residual disease | NC | No | 1 × 108 | Progression | A |
| 10 | Sib | MM residual disease | NC | No | 3 × 106 | Progression | A |
| 11 | Sib | MM residual disease | NC | Yes | 1 × 107 | Progression | D-PTLD |
| 12 | MUD | MM progression | PR | Yes | 1 × 107 | Progression | D-GVH/dis |
| 13 | MUD | MM progression | PR | Yes | 3 × 106 | Progression | D-GVH/dis |
| 14 | Sib | MM progression | PR† | Yes | 1 × 107 | Progression | D-GVH/dis |
| 15 | Sib | MM progression | PR | No | 3 × 108 | Progression | A |
| 16 | Sib | MM progression | NC | No | 1 × 108 | NC | A |
| 17 | Sib | MM progression | NC | No | 1 × 108 | Progression | A |
| 18 | Sib | MM progression | NR | No | 3 × 107 | Progression | D-dis |
| 19 | Sib | MM progression | NR | No | 3 × 106 | Progression | D-dis |
| 20 | MUD | HL residual disease | NR | No | 1 × 106 | Progression | A |
| 21 | MUD | HL progression | CR | Yes | 1 × 107 | CR | A |
| 22 | Sib | HL progression | CR | No | 1 × 107 | CR | A |
| 23 | Sib | HL progression | CR† | Yes | 1 × 108 | Relapse | A |
| 24 | Sib | HL progression | CR† | Yes | 1 × 108 | CR | D-GVH |
| 25 | Sib | HL progression | CR | Yes | 1 × 107 | CR | D-PTLD |
| 26 | Sib | HL progression | PR | No | 1 × 108 | PR | A |
| 27 | Sib | HL progression | PR | No | 3 × 108 | Progression | A |
| 28 | Sib | HL progression | NR | No | 1 × 108 | Progression | A |
| 29 | Sib | MC progression | NR† | Yes | 3 × 107 | Progression | D-dis |
| 30 | Sib | LG-NHL residual disease | PR† | No | 1 × 108 | PR | A |
| 31 | Sib | LG-NHL MC progression | CR | No | 3 × 107 | CR | A |
| 32 | Sib | LG-NHL MC progression | NE | No | 3 × 107 | Progression | A |
| 33 | Sib | CLL MC-MRD | CR | Yes | 3 × 107 | CR | A |
| 34 | Sib | HG-NHL progression | NE | No | 1 × 107 | CR | A |
| 35 | MUD | PLL progression | NR† | No | 1 × 107 | Progression | D-dis |
| 36 | Sib | CML progression | Cyt CR | No | 3 × 106 | Cyt CR | A |
MM indicates multiple myeloma; A, alive; D, dead; dis, underlying disease; MR, minimal response; NC, no change; NR, no response; HL, Hodgkin lymphoma; NE; not evaluable; MC, mixed chimerism; MRD, minimal residual disease; Cyt CR, complete cytogenetic response. Other abbreviations are defined in the text or in Tables 1, 3, and 4.
Including major cause of death.
Patients receiving antitumoral chemotherapy prior to DLI.
Disease responses and response durability
Disease responses are evaluable in 34 of 36 patients treated for residual disease or disease progression (including 4 who progressed after initial treatment for mixed chimerism; Table 5). One of the other 2 patients developed a localized relapse of LG-NHL and was treated with localized radiotherapy as well as DLI. Another had a high-grade lesion removed from the bowel as part of a diagnostic procedure and subsequently received DLI. Although both of the latter attained a complete response (CR), the contribution of DLI is unclear.
Twelve (63%) of 19 patients with multiple myeloma had some disease response. This figure includes 2 patients with progressive disease who had disease stabilization (without reduction in paraprotein level) sustained for over 12 months (one remaining stable 25 months following initial DLI). Only one patient attained CR following DLI. There was no significant difference in response rates between those treated for residual and those for progressive disease (6 of 11 versus 6 of 8; P = 0.6, Fisher exact test). CRs or partial responses (PRs) were significantly more likely in those patients developing GVHD (P = .02, Fisher exact test). Only 3 of the 12 who achieved a response to DLI remain alive and progression free (median follow-up 760 days).
Seven of the 10 patients treated for Hodgkin lymphoma achieved CR or PR. A further patient has received only a single low dose of DLI and continues on the dose-escalation protocol (Table 5). Attainment of CR was significantly associated with the development of GVHD (P = .048, Fisher exact test). Only one CR occurred in the absence of GVHD. At a median follow-up of 700 days 3 of the responding patients remain alive and progression free. None of these had cytoreductive chemotherapy prior to DLI. One patient died secondary to GVHD complications and one of PTLD following ATG therapy for GVHD. Disease responses were maintained for 427 and 827 days after DLI in the other 2 responding patients.
The number of patients treated for other disorders was relatively small. Responses were documented in 3 evaluable patients with LG-NHL and the single patient treated for CML. The latter patient achieved a cytogenetic but not yet a molecular CR. The patient treated for prolymphocytic leukemia (PLL) did not respond. Some of these responses occurred without the development of GVHD (Table 5), but the numbers remain too small for further comment.
Disease responses and preceding chemotherapy
Seven patients received specific antitumoral therapy shortly before DLI (Table 5). In these cases separation of responses to chemotherapy from those to DLI is clearly difficult. Three failed to achieve a clinical response. Two achieved a response but died from complications of GVHD with or without disease progression. One achieved CR (Hodgkin lymphoma) that lasted for 827 days, exceeding any response to chemotherapy achieved prior to transplantation (maximum 330 days) or following transplantation and prior to DLI (615 days). The other patient (LG-NHL) has achieved a PR with continuing reduction in disease mass 378 days following initial DLI, having received a total of 3 escalating doses of DLI. Thus, the inclusion of these patients appears unlikely to have an impact in a major way on the overall results of the study.
Chimerism responses
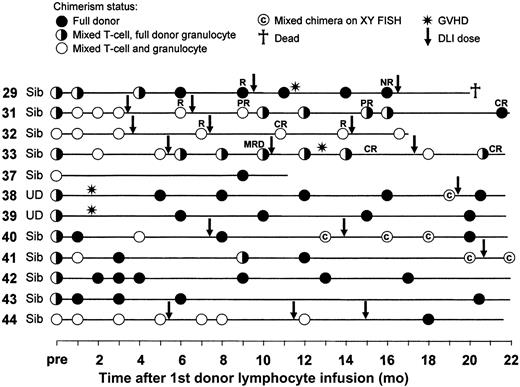
Fourteen patients received DLI for mixed chimerism alone. Twelve are evaluable for response (Figure 2). One died of grade IV GVHD and no chimerism analyses were performed following DLI (unrelated donor). One has not yet had repeat studies performed (no GVHD). Eleven of the other 12 remain alive (1 death due to disease progression). Ten converted to full donor chimerism in all lineages. Although 3 subsequently reverted to mixed status, 2 of these responded to further doses of DLI. Of the remaining 2 cases, one (patient no. 33) converted to mixed chimerism in the granulocyte lineage despite the development of GVHD following incremental DLI dosing, but on further dose escalation is currently full donor in the granulocyte lineage and mixed in the T-cell lineage. The other (patient no. 32) has remained a mixed chimera in both the granulocyte and T-cell lineages (Figure 2), with no increase in the level of donor chimerism throughout the study period.
Chimerism responses and GVHD occurrence following DLI for mixed chimerism. Results are shown starting from the chimerism result immediately prior to the first DLI dose. Subsequent DLI doses are indicated by the arrows. In those patients subsequently receiving DLI for relapse the disease responses are also indicated: CR, complete response; PR, partial response; R, relapse/progression; NR, no response; MRD, minimal residual disease.
Chimerism responses and GVHD occurrence following DLI for mixed chimerism. Results are shown starting from the chimerism result immediately prior to the first DLI dose. Subsequent DLI doses are indicated by the arrows. In those patients subsequently receiving DLI for relapse the disease responses are also indicated: CR, complete response; PR, partial response; R, relapse/progression; NR, no response; MRD, minimal residual disease.
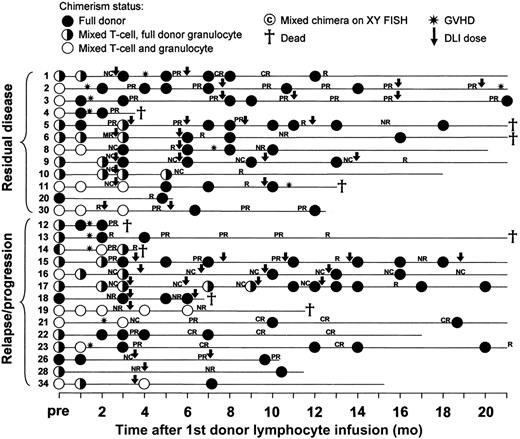
Twelve of the 13 patients treated for residual disease and 14 of 19 treated for progressive or relapsing disease have been serially examined for chimerism status (Figure 3). Three were full donor chimeras and remained so following DLI. Of the remaining 23 patients, 20 converted to full donor status in all lineages and all remain full donor chimeras at latest follow up, including 9 of the 11 patients with mixed chimerism in the granulocyte lineage at the time of initial DLI. Of the other 3 patients, one (patient no. 14) converted to mixed chimerism in the granulocyte lineage with stable levels of mixed chimerism in the T-cell lineage before death due to a combination of GVHD and disease progression, one (patient no. 19) remained a mixed chimera in both lineages before death due to disease progression, and one (patient no. 10) remained mixed in the T-cell lineage and full donor in the granulocyte lineage.
Chimerism responses, GVHD, and disease responses following DLI for residual or progressive disease. Results are shown starting from the chimerism result immediately prior to the first DLI dose. Subsequent DLI doses are indicated by the arrows. Further details on patient characteristics and GVHD treatment are shown in Tables 4 and 5. CR indicates complete response; PR, partial response; R, relapse/progression; NR, no response; NC, no change.
Chimerism responses, GVHD, and disease responses following DLI for residual or progressive disease. Results are shown starting from the chimerism result immediately prior to the first DLI dose. Subsequent DLI doses are indicated by the arrows. Further details on patient characteristics and GVHD treatment are shown in Tables 4 and 5. CR indicates complete response; PR, partial response; R, relapse/progression; NR, no response; NC, no change.
Multilineage full donor chimerism was achieved in 30 of 35 evaluable patients and maintained/re-established in 29 at latest follow-up. Two further patients converted to full donor status in the granulocyte lineage only, 2 remained mixed in the T-cell lineage and full donor in the granulocyte lineage, and one mixed in both lineages. Conversion to full donor T-cell chimerism was not statistically significantly more or less likely to occur if the granulocyte lineage chimerism was mixed at the time of initial DLI (11 of 14 versus 19 of 21; P = .16, Fisher exact test).
GVHD, disease responses, and chimerism status
GVHD developed in 15 patients in whom serial chimerism studies were performed (Figures 2-3). Eleven were mixed chimeras immediately prior to DLI and 4 were full donor chimeras. Only 2 remained mixed chimeras following the development of GVHD (patients no. 13 and 33) although both converted to full donor status in the granulocyte lineage. One hundred and two doses of DLI were administered in those patients in whom chimerism status was known prior to infusion. In total, 67 doses of DLI were administered in the presence of mixed chimerism and 35 doses in the presence of full donor chimerism. GVHD occurred in 10 of the former and 8 of the latter (P = .41, Fisher exact test). Thus GVHD did not appear significantly more common according to the peripheral blood chimerism status of the recipient at the time of DLI, even if analysis was restricted to the myeloid series (6 of 30 mixed versus 12 of 72 full donor; P = .78).
Disease responses are evaluable in 27 patients in whom serial chimerism data are available. Seventeen had DLI-responsive disease. Of these, 2 remained mixed chimeras in the T-cell lineage but converted to full donor status in the granulocyte lineage (patients no. 14 and 33) and 15 converted to multilineage full donor chimerism. Of the nonresponding patients, 2 remained mixed chimeras (patients 10 and 19) and 8 converted to full donor chimerism. Thus disease responses were not predicted by conversion of chimerism status. The positive predictive value of conversion to full donor status in both lineages for disease response was 65%, and the negative predictive value 50%. Only one patient who was evaluable for disease response failed to achieve full donor pattern in the granulocyte lineage and this patient did not have a disease response. More cases would be required to confirm the negative predictive value of this situation.
Discussion
Murine models have demonstrated that establishment of mixed chimerism may be accompanied by bilateral transplantation tolerance with graft acceptance and no GVHD.13 In the T cell–depleted murine model described by Sykes et al, newly formed T lymphocytes acquire central tolerance to host and donor antigens present in the thymus and delayed DLI is able to induce complete lymphohematopoietic chimerism with no GVHD.14-16 Theoretically, however, bilateral tolerance may also tolerize against the underlying malignancy such that the relapse risk may be higher. Despite this, the persistence of host APCs has been shown in murine models to result in superior GVL effects following DLI.17 They have also been shown to be responsible for initiation of GVH reactivity leading to the suggestion that strategies aimed at inactivation or destruction of host APCs could be effective in reducing the incidence of GVHD following allogeneic HSCT.18 However, there may be critical differences between induction of tolerance in these rodent models and the situation in humans, prompting the need for caution in the extrapolation of these observations. Most rodent models have used young, highly inbred animals housed in clean facilities presenting a largely naive peripheral T-cell repertoire. In humans, or indeed most large animal models, the presence of a greater proportion of memory T cells resultant on age and environmental antigenic exposure provides a population of cells that are relatively more resistant to tolerance induction than their naive counterparts. Incorporation of in vivo T-cell depletion of both recipient and graft results in the combination of low incidences of both graft failure and GVHD, providing a platform for the study of subsequent DLI. The relative contribution of alemtuzumab-mediated depletion of T cells and of APCs19 to these clinical outcomes is unclear.
The current data suggest that GVHD is more frequent, is more severe, and occurs at lower DLI doses in unrelated donor transplant recipients than in sibling donor transplant recipients. Indeed, the toxicities observed with the application of DLI in accordance with the protocol outlined in this study were prohibitive in the cohort with unrelated donors. Previous studies reporting the toxicities of DLI in the unrelated donor setting have generally found no evidence of increased toxicity compared to use in the sibling donor setting.9-11,20 There may be a number of reasons for these differences. Conventional myeloablative unrelated donor transplants have been associated with relatively high incidences of GVHD and levels of transplant-related mortality (TRM). Thus a significant number of patients undergoing these procedures would have either died prior to or be considered ineligible for DLI. The combination of reduced-intensity conditioning and T-cell depletion results in reduced GVHD (6.4% grades II-IV acute GVHD, 7.9% chronic GVHD) and TRM (19.8% at 1 year)2 in unrelated donor transplants. Thus the patients in the current study may be less heavily preselected. In addition it has often not been possible to draw direct comparison with sibling DLI because of differences in transplantation conditioning regimens and differences in the timing of equivalent DLI doses following transplantation. Van Rhee et al attempted to directly compare sibling and unrelated donor DLI.10 Median T-cell doses were similar for the 2 cohorts. Although there was not a significant excess of GVHD in the unrelated DLI group, there was a trend toward this (58.3% versus 38.9% acute grades II-IV GVHD; P = .09) and the influence of differing conditioning regimens remained impossible to assess. Marks et al reported that GVHD following DLI in patients undergoing RIT with T-cell depletion was not demonstrably more common in unrelated compared to sibling donor recipients.11 However, interpretation is complicated by the multiplicity of conditioning regimens (n = 6) and the lack of detailed data demonstrating equivalence in terms of dosing and timing after transplantation in these groups. It is not possible to exclude some impact of the source of stem cells at the time of transplantation on subsequent outcomes in the current study. The majority of related donor transplant recipients were given PBSC grafts, whereas all the matched unrelated donor recipients were given bone marrow. This practice reflected the lack of availability of unrelated donor PBSCs in the United Kingdom at the time of the study. However, the same is also true for a number of the earlier studies that have reported no difference between donor types in terms of post-DLI GVHD, and is therefore unlikely to have been a significant variable in the currently observed differences.
The toxicity developing following the initial DLI dose limited escalation in the unrelated donor cohort. However, the majority of those with matched related donors were eligible for dose escalation. Durable disease responses were not obtained following the lowest dose of DLI (1 × 106/kg; Table 5) suggesting that a higher starting dose may be appropriate in this group with matched related donors treated for residual/progressive disease, although the toxicity of such doses at earlier time points is likely to be higher (as illustrated in Figure 1A for the higher doses given at relatively early time points). Conversion of chimerism status was documented at the lowest doses and the use of such doses may therefore be more appropriate if this is the desired therapeutic outcome. There was a trend toward less GVHD in recipients of matched related transplants receiving lower initial DLI doses, and the severity of GVHD developing in this group also appeared lower, supporting the principle of dose escalation as a means of limiting DLI toxicity.
The lack of graft hypoplasia in the current study probably reflects the underlying disease diagnoses and the use of serial multilineage chimerism analyses to act as a trigger for intervention with DLI (hypoplasia being most commonly reported in patients treated for myeloid malignancies and in circumstances when there is a lack of residual donor hematopoiesis). The majority of evaluable patients (86%) documented to be mixed chimeras prior to DLI converted to full donor chimeric status in all lineages and in many of the sibling DLI recipients this occurred without the development of GVHD, confirming the ability to dissociate graft-versus-lymphohematopoietic system effects from GVHD (Figures 2-3). A further 6% converted to full donor chimeric status in the granulocyte but not T-lymphocyte lineage. As in all studies of DLI it can be difficult to dissociate tumor responses following GVHD induction from the effects of GVHD treatment, particularly with the use of steroids in lymphoid malignancies. However, the demonstration of durable responses that exceeded the response durations seen both after transplantation and following previous chemotherapy cycles in some patients suggests that immune-mediated effects are contributory in many cases, particularly those in the heavily pretreated cohort of patients with Hodgkin lymphoma. Although a minority of disease responses was documented without coincident development of GVHD, there was a clear association between the induction of response and the development of alloreactive toxicity.
The existence of significant graft-versus-Hodgkin lymphoma activity following allogeneic transplantation has proven difficult to establish firmly. The particularly high TRM rates associated with this treatment modality in patients with Hodgkin lymphoma have resulted in relatively low numbers of patients being evaluable for relapse incidence according to whether or not they developed GVHD. The available data from 2 large registry-based retrospective studies suggest that relapse rates may be lower in patients developing GVHD compared to allogeneic recipients without GVHD or autologous transplant recipients.21,22 In addition, several case reports of disease responses following DLI have added further support to the existence of a graft-versus-Hodgkin effect.23 The current data add to this evidence and suggest that durable responses may be achievable although further follow-up and larger numbers of patients are required to confirm this.
The response rates in the patients with myeloma are consistent with those previously reported following conventional allogeneic transplantation.24-26 The outcomes illustrate the lack of durability of responses in the majority of cases with myeloma and the difficulty in separating graft-versus-myeloma from GVH activities with current approaches to the application of DLI. Dose escalation of DLI allowed separation of graft-versus-tumor effects from GVHD in only a minority of the patients with lymphoid neoplasms described in this study, unlike earlier studies in CML. The approach may have limited toxicity in the sibling cohort, but direct comparison with a bulk DLI protocol is required to substantiate this.
Interestingly, both GVHD and tumor responses developed in 2 patients with persisting mixed chimerism, although both converted to full donor status in the granulocyte lineage. The development of GVHD and graft-versus-tumor effects in the presence of mixed T-cell chimerism has been reported previously.27 However, conversion to full donor status occurred in all patients with GVHD by a median of 76 days,27 as occurred in the majority of patients in the current study. Conversion to full donor chimerism had neither a very high positive or negative predictive value for disease response. Similarly, the presence of mixed chimerism prior to DLI did not appear to be strongly predictive of the development of GVHD. In addition, subsequent relapse or progression usually occurred in the presence of persisting full donor chimerism. Whether the chimerism status of tissue-restricted APCs may be more predictive merits further evaluation. The data reinforce the need to remain cautious about extrapolation of findings in murine models to patients, although it is clear that such studies can be instructive about the immune processes involved. Further investigations of allogeneic approaches in Hodgkin lymphoma are clearly warranted, but the need for the development of more refined, antigen-specific strategies remains critical for more successful application in many malignancies.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-05-1513.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal