Abstract
Recently, it was demonstrated that transfection of dendritic cells (DCs) with tumor-derived RNA can elicit effective T-cell responses. This technique does not require the definition of the tumor antigen or HLA haplotype of the patients. We applied this approach to induce HLA class I– and class II–restricted T-cell responses directed against malignant cells from patients with chronic lymphocytic leukemia (B-CLL). Here, we show that DCs generated from monocytes of patients with B-CLL induce leukemia-specific cytotoxic and proliferative T-cell responses on transfection with total RNA isolated from autologous leukemic B lymphocytes. Standard 51Cr-release assays showed specific major histocompatibility complex (MHC) class I–restricted cytotoxic activity against the autologous leukemic B cells and DCs transfected with CLL-RNA, whereas nonmalignant B cells were spared. The specificity of the cytotoxic T-lymphocyte (CTL) response was confirmed using cold target inhibition assays and by blocking HLA class I molecules. Furthermore, we established a protocol for the amplification of whole B-CLL mRNA. The use of DCs transfected with in vitro amplified B-CLL mRNA elicited specific T-cell responses similar to the results obtained with native mRNA. These data suggest that vaccinations using DCs transfected with RNA might be a potent new strategy in the treatment of CLL.
Introduction
Chronic lymphocytic leukemia (CLL) remains largely incurable despite major advancements in our understanding of the pathology and treatment of CLL and its variants.1-4 New drugs like the purine analogues5-7 and the monoclonal antibodies rituximab8,9 and alemtuzumab (Campath-1H; Medac-Schering, Munich, Germany)10-13 have demonstrated to be more active than conventional therapy in achieving complete remission and a prolonged remission duration in this disease. Aggressive treatments with curative intention such as high-dose chemotherapy14-17 or nonmyeloablative allogeneic transplantation18-21 for selected high-risk patients are currently under investigation in randomized clinical trials. However, even after the achievement of a complete clinical and molecular remission most of the patients will eventually relapse, and it remains unclear whether some of these patients will actually be cured.
There are several aspects deduced from recent studies that make CLL a very attractive candidate for the application of immunotherapeutic treatment strategies. It is usually a slowly growing malignancy, giving the time to allow the induction of immune responses against the malignant cells on vaccinations.1-3 Although a number of defects and alterations have been characterized in T cells from patients with CLL,22-25 it has been previously demonstrated that autologous cytotoxic and proliferative T-cell responses directed against the CLL cells can be generated in vitro and that these T cells appear to be functionally active and capable of killing the malignant cells.26,27 According to these reports, it seems likely that a potent stimulus and an enhanced antigen presentation provided by professional antigen-presenting cells might overcome the observed T-cell alterations and possible tolerance mechanisms described in patient with CLL. Furthermore, there is evidence that CLL appears to represent a good target for a graft-versus-leukemia effect following allogeneic stem cell transplantation.21,28,29
Dendritic cells (DCs) are recognized as the most powerful antigen-presenting cells capable of the induction of primary immune responses.30-32 The development of protocols for the ex vivo generation of DCs33,34 led to the design and clinical application of vaccination therapies using DCs pulsed with antigenic synthetic peptides derived from tumor-associated antigens (TAAs).35-37 However, this approach, which was shown to be effective as a means to induce clinical and immunologic responses in cancer patients, is limited to defined antigens and certain HLA types. So far, there are only a few target antigens like survivin or MUC1 that have been characterized in CLL cells.38,39 In addition, whole-tumor strategies that can be applied without the knowledge of expressed tumor antigens were developed by using DCs loaded with dying tumor cells, heat shock proteins or exosomes, and DCs fused with tumor cells.40-42 Alternatively, gene-based delivery using recombinant viruses or liposomes can be used to introduce a TAA into the processing and presenting machinery of DCs, resulting in the induction of a protective antitumor immune response.43-45
Another effective way to deliver antigens into DCs is the transfection with mRNA coding for a certain TAA or with total tumor RNA. This approach was shown to be capable of eliciting potent tumor-specific cytotoxic T-cell responses against a broad variety of malignant cells.45-51 Additional induction of HLA class II–restricted T-cell responses might further increase and prolong the effects of the generated cytotoxic T lymphocytes. In contrast to other whole-tumor vaccines using tumor cell lysates or dead cells, RNA transfection could be performed even in patients with small tumors by simply amplifying the RNA templates isolated from few malignant cells.48,49
In our study, we analyzed the efficiency of RNA-transfected DCs to generate leukemia-specific autologous T-cell responses in vitro and show that monocyte-derived DCs transfected with total tumor RNA or PCR-amplified RNA derived from CLL cells are able to elicit potent autologous cytotoxic and proliferative T-cell responses. These results indicate that both functional DCs and T cells can be generated from the peripheral blood of patients with CLL. We demonstrate that the DCs of patients with CLL are capable of the induction of CLL-directed CD8- and CD4-mediated immunity. The induced cytotoxic T lymphocyte (CTL) and T-helper cells recognized autologous unstimulated CLL cells and DCs transfected with the RNA isolated from autologous malignant cells while ignoring autologous nonmalignant B lymphocytes or DCs transfected with irrelevant RNA. Furthermore, these CTLs recognized autologous DCs transfected with RNA derived from different patients with CLL, indicating that their cytotoxic activity was directed against antigens shared among the malignant leukemic cells. Taken together, our results show that transfection of DCs with RNA isolated from CLL cells may overcome the immune defects and alterations described in the antigen-presenting and effector pathways of patients with CLL and represent a promising option to design CLL-specific immunotherapies.
Materials and methods
Cell isolation
CD19+ B cells were isolated from nonadherent peripheral blood mononuclear cells (PBMNCs) from patients with CLL using the B Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the protocol provided by the manufacturer. CD5+CD19+ peripheral leukemic lymphocytes (CLL cells) were separated from CD5–CD19+ B cells (nonmalignant) by application of the AntiFITC Multisort Kit (Miltenyi Biotec) after staining the CD19+ cell fraction with a fluorescein isothiocyanate (FITC)–labeled monoclonal antibody (mAb) directed against CD5 (Becton Dickinson, Heidelberg, Germany). Anti-FITC microbeads were removed by using the magnetically activated cell sorter (MACS) MultiSort Release Reagent provided by the manufacturer before using the cells for RNA preparation or in standard 51Cr-release and proliferation assays. Cell populations were characterized by flow cytometry after isolation procedures, and purity was routinely found to be approximately 95%. CLL cells and nonmalignant B cells were cryopreserved at –80°C. Prior to their use in 51Cr-release or proliferation assays cells were thawed and grown in RP10 (RPMI 1640 with glutamate supplemented with 10% heat-inactivated fetal calf serum [FCS], 50 μM 2-mercaptoethanol and antibiotics) medium for 24 hours.
Generation of DCs from adherent PBMNCs from patients with CLL
Generation of DCs from peripheral blood monocytes was performed as described previously.36,52 In brief, PBMNCs were isolated by Ficoll/Paque (BD Falcon, Heidelberg, Germany) density gradient centrifugation of 50 mL heparinized blood obtained from patients with CLL treated at the University of Tübingen. Cells were seeded (1 × 107 cells/3 mL/well) into 6-well plates (Corning, Cambridge, MA) in RP10 medium. After 2 hours of incubation at 37°C, the nonadherent cells were removed and cryopreserved at –80°C to be used later for cell isolation. The adherent blood monocytes were cultured in RP10 medium supplemented with the following cytokines: human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; Leukomax; 100 ng/mL; Novartis, Nürnberg, Germany) and interleukin 4 (IL-4; 1000 IU/mL; R&D Systems, Wiesbaden, Germany) for the generation of immature DCs. For maturation, DCs were additionally cultured with tumor necrosis factor-alpha (TNF-α; 10 ng/mL; R&D Systems) for 24 hours after day 6. The phenotype of DCs was analyzed by flow cytometry (FACSCalibur; Becton Dickinson) after 7 days of culture.
Immunostaining
Cell staining was performed using FITC- or phycoerythrin (PE)–conjugated mouse monoclonal antibodies against CD86, CD40 (PharMingen, Hamburg, Germany), CD4, CD5, CD8, CD14, CD19, CD20, CD54, CD56, CD80, HLA-DR (Becton Dickinson), CD83 (Coulter-Immunotech, Hamburg, Germany), and CD1a (DAKO Diagnostics, Hamburg, Germany). Appropriate mouse immunoglobulin G (IgG) isotypes were used as controls (Becton Dickinson). The samples were analyzed by flow cytometry.
RNA isolation
Total RNA was isolated from cell lysates using RNeasy Maxi anion-exchange spin columns (Qiagen, Hilden, Germany) according to the protocol for isolation of total RNA from animal cells provided by the manufacturer. Quantity and purity of RNA was determined by UV spectrophotometry.
RNA amplification
Amplification of RNA derived from CLL cells was performed as previously described.49
Electroporation of DCs
Prior to electroporation on day 6, immature DCs were washed twice with serum-free X-VIVO 20 medium (BioWhittaker, Walkersville, MD) and resuspended to a final concentration of 2 × 107 cells/mL. Subsequently, 200-μL cell suspension was mixed with 10 μg total tumor RNA or polymerase chain reaction (PCR)–amplified mRNA, respectively, and electroporated in a 4-mm cuvette using an Easyject Plus unit (Peqlab, Erlangen, Germany). The physical parameters used were as follows: voltage of 300 V, capacitance of 150 μF, resistance of 1540 Ω, and pulse time of 231 ms. After electroporation the cells were immediately transferred into RP10 medium and returned to the incubator.
Induction of CLL-specific cytotoxic T lymphocytes using DCs transfected with total RNA derived from CLL leukemic lymphocytes
DCs were transfected with CLL RNA by using electroporation as described in “Electroporation of DCs.” After transfection, DCs were incubated for 24 hours in RP10 medium containing 10 ng/mL TNF-α for maturation of DCs. For CTL induction, 5 × 105 DCs (transfected with CLL RNA) were incubated with 2.5 × 106 autologous PBMNCs in RP10 medium. Additional DCs transfected with CLL RNA were stored at –80°C to be used later for restimulation or as target cells. After 7 days of culture, cells were restimulated with autologous CLL RNA-transfected DCs, and 2 ng/mL human recombinant IL-2 (R&D Systems) was added on days 1, 3, and 5. The cytolytic activity of induced DCs was analyzed on day 5 after restimulation in a standard 51Cr-release assay.
CTL assay
The standard 51Cr-labeled release assay was performed as described.34 Target cells were pulsed with 50 μg/mL synthetic peptide for 2 hours and labeled with 51Cr sodium chromate in RP10 medium for 1 hour at 37°C. Target cells (104) were transferred to a well of a round-bottomed 96-well plate. Varying numbers of CTLs were added to give a final volume of 200 μL and incubated for 4 hours at 37°C. At the end of the assay, supernatants (50 μL/well) were harvested and counted in a beta-plate counter. The percentage of specific lysis was calculated as 100 × (experimental release – spontaneous release)/(maximal release – spontaneous release). Spontaneous and maximal releases were determined in the presence of either RP10 medium or 2% Triton X-100, respectively. Antigen specificity of tumor cell lysis was further assessed in a cold target inhibition assay by analyzing the capacity of “cold” (unlabeled) CLL cells to block lysis of “hot” (labeled) CLL cells and by blocking HLA class I molecules using a monoclonal antibody (W06/32, 20 μg/mL; generously provided by S. Stevanovič,Tübingen, Germany).
IFN-γ ELISPOT assay
Cytotoxic T cells from patient no. 1 generated in vitro using autologous DCs transfected with CLL-RNA were incubated at a concentration of 2 × 105 cells/well in an antihuman interferon γ (IFN-γ) antibody (mAb 1-D1K, 10 μg/mL; Mabtech AB, Hamburg, Germany)–coated 96-well plate with autologous irradiated PBMNCs pulsed with the HLA-A2 binding peptides derived from the tumor antigens survivin or MUC1. For the detection of spots, a biotin-labeled antihuman IFN-γ antibody (Mab 7-B6-1-Biotin, 2 μg/mL; Mabtech AB) was used. Spots were counted after an incubation period of 36 hours by using an automated enzyme-linked immunospot (ELISPOT) reader (IMMUNOSPOT ANALYZER; CTL Analyzers LLC, Cleveland, OH).
Synthetic peptides
The HLA-A2 binding peptides derived from the tumor antigens survivin (ELTLGEFLKL),53 adipophilin (SVASTITGV),54 telomerase (ILAKFLHWL),55 and MUC1 (LLLLTVLTV)52 were synthesized by using standard Fmoc chemistry on a peptide synthesizer (432A; Applied Biosystems, Weiterstadt, Germany) and analyzed by reversed-phase high-performance liquid chromatography (HPLC) and mass spectrometry.
Induction of CLL-specific T-helper cells (CD4+) using DCs transfected with CLL RNA
CD4+ T lymphocytes were isolated from PBMNCs by using the CD4+ T-Cell Isolation Kit (Miltenyi Biotec). Purity of CD4+ T cells was assessed after isolation by flow cytometry. DCs were transfected with CLL total RNA by electroporation on day 6 as described in “Induction of CLL-specific cytotoxic T lymphocytes using DCs transfected with total RNA derived from CLL leukemic lymphocytes.” After transfection, DCs were incubated for 24 hours in RP10 medium containing 10 ng/mL TNF-α for maturation of DCs. For T-helper cell induction, 2.5 × 106 CD4+ T lymphocytes were coincubated with 5 × 105 autologous DCs (transfected with CLL RNA). On days 7 and 14 after T-cell induction, restimulations were performed by using 5 × 105 autologous DCs (transfected with CLL RNA) each time. IL-2 was added every other day following the first restimulation (2 ng/mL). The antigen specificity of the induced CD4-mediated immune response was assessed on day 20 after T-cell induction in a 3H-Thymidine proliferation assay as described in “Proliferation assay.”
Proliferation assay
A total of 2 × 105 responding cells (CD4+ T lymphocytes) were cultured in 96 flat-bottom well microplates (Nunc, Wiesbaden, Germany) with 105 DCs. Stimulation with phorbol 12-myristate 13-acetate (PMA)/Ionomycin was used as a positive control, whereas unstimulated CD4+ T lymphocytes and stimulation with DCs electroporated with irrelevant enhanced green fluorescence protein (EGFP) RNA served as negative controls. Inhibition of HLA class I or class II molecules was achieved by incubating DCs for 1 hour prior to the assay either with the monoclonal antibodies W6/32 (20 μg/mL) directed against HLA-class I molecules or Tü39 (20 μg/mL) directed against HLA class II molecules (both antibodies were kindly provided by S. Stevanovič, Tübingen, Germany). Thymidine incorporation was measured on day 5 by a 16-hour pulse with 3H-thymidine (1 μCi/well [0.037 MBq/well]; Amersham Life Science, Braunschweig, Germany).
Results
Induction of CLL-specific CTL using RNA-transfected DCs
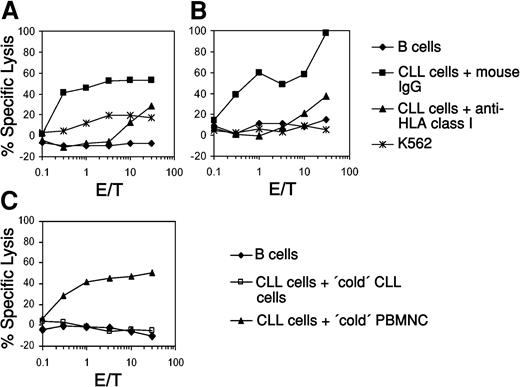
To analyze the induction of autologous CTL responses against CLL in vitro we generated monocyte-derived DCs from patients with CLL and used them as antigen-presenting cells after transfection with total tumor RNA isolated from autologous leukemic lymphocytes. As shown in Figure 1, transfection of DCs generated from 2 patients with total RNA resulted in the induction of CTL that recognized the malignant autologous CLL cells but spared the nonmalignant purified autologous B lymphocytes in a standard 51Cr-release assay (Figure 1A, patient no. 1; Figure 1B, patient no. 2). The lysis of CLL cells could be blocked by a monoclonal antibody directed against HLA class I molecules, demonstrating that the cytolytic activity of the in vitro–induced CTL was HLA class I restricted. There was no lysis of K562 cells, demonstrating that the induced cytolytic activity was not natural killer (NK) cell mediated. To further analyze the specificity of these CTLs, we performed cold target inhibition assays. As demonstrated in Figure 1C, the lysis of autologous CLL cells (pulsed with 51Cr, hot targets) could be blocked by addition of unpulsed autologous CLL cells (cold targets) but not by nonmalignant autologous peripheral blood mononuclear cells.
Induction of autologous CLL-specific CTL responses by DCs transfected with whole-tumor RNA. Immature DCs generated from the peripheral blood of 2 patients with CLL (A, patient no. 1; B, patient no. 2) were electroporated with 10 μg whole-tumor RNA isolated from autologous malignant B cells. After further incubation with 10 ng/mL TNF-α for 24 hours DCs were used as APCs for in vitro CTL induction. Cytolytic activity of the CTLs was determined on day 5 after the last restimulation by using autologous purified leukemic cells or nonmalignant B lymphocytes as targets in a standard 51Cr-release assay. Blocking of HLA class I molecules with the monoclonal antibody W06/32 was performed to analyze the HLA class I restriction of the elicited CTL responses. K562 tumor cells were included to determine the NK cell activity. A cold target inhibition assay was performed to further assess the specificity of the CTLs induced with the DCs from patient no. 1 (C). The lysis of autologous CLL cells (pulsed with 51Cr, hot targets) could be blocked by addition of unpulsed autologous CLL cells (cold targets) but not by nonmalignant autologous peripheral blood mononuclear cells (PBMNCs).
Induction of autologous CLL-specific CTL responses by DCs transfected with whole-tumor RNA. Immature DCs generated from the peripheral blood of 2 patients with CLL (A, patient no. 1; B, patient no. 2) were electroporated with 10 μg whole-tumor RNA isolated from autologous malignant B cells. After further incubation with 10 ng/mL TNF-α for 24 hours DCs were used as APCs for in vitro CTL induction. Cytolytic activity of the CTLs was determined on day 5 after the last restimulation by using autologous purified leukemic cells or nonmalignant B lymphocytes as targets in a standard 51Cr-release assay. Blocking of HLA class I molecules with the monoclonal antibody W06/32 was performed to analyze the HLA class I restriction of the elicited CTL responses. K562 tumor cells were included to determine the NK cell activity. A cold target inhibition assay was performed to further assess the specificity of the CTLs induced with the DCs from patient no. 1 (C). The lysis of autologous CLL cells (pulsed with 51Cr, hot targets) could be blocked by addition of unpulsed autologous CLL cells (cold targets) but not by nonmalignant autologous peripheral blood mononuclear cells (PBMNCs).
Antigens expressed in CLLs are shared among the leukemic cells
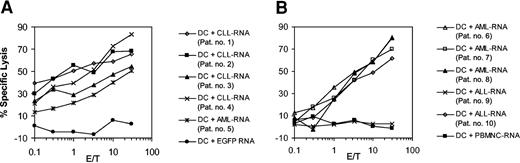
To determine whether the epitopes recognized by CTL lines induced with RNA-transfected DCs are also expressed and presented by CLL cells obtained from different donors we used autologous DCs that were transfected with the whole-tumor RNA isolated from leukemic cells of several patients as targets in a standard 51Cr-release assay. As demonstrated in Figure 2A, the CTLs efficiently lysed autologous DCs transfected with the cognate RNA. However, they were also capable of recognizing autologous DCs transfected with RNA derived from 3 other patients with CLL and from one patient with acute myelogenous leukemia (AML), indicating that shared tumor-associated antigens might contribute to the observed cytotoxicity. In a subsequent experiment we used autologous DCs electroporated with whole-tumor RNA derived from 3 patients with AML and 2 patients with acute lymphoblastic leukemia (ALL) as target cells. As depicted in Figure 2B, the CTLs efficiently lysed DCs electroporated with AML (3 of 3 samples) or ALL RNA (1 of 2 samples) but spared autologous DCs electroporated with RNA derived from allogeneic PBMNCs, showing that the observed cross-reactivity was not due to presentation of allo-MHC.
Cross-reactivity of the induced leukemic CTLs. Immature monocyte-derived DCs generated from the peripheral blood of patient no. 1 were electroporated with 10 μg total tumor RNA isolated from CLL cells. After further incubation with 10 ng/mL TNF-α for 24 hours DCs were used as APCs for in vitro CTL induction. Cytolytic activity of the CTL was determined on day 5 after the last restimulation by using autologous monocyte-derived DCs transfected with autologous CLL-RNA or RNA isolated from 3 other patients with CLL (patients no. 2 to no. 4) and from one patient with AML (patient no. 5) as targets in a standard 51Cr-release assay (A). DCs electroporated with in vitro–transcribed EGFP RNA were included as a control. The cytolytic activity of CTLs induced from patient no. 1 in a similar fashion was tested in a subsequent experiment (B) against autologous DCs electroporated with RNA isolated from 3 patients with AML (patients no. 6 to no. 8) and from 2 patients with ALL (patients no. 9 and no. 10). Autologous DCs electroporated with allogeneic PBMNC-RNA were used as a control to exclude allo-MHC reactivity.
Cross-reactivity of the induced leukemic CTLs. Immature monocyte-derived DCs generated from the peripheral blood of patient no. 1 were electroporated with 10 μg total tumor RNA isolated from CLL cells. After further incubation with 10 ng/mL TNF-α for 24 hours DCs were used as APCs for in vitro CTL induction. Cytolytic activity of the CTL was determined on day 5 after the last restimulation by using autologous monocyte-derived DCs transfected with autologous CLL-RNA or RNA isolated from 3 other patients with CLL (patients no. 2 to no. 4) and from one patient with AML (patient no. 5) as targets in a standard 51Cr-release assay (A). DCs electroporated with in vitro–transcribed EGFP RNA were included as a control. The cytolytic activity of CTLs induced from patient no. 1 in a similar fashion was tested in a subsequent experiment (B) against autologous DCs electroporated with RNA isolated from 3 patients with AML (patients no. 6 to no. 8) and from 2 patients with ALL (patients no. 9 and no. 10). Autologous DCs electroporated with allogeneic PBMNC-RNA were used as a control to exclude allo-MHC reactivity.
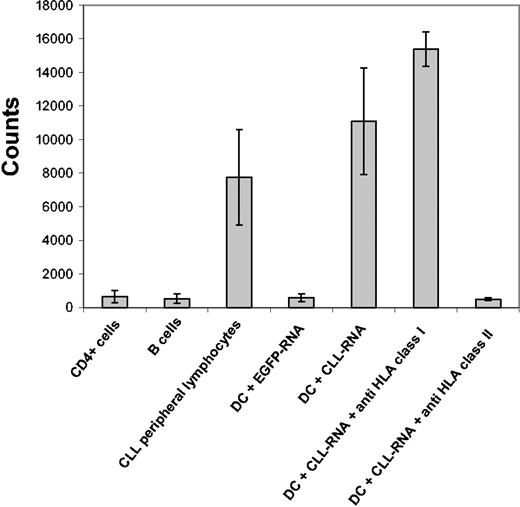
In the next set of experiments we analyzed the specificity of the in vitro–induced CTLs. To test this specificity, we used a CTL line induced from a patient with HLA-A2–positive CLL by RNA-transfected DCs as effector cells and autologous DCs pulsed with different antigenic HLA-A2 binding peptides derived from tumor-associated antigens known to be expressed in CLL and on a broad variety of other malignancies as target cells. As shown in Figure 3A, the CTLs recognized target cells pulsed with a peptide derived from the survivin protein while ignoring other peptide antigens, thus suggesting that survivin-directed cytotoxic activity might contribute to the elicited CTL responses. In line with these results, an expansion of survivin-reactive T cells could be observed in an ELISPOT assay (Figure 3B-C).
Specificity of the CTL responses induced by DCs transfected with CLL-derived RNA. The specificity of CTLs induced with RNA-transduced DCs was analyzed by using autologous DCs from patient no. 1 pulsed with HLA-A2 binding peptides derived from the adipophilin, survivin, MUC1 and telomerase tumor-associated antigens as targets (A). Autologous DCs electroporated with EGFP mRNA were used as a negative control. Survivin-specific IFN-γ production was determined in an IFN-γ ELISPOT assay (B). The number of spots was determined by using an automatic ELISPOT reader (C). Values represent number of spots per 2 × 105 T cells after subtraction of spots counted in wells stimulated with unpulsed PBMNCs (dimethyl sulfoxide [DMSO] control).
Specificity of the CTL responses induced by DCs transfected with CLL-derived RNA. The specificity of CTLs induced with RNA-transduced DCs was analyzed by using autologous DCs from patient no. 1 pulsed with HLA-A2 binding peptides derived from the adipophilin, survivin, MUC1 and telomerase tumor-associated antigens as targets (A). Autologous DCs electroporated with EGFP mRNA were used as a negative control. Survivin-specific IFN-γ production was determined in an IFN-γ ELISPOT assay (B). The number of spots was determined by using an automatic ELISPOT reader (C). Values represent number of spots per 2 × 105 T cells after subtraction of spots counted in wells stimulated with unpulsed PBMNCs (dimethyl sulfoxide [DMSO] control).
Induction of CLL-specific T cells using amplified mRNA
One limitation of the whole-tumor approaches in vaccination studies is the availability of tumor samples, especially in patients with low tumor burden or limited disease. Recently, it was demonstrated that this obstacle can be overcome by using amplified mRNA. We used this methodology to generate CLL-specific CTLs in vitro. Monocyte-derived DCs were electroporated either with whole-tumor RNA or amplified mRNA isolated from autologous CLL cells and used as antigen-presenting cells (APCs) for CTL induction. As shown in Figure 4A-B CLL-specific CTL responses could be obtained with both RNA sources. The cytolytic activity could be blocked by anti–HLA class I antibody but not by anti–HLA class II antibody, showing that the induced CTLs were entirely HLA class I restricted.
Induction of CTLs in vitro by using amplified CLL RNA. The cytotoxicity of CTLs induced with autologous DCs that were electroporated with native total CLL RNA or amplified CLL mRNA isolated from autologous CLL cells was analyzed in a standard 51Cr-release assay by using autologous purified leukemic cells or nonmalignant B lymphocytes as targets. Inhibition of HLA class I or class II molecules was performed by incubating DCs for 1 hour prior to the assay either with the mAb W06/32 (directed against HLA class I) or Tü39 (directed against HLA class II molecules).
Induction of CTLs in vitro by using amplified CLL RNA. The cytotoxicity of CTLs induced with autologous DCs that were electroporated with native total CLL RNA or amplified CLL mRNA isolated from autologous CLL cells was analyzed in a standard 51Cr-release assay by using autologous purified leukemic cells or nonmalignant B lymphocytes as targets. Inhibition of HLA class I or class II molecules was performed by incubating DCs for 1 hour prior to the assay either with the mAb W06/32 (directed against HLA class I) or Tü39 (directed against HLA class II molecules).
Induction of CD4+ T-helper lymphocytes using RNA-transfected DCs
We next analyzed the possible induction of a CD4-mediated T-helper cell response by RNA-transfected DCs. CD4+ T lymphocytes were isolated by using magnetic bead technology and stimulated with autologous DCs transfected with total RNA isolated from autologous CLL cells. As demonstrated in Figure 5, 3H-thymidine proliferation assays performed after 2 restimulations revealed a CLL-specific proliferation pattern. The induced proliferative response could be blocked by using antibodies directed against HLA class II but not with monoclonal antibody against HLA class I molecules, thus demonstrating that the induced T-cell population was entirely HLA class II restricted.
Induction of proliferative T-helper cell responses by DCs transfected with CLL-derived RNA. CD4+ T lymphocytes were isolated by using magnetic bead technology. T-helper cell induction was performed by coincubating DCs electroporated with total RNA isolated from CLL cells and autologous CD4+ T lymphocytes. Two restimulations with autologous RNA-transfected DCs were performed on days 7 and 14 after T-cell induction. The induced proliferation was determined by a 16-hour pulse with 3H-thymidine and subsequent measurement of thymidine incorporation. Autologous unstimulated CLL cells, B lymphocytes, or monocyte-derived DCs electroporated with the cognate CLL RNA were used as stimulating cells. Unstimulated CD4+ T lymphocytes and stimulation with DCs electroporated with EGFP RNA served as negative controls. Inhibition of HLA class I or class II molecules was performed by incubating DCs for 1 hour prior to the assay either with the mAb W06/32 (directed against HLA class I) or Tü39 (directed against HLA class II molecules).
Induction of proliferative T-helper cell responses by DCs transfected with CLL-derived RNA. CD4+ T lymphocytes were isolated by using magnetic bead technology. T-helper cell induction was performed by coincubating DCs electroporated with total RNA isolated from CLL cells and autologous CD4+ T lymphocytes. Two restimulations with autologous RNA-transfected DCs were performed on days 7 and 14 after T-cell induction. The induced proliferation was determined by a 16-hour pulse with 3H-thymidine and subsequent measurement of thymidine incorporation. Autologous unstimulated CLL cells, B lymphocytes, or monocyte-derived DCs electroporated with the cognate CLL RNA were used as stimulating cells. Unstimulated CD4+ T lymphocytes and stimulation with DCs electroporated with EGFP RNA served as negative controls. Inhibition of HLA class I or class II molecules was performed by incubating DCs for 1 hour prior to the assay either with the mAb W06/32 (directed against HLA class I) or Tü39 (directed against HLA class II molecules).
Discussion
In our study we analyzed the efficiency of RNA-transfected DCs to elicit autologous CLL-specific T-cell mediated immune responses. Monocyte-derived DCs were generated from peripheral blood of patients with CLL and used as antigen-presenting cells for generation of cytotoxic and proliferative T-cell responses on transfection with RNA isolated from malignant CLL cells.
We show that RNA transfection of DCs is a feasible approach capable of the induction of HLA class I– and HLA class II–restricted immune responses. The in vitro–generated cytotoxic T cells exhibited leukemia-specific killing of autologous nonactivated malignant cells while sparing the nonmalignant autologous B lymphocytes and DCs. The specificity of the observed cytotoxic activity could be confirmed in cold target inhibition assays. The lysis of CLL cells could be blocked by MHC class I mAbs, indicating that HLA class I–restricted epitopes are presented and recognized by autologous CTLs. To further analyze the feasibility and possible use of RNA-transfection in the setting of low tumor burden or minimal residual disease we established a method to amplify the isolated tumor RNA.49 In line with previous studies39,49 we found that by using this approach autologous CLL-specific CTL responses can be generated in vitro.
Another important finding of our study is the demonstration that electroporation of DCs with whole-tumor RNA can elicit HLA class II–mediated T-cell responses. Similar to the results obtained with HLA class I–restricted CTLs the induced CD4+ T cells were entirely HLA class II restricted. This observation that cytosolic antigens can access the MHC class II pathway is somewhat contradictory to the classic concepts of antigen presentation but in line with previous reports demonstrating that cytosolic proteins can be processed for MHC class II presentation. So far, several possible pathways were presented and analyzed. However, the underlying mechanisms involved are still elusive. MHC class II–restricted presentation of endogenous antigens was shown to depend on the actions of cytoplasmatic proteases, including proteasomal and nonproteasomal proteolysis, and was independent of endosomal acidification.56,57 More recently, another pathway for the MHC class II presentation of a cytosolic antigen by autophagy was introduced.58 This presentation pathway involves the uptake of the proteins into autophagosomes and their subsequent processing in the endosomal/lysosomal compartment, in which the cytoplasmatic degradation and turnover of cellular proteins by autophagy converges with MHC class II–restricted presentation.
Our results demonstrate that functionally active antigen-presenting cells (DCs) and T lymphocytes capable of recognizing autologous leukemic B cells can be generated in patients with CLL despite the described immune alterations and peripheral T-cell tolerance in tumor-bearing hosts,22-25 thus suggesting that efficient delivery and processing of antigens and their enhanced presentation by professional APCs may overcome the immunologic defects in CLL on the level of antigen presentation and effector functions.
Interestingly, when different HLA-A2–matched malignant CLL cells were used as targets in the 51Cr-release assay, we found that the CTLs obtained after several restimulations with RNA-transfected DCs recognized not only the autologous CLL cells but also autologous DCs transfected with RNA derived from leukemic cells of different patients with CLL, thus indicating that at least some of the antigens that contribute to the observed lytic activity are shared among these cells.
In the next set of experiments we analyzed the specificity of the in vitro–induced CTLs by using autologous DCs pulsed with HLA-A2–binding peptides derived from tumor-associated antigens known to be expressed in a broad variety of malignancies, including CLL, as targets in a standard 51Cr-release assay. Surprisingly, we found that these polyclonal CTL lines induced with whole RNA-transfected DCs were able to recognize target cells loaded with a survivin peptide while sparing cells pulsed with synthetic peptides deduced from the telomerase or MUC1 antigens, indicating that cytotoxic activity directed against the survivin protein contributes to the observed lytic activity of the CTL lines. We have recently shown that autologous survivin-specific CTLs capable of recognizing autologous malignant cells can be induced in patients with CLL,39 thus indicating that survivin is an interesting tumor rejection antigen suitable for the design of immunotherapeutic approaches in the treatment of CLL.
In summary, our data demonstrate that specific autologous CD8- and CD4-mediated T-cell responses can be generated in vitro by using DCs transfected with whole-tumor RNA. These results may provide the rationale for the development of novel vaccination strategies in this disease.
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2003-06-2097.
Supported by grants from the Deutsche Forschungsgemeinschaft, the Deutsche Krebshilfe, and the Fortüne Program of the University of Tübingen.
M.R.M. and G.T. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Sylvia Stephan and Bruni Schuster for excellent technical assistance.

![Figure 3. Specificity of the CTL responses induced by DCs transfected with CLL-derived RNA. The specificity of CTLs induced with RNA-transduced DCs was analyzed by using autologous DCs from patient no. 1 pulsed with HLA-A2 binding peptides derived from the adipophilin, survivin, MUC1 and telomerase tumor-associated antigens as targets (A). Autologous DCs electroporated with EGFP mRNA were used as a negative control. Survivin-specific IFN-γ production was determined in an IFN-γ ELISPOT assay (B). The number of spots was determined by using an automatic ELISPOT reader (C). Values represent number of spots per 2 × 105 T cells after subtraction of spots counted in wells stimulated with unpulsed PBMNCs (dimethyl sulfoxide [DMSO] control).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/5/10.1182_blood-2003-06-2097/6/m_zh80050457280003.jpeg?Expires=1764972542&Signature=KnB0FTolkaDrffgtYbH9Audp5WdvNrdb7~m6Rfwedxt7U23rDe7TDQDPBVHQuJo1FgiP8YH-8HN2MU63eWOioG5kZnfuAOZqT~5N6am4U23dISveadrx6bfOJp0elCgdzBgILet2w7YukU6sQWVH9Bw~XVdK0sr6WxJdrRB7H~B8YV6KntXXAEaKFhJ79w0keYRQG2co2PiHJtU9CMD-Q4QOap7SMJQ710EPkiYzTuTUQ5ntCL0K9WO7-CUh3-SImo5htMO3axLlGFK6iJk8znFpHVZ9Dwv55IccmYFU7yVocpbiQTqAKDaN8tZae4NsHTqgQyOsjZweNnzdkOmfnQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal