By all accounts, P-selectin and von Willebrand factor (VWF) have been dating for quite some time. Both glycoproteins can be found in the same location (namely, Weibel-Palade [WP] bodies of endothelial cells and α-granules of platelets), they travel together (exported to the cell surface), and they share the same activities (both can mediate platelet rolling and participate in hemostasis). The word on a consummated relationship recently became apparent when it was shown that mice lacking VWF displayed reduced P-selectin–mediated leukocyte rolling in calcium ionophore–stimulated mesenteric venules.1 In addition, transfection of VWF into a cell line that expressed P-selectin, but not VWF, induced formation of WP-like structures and sorting of P-selectin into these granules.2 Although the interpretation of the data is confounded by the fact that VWF is necessary for the formation of WP bodies, the results suggested that VWF multimers might somehow be important in the storage of P-selectin in these organelles.
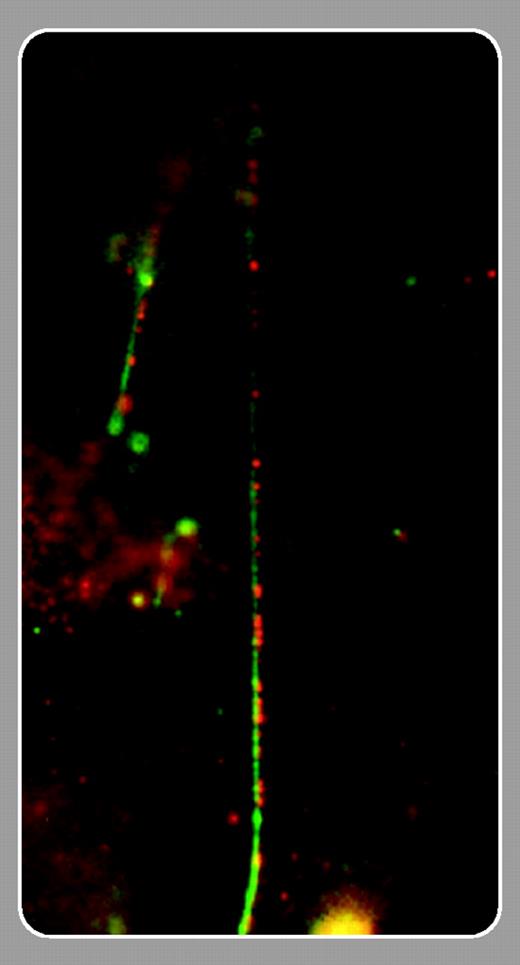
VWF is secreted through either a constitutive or a regulated pathway. Constitutively secreted VWF, consisting of dimers or low-molecular-weight multimers, is rapidly transported toward the plasma membrane. High-molecular-weight (ultralarge) VWF (ULVWF) is stored in WP bodies. These ULVWF multimers are highly active and may lead to platelet aggregation and thrombus formation when secreted without-further alteration. Proteolytic processing of VWF by ADAMTS13, a metalloproteinase deficient in thrombotic thrombocytopenic purpura (TTP), likely prevents spontaneous thrombosis. Previous studies by Dong et al revealed that ULVWF multimers can form long strings on endothelial cells that are efficiently cleavable by ADAMTS13 under shear stress.3 In this issue of Blood, Padilla and colleagues (page 2150) show that these VWF strings are anchored to the endothelial surface by clustered P-selectin, which appears to tie the strings by forming a series of small P-selectin and VWF-enriched “knots.” A direct interaction between P-selectin and VWF is strongly suggested by adhesion assays, colocalization using immunofluorescence staining, and coimmunoprecipitation experiments. Strikingly, string formation was completely prevented by incubation of endothelial cells with anti–P-selectin antibody or soluble P-selectin. Whether these strings play a role in the pathogenesis of TTP remains to be demonstrated, however. These results nevertheless raise the interesting possibility of therapeutic interventions in TTP directed at inhibiting P-selectin. In addition, ULVWF presented by endothelial P-selectin may conceivably contribute to some P-selectin–mediated plateletendothelial interactions, although platelet translocation on VWF in vivo was shown to be independent of P-selectin.4
The study is also notable because P-selectin had only one major physiologic counterreceptor, P-selectin glycoprotein ligand-1 (PSGL-1), which interacts through the coordinated presentation of sialyl Lewis X on O-glycans in the context of sulfated tyrosines. The restricted binding to ULVWF suggests a cryptic binding site for P-selectin within ULVWF. Carbohydrates comprise a significant fraction (about 20%) of the molecular weight of VWF; the VWF amino acid sequence indeed contains 22 probable glycosylation sites, of which 10 are O-glycans.5 Although the authors' results suggest that the interaction between P-selectin and VWF occursonly after endothelial activation, the possibility that this interaction plays a role in the physiologic targeting of P-selectin to WP bodies still remains and will undoubtedly be the subject of future studies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal