Abstract
In human follicular lymphoma the t(14; 18) chromosome translocation activates the antiapoptotic oncogene Bcl2 by linking it to the immunoglobulin heavy chain (IGH) locus. Transgenic mice expressing Bcl2 controlled by an Igh enhancer (Eμ) do not develop follicular lymphoma, although they do have an increased incidence of other B-lymphoid neoplasms. We have now analyzed tumorigenesis in mice bearing a Bcl2 transgene controlled by Vav gene regulatory sequences (VavP), which confer expression in multiple hematopoietic lineages. Unlike Eμ-Bcl2 mice, many VavP-Bcl2 mice older than 10 months developed follicular lymphoma. Young VavP-Bcl2 mice had an overabundance of enlarged germinal centers and greatly elevated numbers of cycling B cells that had undergone IgH class switching and V-gene hypermutation. The peripheral T-cell compartment was larger in the VavP-Bcl2 mice than in Eμ-Bcl2 strains and, notably, CD4 T cells were 5-fold increased over normal. The germinal center hyperplasia required CD4 T cells, because it could be abolished by anti-CD4 antibody in vivo. VavP-Bcl2 mice also had a propensity to develop kidney disease of the autoimmune type. We suggest that the increased survival capacity of B and T cells fosters prolonged germinal center reactions, and that autoreactivity and hypermutation conspire to generate follicular lymphoma.
Introduction
Follicular lymphoma is a relatively indolent disease, but ultimately it progresses to a more aggressive phase, which is invariably fatal. Its characteristic t(14;18) chromosome translocation results from linkage of the gene Bcl2 to the immunoglobulin heavy chain (IGH) locus.1-3 Although 75% of follicular lymphoma cases assessed by conventional karyotyping carry the chromosome translocation,4 approximately 90% of cases show high levels of Bcl2 protein. Thus, overexpression of Bcl2 is a hallmark of follicular lymphoma.
Unlike most other oncogenes, Bcl2 promotes cell survival rather than cell proliferation.5 The tumorigenic potential of Bcl2 has been explored in transgenic mouse models that mimic the translocation by expressing the gene in B cells under the control of an Igh enhancer (Eμ).6-9 Enforced expression of Bcl2 was found to increase the risk of B-lymphoid neoplasia, but the incidence was low, and the tumors were pre-B lymphomas, immunoblastic lymphomas, and plasmacytomas rather than follicular lymphomas.
Although Bcl2 activation is a prerequisite for human follicular lymphoma, it does not appear to be sufficient to initiate the tumor, because sensitive polymerase chain reaction (PCR) procedures can detect the t(14;18) translocation at low frequency in the B cells of a substantial proportion of healthy individuals.10,11 The early events leading from the translocation to follicular lymphoma are not clear, and, although the use of cDNA array technology has started to provide some insight,12 this approach is limited by the cellular complexity of the tumor tissue and the possible involvement of cell-cell interactions. A mouse model that closely models the human disease would, therefore, be of significant benefit.
We report here that VavP-Bcl2 transgenic mice,13 engineered to overexpress Bcl2 in cells of all hematopoietic lineages, are highly predisposed to develop follicular lymphoma. We have, therefore, investigated the pretumor phenotype of this new mouse model in the hope of gaining insight into the enigmatic early stages of human follicular lymphoma.
Materials and methods
Mice
C57BL/6J.VavP-Bcl213 and C57BL/6J.Eμ-Bcl2 mouse strains7 were propagated as transgene heterozygotes through matings with healthy C57BL/6J mice. Transgenic offspring were identified at 4 weeks of age from their elevated peripheral blood leukocyte counts, using a Z2 Coulter counter (Beckman Coulter, Fullerton, CA). GK5 mice,14 which transgenically express an antimouse CD4 antibody and consequently lack all peripheral CD4 T cells, were on a C57BL/6By.C-H2bm1/ByJ background. Littermates were used for the analysis of all genotypes. All mice were maintained under specific pathogen free (SPF) conditions, the only pathogen present being an endemic rotavirus.
Pathology
Mice were monitored daily for morbidity and killed when clinically ill. Conventional histopathology was performed on hematoxylin- and eosinstained sections prepared from tumor tissue, thymus, lymph nodes, spleen, kidney, lung, heart, and liver fixed in Bouin solution and decalcified sternum fixed in neutral-buffered 10% formalin. Tumor-invaded tissues, usually spleen and/or lymph nodes, were scored for mitotic activity by counting mitotic figures in 6 microscopic fields per tissue viewed through a 40 × objective.
Statistics
The StatView software package (SAS Institute, Cary, NC) was used for generating Kaplan-Meyer survival plots and performing all statistical analyses.
Flow cytometry and cell sorting
Antibodies used for the surface phenotyping of hematopoietic cells by flow cytometry have been described.15 Cells (106 per analysis) were stained with relevant antibodies labeled with fluorochromes (fluorescein isothiocyanate [FITC], phycoerythrin [PE], or cyanin 5 [Cy5]) or biotin using 1% normal rat serum to block Fc receptors. For intracellular staining the cells were fixed in 1% paraformaldehyde/phosphate-buffered saline (PBS) and permeabilized with 0.3% Saponin (Sigma, St. Louis, MO). Analysis of transgenic Bcl2 expression used biotinylated Bcl2-100 monoclonal antibody.16 Streptavidin conjugated to FITC or PE (Caltag Laboratories, Burlingame, CA) was used as a secondary reagent for biotinylated antibodies. Analyses were performed on a Life Sciences Research (LSR) or a FACStar II flow cytometer (Becton Dickinson, San Jose, CA).
To prepare populations of immunoglobulin class–switched and control B cells, erythrocytes were removed from spleen cell suspensions by lysis in NH4Cl, and the cells were then stained with PE-labeled anti-B220, FITC-labeled anti-IgM and anti-IgD antibodies, and Cy5-labeled anti-κ light chain antibody, washed, filtered through cotton wool to remove aggregates, and stained with propidium iodide to enable dead cell exclusion. B220+ IgM- IgD- κ+ cells and control B220+ IgM+ cells were sorted in a modified MoFlo cell sorter (DakoCytomation, Fort Collins, CO). Total genomic DNA was extracted and used for PCR cloning as detailed in “Hypermutation analysis.”
Immunohistochemistry
Bouin-fixed, paraffin-embedded tissues were sectioned and deparaffinized. Endogenous peroxidase activity and nonspecific antibody binding were blocked by incubation with 3% H2O2 and 10% normal goat serum in PBS, respectively. Primary biotinylated reagents (peanut agglutinin[AI7] [PNA], anti-mouse IgG, and anti-mouse IgM antibodies from Vector, Burlingame, CA; anti-PCNA [proliferating cell nuclear antigen] antibody from BD Biosciences, San Diego, CA), titrated and used at optimized dilutions, were detected by the avidin-biotin-horseradish peroxidase complex (ABC) system (Vector) using horseradish peroxidase. Sections were counterstained with hematoxylin.
BrdU uptake
Mice were fed 5-bromodeoxyuridine (BrdU) at 1 mg/mL in their drinking water (plus 2% sucrose to overcome taste aversion) as described previously17 and analyzed after 1, 3, and 5 days. Spleen cells were stained for surface markers and BrdU, and defined lymphoid subsets were analyzed by flow cytometry as described previously.17,18
Immunoglobulin heavy chain CDR3 spectratyping
Genomic DNA was prepared from B220+ IgM+ cells sorted from C57BL/6 spleen or tumor samples, using proteinase K digestion.19 A DNEasy Tissue Kit (Qiagen, Hilden, Germany) was used to extract genomic DNA from fixed, paraffin-embedded samples. The DNA was subjected to 30 cycles of PCR by using a degenerate primer set (MH1-7, below) designed by Wang et al20 to amplify all mouse VH genes, and a degenerate primer (JHR, below) for JH segments 1 to 4. The MH primer set had been designed for use with cDNA, but adjustment of the annealing temperature enabled amplification of sequences from 11 of 15 (73%) clonal tumors from Eμ-Myc mice, indicating a reduced, but acceptable 5-gene coverage. The PCR was performed in 20 μL by using 2 μL 10 × PCR buffer, 0.8 μL 50 mM MgSO4, 0.5 μL 10 mM deoxynucleotide triphosphates (dNTPs), 6 μL 10 pM MH primer mix, 2 μL 10 pM FAM-labeled JHR primer (GeneWorks, Adelaide, South Australia), and 0.14 μL Taq polymerase (Invitrogen, Carlsbad, CA) for 30 cycles with an annealing temperature of 63°C. The primers were as follows: MH1, 5′-SARGTNMAGCTGSAGSAGTC-3′; MH-2, 5′-SARGTNMAGCTGSAGSAGTCWGG-3′; MH-3, 5′-CAGGTTACTCTGAAAGWGTSTG-3′; MH-4, 5′-GAGGTCCARCTGCAACARTC-3′; MH-5, 5′-CAGGTCCAACTVCAGCARCC-3′; MH-6, 5′-GAGGTGAASSTGGTGGAATC-3′; MH-7, 5′-GATGTGAACTTGGAAGTGTC-3′; JHR, 5′-CCTGMRGAGACDGTGASHRDRGTBCCTKKRCCCC-3′ (using the IUB mixed base nomenclature in the degenerate primers). The PCR product was analyzed in an ABI Prism 377 sequencer (Applied Biosystems, Foster City, CA) using Gene-Scan-500 size markers (Applied Biosystems) in each lane. Graphs were produced by using Genotyper (Applied Biosystems) software.
Hypermutation analysis
Genomic DNA was extracted from immunoglobulin class–switched VavP-Bcl2 B220+ IgM- κ+ splenic germinal center cells and control C57BL/6 B220+ IgM+ splenic B cells. S107 V-gene sequences recombined to JH1 were amplified by using modified primers21 and Expand High Fidelity PCR enzyme (Roche Diagnostics, Mannheim, Germany); VH1E primer, 5′TGGTAATTATGGGCAA-3′; modT15-3 primer, 5′-CCGTTTCAGAATGGAATGTGC-3′. The PCR products were gel purified, cloned into the pGEM-T TA-cloning vector (Promega, Madison, WI), and sequenced using published vector primers. Sequence comparisons were assessed by chi-square tests in the StatView program. Five randomly picked S107 clones obtained from control C57BL/6 B220+ IgM+ cells lacked any mutations, validating the procedure.
Results
VavP-Bcl2 mice develop follicular lymphoma
C57BL/6 mice expressing the human Bcl2 gene under the control of the panhematopoietic Vav promoter accumulate increased numbers of B cells, T cells, and myeloid cells.13 To assess the long-term pathologic consequences, we monitored cohorts of 3 independent strains (VavP-Bcl2 45, 68, and 69) to up to 18 months of age. Some mice became terminally ill with a kidney disease with the histologic appearance of autoimmune glomerulonephritis. The glomeruli were hypercellular and contained amorphous, eosinophilic deposits, most capillaries were no longer patent, and Bowman epithelium had proliferated to form crescents in the most advanced cases. The overall incidence of this disease at 40 weeks was 15% to 25%, depending on the strain. We have previously reported that Eμ-Bcl2 mice with a mixed (C57BL/6J × SJL/J)F2 background develop a lupuslike glomerulonephritis,7 but on an inbred C57BL/6J background they do not.22
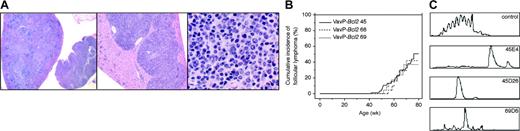
Most of the VavP-Bcl2 mice that were spared from developing advanced glomerulonephritis went on to become ill with hematopoietic neoplasms, the most frequent of which was diagnosed according to published criteria23,24 as follicular B-cell lymphoma. These mice presented on autopsy with enlargement of the spleen and various (frequently most) lymph nodes, often together with an abdominal tumor mass that appeared to have developed from a Peyer patch or the mesenteric lymph node. The liver, lungs, and bone marrow usually showed gross evidence of infiltration, predominantly in a nodular pattern. Twenty-one cases were examined in detail histologically. The abnormal tissues were extensively populated by a variable mixture of centrocytes and centroblasts (Figure 1A). In 12 cases, centrocytes were greatly predominant, whereas centroblasts greatly outnumbered centrocytes in 5 cases. The frequency of mitotic figures correlated approximately with centroblast content, ranging from a mean of 2 per high-power field in the 12 centrocytic cases up to 12 per high-power field in the 5 centroblast-dominated cases. The cumulative incidence of follicular lymphoma was between 37% and 50% at 18 months of age (Figure 1B). A few mice instead developed plasma cell tumors (up to 12% cumulative incidence) or, more rarely, lymphoblastic or large cell B lymphoma, thymic lymphoma, or histiocytic sarcoma (together, < 10%).
VavP-Bcl2 transgenic mice develop follicular lymphoma. (A) Histopathology of lymphoma in a 335-day-old VavP-Bcl2 45 mouse, which had massive enlargement of the spleen and lymph nodes and extensive infiltration of the liver and kidneys. Low-power (× 10) views of spleen and a lymph node (left) show nodular and diffuse infiltration, and a section of the liver (middle) with extensive infiltrates in the portal tract. High-power (× 100) view of the tumor tissue (right) shows centrocytes, with their small, darkly stained nuclei, among the larger centroblasts with their typical vesicular nucleus containing a prominent nucleolus and dispersed chromatin. (B) Cumulative incidence of follicular lymphoma in VavP-Bcl2 transgenic mice. A total of 69, 40, and 49 mice of strains 45, 68, and 69, respectively, were monitored up to 18 months of age. Follicular lymphoma was diagnosed from histology of enlarged lymphoid organs of sick mice. The incidence in each strain was determined by censoring all cases of other disease at the time of occurrence, as well as any mice removed for analysis. There were no significant differences among the 3 strains by log-rank test (chi-square = 0.651; P = .722). (C) Rearranged immunoglobulin VDJ sequences were amplified as described in “Materials and methods” and analyzed at high resolution in an automated sequencer. Control B cells sorted from healthy C57BL/6 spleen yielded, as expected, multiple clusters of bands separated by 3 base pairs. The 3 lower panels show VDJ genes from individual follicular lymphomas, each yielding a single predominant band indicative of clonality.
VavP-Bcl2 transgenic mice develop follicular lymphoma. (A) Histopathology of lymphoma in a 335-day-old VavP-Bcl2 45 mouse, which had massive enlargement of the spleen and lymph nodes and extensive infiltration of the liver and kidneys. Low-power (× 10) views of spleen and a lymph node (left) show nodular and diffuse infiltration, and a section of the liver (middle) with extensive infiltrates in the portal tract. High-power (× 100) view of the tumor tissue (right) shows centrocytes, with their small, darkly stained nuclei, among the larger centroblasts with their typical vesicular nucleus containing a prominent nucleolus and dispersed chromatin. (B) Cumulative incidence of follicular lymphoma in VavP-Bcl2 transgenic mice. A total of 69, 40, and 49 mice of strains 45, 68, and 69, respectively, were monitored up to 18 months of age. Follicular lymphoma was diagnosed from histology of enlarged lymphoid organs of sick mice. The incidence in each strain was determined by censoring all cases of other disease at the time of occurrence, as well as any mice removed for analysis. There were no significant differences among the 3 strains by log-rank test (chi-square = 0.651; P = .722). (C) Rearranged immunoglobulin VDJ sequences were amplified as described in “Materials and methods” and analyzed at high resolution in an automated sequencer. Control B cells sorted from healthy C57BL/6 spleen yielded, as expected, multiple clusters of bands separated by 3 base pairs. The 3 lower panels show VDJ genes from individual follicular lymphomas, each yielding a single predominant band indicative of clonality.
Each of 8 follicular lymphoma samples analyzed by immunohistochemistry had the phenotype of immunoglobulin class–switched B cells (B220+ IgM-) and 7 of 8 appeared to be surface IgG+, although there was a high background because of secreted IgG trapped in the tissues (data not shown). To assess clonality, VDJ sequences from Igh genes of 3 randomly selected tumors were PCR-amplified using a set of degenerate primers,20 and the length of the complementarity-determining region (CDR) was determined in an automated sequencer. Although polyclonal B-cell templates generated multiple bands having peaks 3 base pairs apart, as expected for multiple productively rearranged V genes (Figure 1C, upper panel), the tumor samples displayed a single predominant band, indicating that they were monoclonal (Figure 1C, 3 lower panels).
Healthy VavP-Bcl2 mice have prominent germinal centers
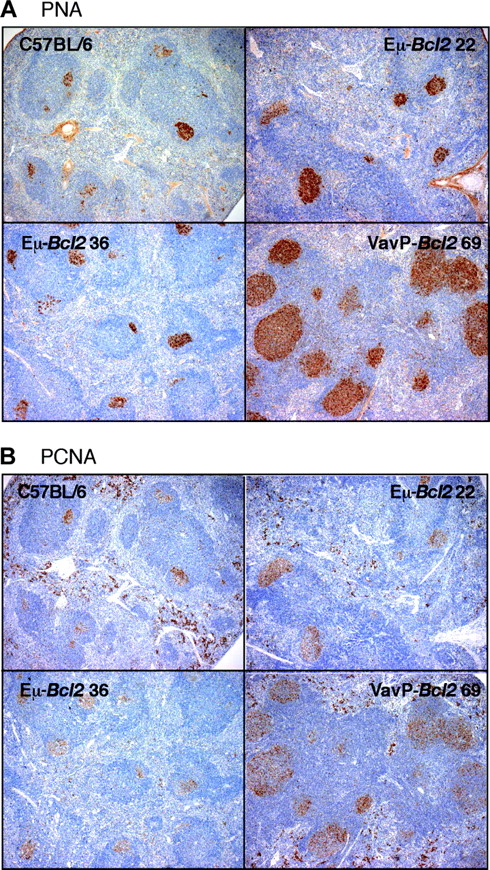
To gain insight into any changes occurring prior to the onset of follicular lymphoma, we analyzed young healthy mice of the 3 VavP-Bcl2 strains and compared them with aged-matched mice from 2 well-studied strains of Eμ-Bcl2 mice (strains 22 and 36).25,26 No major differences between the 5 strains in lymphoid architecture were detected in 6- to 8-week-old mice (data not shown). By 18 weeks of age, however, there were striking differences. The VavP-Bcl2 animals showed numerous grossly enlarged germinal centers replete with cells staining for the classic germinal center cell marker, PNA (Figure 2A). Consistently, more than 5 such germinal centers were found per nodule of white pulp in the spleen. By contrast, germinal centers in healthy and Eμ-Bcl2 mice were smaller and less frequent (1 or 2 per nodule).
Pretumor VavP-Bcl2 transgenic mice display grossly enlarged germinal centers. Consecutive sections of the spleen from 18-week-old mice of the indicated strains are stained brown for (A) PNA, a marker of germinal center cells, and (B) PCNA. Stains for PCNA and PNA are coincident. Images are magnified × 20.
Pretumor VavP-Bcl2 transgenic mice display grossly enlarged germinal centers. Consecutive sections of the spleen from 18-week-old mice of the indicated strains are stained brown for (A) PNA, a marker of germinal center cells, and (B) PCNA. Stains for PCNA and PNA are coincident. Images are magnified × 20.
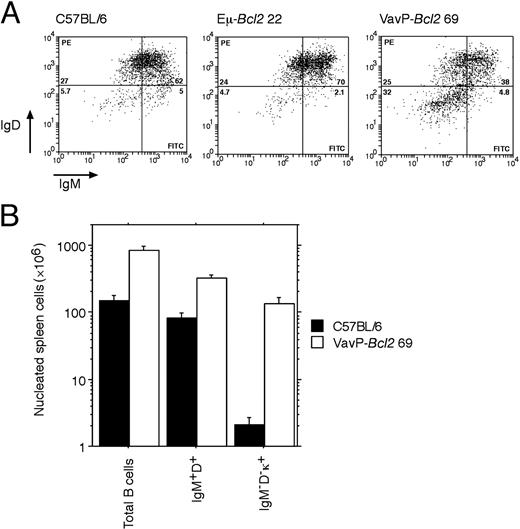
By immunohistochemical staining, the PNA-positive spleen cells in VavP-Bcl2 mice were negative for IgM, suggesting that they had undergone immunoglobulin class switching. Flow cytometry confirmed that the mice had far more “switched” B cells than did either C57BL/6 or Eμ-Bcl2 22 mice (Figure 3A). Between 30% and 50% of the B220+ cells in VavP-Bcl2 mice were negative for both IgM and IgD (eg, third panel in Figure 3A), and essentially all IgM/IgD double-negative B cells expressed κ light chain on their surface (data not shown). On average, the total number of switched (IgM- IgD- κ+) B cells was approximately 50-fold higher in VavP-Bcl2 mice than in C57BL/6 mice, whereas IgM+ IgD+ cells were only 4- to 5-fold elevated (Figure 3B).
Flow cytometric analysis of B-lymphoid cell populations in the spleen. (A) Gated B220+ spleen cells analyzed for IgM and IgD expression. The total numbers of B220+ IgM- IgD- cells per spleen were 7.1, 20, and 170 × 106 for the C57BL/6, Eμ-Bcl2 22, and VavP-Bcl2 69 mice, respectively. (B) Quantification of B-lymphoid cell populations in C57BL/6 and VavP-Bcl2 spleen (n = 4 for each strain).
Flow cytometric analysis of B-lymphoid cell populations in the spleen. (A) Gated B220+ spleen cells analyzed for IgM and IgD expression. The total numbers of B220+ IgM- IgD- cells per spleen were 7.1, 20, and 170 × 106 for the C57BL/6, Eμ-Bcl2 22, and VavP-Bcl2 69 mice, respectively. (B) Quantification of B-lymphoid cell populations in C57BL/6 and VavP-Bcl2 spleen (n = 4 for each strain).
How does the VavP-Bcl2 transgene promote germinal center activity?
The florid germinal center activity in the VavP-Bcl2 mice seemed likely to be the factor predisposing them to follicular lymphoma. Why are germinal center reactions so much stronger in these mice than in Eμ-Bcl2 mice? To address this issue, we compared the level of expression of the transgenes in the major lymphoid and myeloid compartments and determined the size of these populations within the 3 strains. Cells labeled with various cell surface markers were fixed and stained for cytoplasmic Bcl2 protein using the Bcl2-100 monoclonal antibody,16 which recognizes human but not mouse Bcl2 and is, therefore, specific for Bcl2 encoded by the transgene. There were no significant differences among the 3 VavP-Bcl2 strains in any cell type examined, so these results have been pooled.
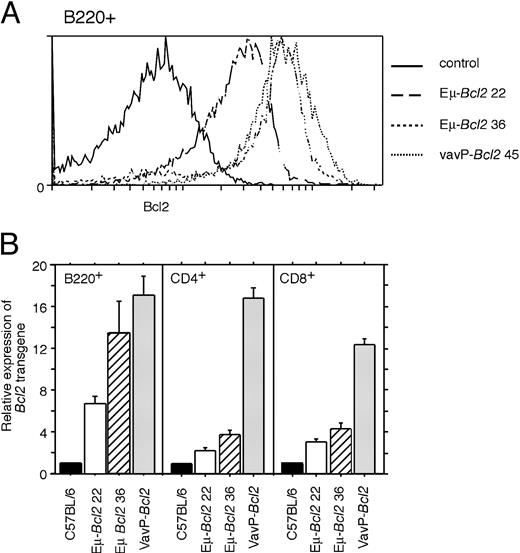
Both types of transgene were expressed at high levels in splenic B-lymphoid cells (Figure 4A and 4B left panel). The VavP-Bcl2 transgene was also highly expressed in peripheral CD4 and CD8 T cells (Figure 4B, center and right panels) and in myeloid (Mac-1+ Gr-1- and Gr-1+) cells (data not shown). The T cells from the Eμ-Bcl2 strains also expressed significant levels of Bcl2, but at far lower levels than those from VavP-Bcl2 mice (Figure 4B, center and right panels).
Expression of Eμ- and VavP-Bcl2 transgenes in lymphoid cells. (A) Flow cytometric analysis of transgenic Bcl2 detected by immunofluorescence staining with Bcl2-100 antibody in B220+ spleen cells. Cells from C57BL/6 mice served as the negative control. (B) Transgenic Bcl2 in B220+ B cells and Thy-1+ CD4+ and CD8+ T cells from the spleen. C57BL/6 (n = 10), Eμ-Bcl2 22 (n = 5), and Eμ-Bcl2 36 (n = 5) mice were compared with pooled data from 3 strains of VavP-Bcl2 mice (total n = 16). Fluorescence intensity values were normalized for nonspecific staining using nontransgenic C57BL/6 controls.
Expression of Eμ- and VavP-Bcl2 transgenes in lymphoid cells. (A) Flow cytometric analysis of transgenic Bcl2 detected by immunofluorescence staining with Bcl2-100 antibody in B220+ spleen cells. Cells from C57BL/6 mice served as the negative control. (B) Transgenic Bcl2 in B220+ B cells and Thy-1+ CD4+ and CD8+ T cells from the spleen. C57BL/6 (n = 10), Eμ-Bcl2 22 (n = 5), and Eμ-Bcl2 36 (n = 5) mice were compared with pooled data from 3 strains of VavP-Bcl2 mice (total n = 16). Fluorescence intensity values were normalized for nonspecific staining using nontransgenic C57BL/6 controls.
The total number of B cells in the spleen of 18-week-old VavP-Bcl2 mice was about 5-fold higher than in healthy littermates (Table 1). However, Eμ-Bcl2 22 mice also had very high levels of B cells, indeed more than Eμ-Bcl2 36 mice even though transgene expression was higher in the latter (Figure 4B). These results suggested that the excessive germinal center formation in the VavP-Bcl2 mice could not be simply a function of the amount of transgenic Bcl2 in B cells.
Quantification of splenic lymphoid cell populations
. | Mouse strain . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Cell phenotype . | C57BL/6 . | Eμ-Bcl2 22 . | Eμ-Bcl2 36 . | VavP-Bcl2 . | |||
| B220+ | 150 ± 10 | 520 ± 120 | 260 ± 20* | 780 ± 60† | |||
| CD4+ | 30 ± 3 | 53 ± 13* | 38 ± 5* | 170 ± 20† | |||
| CD8+ | 26 ± 3 | 35 ± 8* | 39 ± 2* | 88 ± 11† | |||
| Ratio of CD4:CD8 | 1.2 ± 0.1 | 1.3 ± 0.2* | 0.9 ± 0.1* | 2.1 ± 0.2† | |||
| Ratio of CD4:B220 | 0.20 ± 0.01 | 0.11 ± 0.01‡ | 0.15 ± 0.02‡ | 0.21 ± 0.01* | |||
. | Mouse strain . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Cell phenotype . | C57BL/6 . | Eμ-Bcl2 22 . | Eμ-Bcl2 36 . | VavP-Bcl2 . | |||
| B220+ | 150 ± 10 | 520 ± 120 | 260 ± 20* | 780 ± 60† | |||
| CD4+ | 30 ± 3 | 53 ± 13* | 38 ± 5* | 170 ± 20† | |||
| CD8+ | 26 ± 3 | 35 ± 8* | 39 ± 2* | 88 ± 11† | |||
| Ratio of CD4:CD8 | 1.2 ± 0.1 | 1.3 ± 0.2* | 0.9 ± 0.1* | 2.1 ± 0.2† | |||
| Ratio of CD4:B220 | 0.20 ± 0.01 | 0.11 ± 0.01‡ | 0.15 ± 0.02‡ | 0.21 ± 0.01* | |||
Mean cell number × 106 ± SEM per spleen. For C57BL/6, n = 10; for Eμ-Bcl2 22, n = 5; for Eμ-Bcl2 36, n = 5; and for VavP-Bcl2, n = 16.
Not different from C57BL/6 by Fisher's PLSD P > 0.45
Different from all other groups by Fisher's PLSD P < 0.03
Different from C57BL/6 and VavP Bcl2 by Fisher's PLSD P < 0.03
Two other cell types that contribute to the formation of germinal centers are dendritic cells and CD4 T cells. In a collaboration with K. Shortman (A.E., A.W.H., and S.C., unpublished results, 2002), we found that spleen dendritic cells expressed transgenic Bcl2 in Eμ-Bcl2 36 and VavP-Bcl2 69 mice, the latter at higher levels. The total number of dendritic cells per spleen was unchanged, however, suggesting that the germinal center hyperplasia was not provoked by increased dendritic cell help.
T-cell numbers were considerably higher in the spleen of VavP-Bcl2 mice than in either of the Eμ-Bcl2 strains and, significantly, the increase in CD4 T cells was greater than that in CD8 T cells (Table 1), in line with the higher expression of Bcl2 in the former (Figure 4B). A similar skewing of the T-cell ratio toward CD4 cells was noted in lymph nodes (not shown). Intriguingly, the ratio of CD4 T cells to B cells in VavP-Bcl2 mice was comparable to that in healthy C57BL/6 mice, whereas in Eμ-Bcl2 mice the number of CD4 cells per B cell was 25% to 50% lower (Table 1). Thus, although B-cell numbers are elevated in both VavP-Bcl2 mice and Eμ-Bcl2 mice, presumably because largely of Bcl2-induced resistance to apoptosis, only the VavP-Bcl2 mice may have sufficient CD4 T-cell help to sustain the further maturation of the excess B cells (see “Discussion”).
Spontaneous, enlarged germinal centers are dependent on CD4 T-cell help
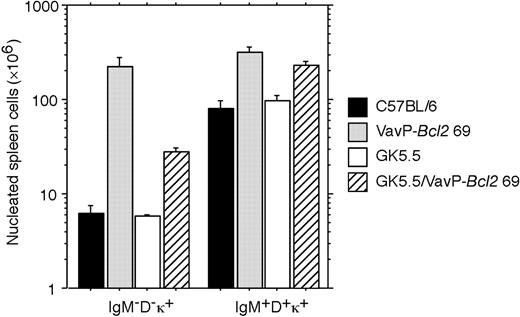
To test the hypothesis that the robust germinal center reactions are driven by CD4 T cells, we crossed VavP-Bcl2 mice with GK5 mice, which lack CD4 cells because of expression of a transgene encoding a monoclonal anti-CD4 antibody under the control of a pancreas-specific promoter.14 Like GK5 mice, bitransgenic VavP-Bcl2-GK5 mice had no CD4 T cells. No cells were detectable with the monoclonal anti-CD4 antibody H129.19 in the spleen or blood at 4, 10, or 18 weeks of age (n > 4 at each time point), and there were no Thy1+ CD8- cells, confirming that the lack of CD4 staining was not due to masking by the transgenic GK1.5 antibody (data not shown). When age-matched littermates of all 4 genotypes were analyzed at 18 weeks of age, it became clear that the absence of CD4 cells in the bitransgenic mice had not greatly altered the total numbers of mature IgM+ IgD+ B cells in the spleen. However, the number of IgM- IgD- κ+ B cells was reduced by almost 10-fold (Figure 5), and germinal centers were not detectable by histology. This finding suggests that expansion of the germinal centers in VavP-Bcl2 mice and the substantial increase in the frequency of switched B cells is dependent on CD4 T-cell help.
The VavP-Bcl2 germinal center phenotype depends on CD4 T cells. Total number of splenic B cells of the indicated surface phenotype in C57BL/6 (n = 3), VavP-Bcl2 69 (n = 5), GK5 (n = 3), and GK5-VavP-Bcl2 69 (n = 5) mice. Although the expression of the GK5 transgene in otherwise healthy mice did not alter the number of B220+ B cells of either mature (IgM+ IgD+ κ+) or immunoglobulin class–switched (IgM- IgD- κ+) phenotype, it reduced the number of class-switched B cells in VavP-Bcl2 transgenic mice approximately 10-fold, while reducing the total number of mature B cells less than 2-fold.
The VavP-Bcl2 germinal center phenotype depends on CD4 T cells. Total number of splenic B cells of the indicated surface phenotype in C57BL/6 (n = 3), VavP-Bcl2 69 (n = 5), GK5 (n = 3), and GK5-VavP-Bcl2 69 (n = 5) mice. Although the expression of the GK5 transgene in otherwise healthy mice did not alter the number of B220+ B cells of either mature (IgM+ IgD+ κ+) or immunoglobulin class–switched (IgM- IgD- κ+) phenotype, it reduced the number of class-switched B cells in VavP-Bcl2 transgenic mice approximately 10-fold, while reducing the total number of mature B cells less than 2-fold.
VavP-Bcl2 mice have an expanded pool of proliferating germinal center cells
CD4 T cells probably promote the germinal center phenotype of VavP-Bcl2 mice at least in part by fostering proliferation and immunoglobulin class switching. When spleen sections were stained for the PCNA (Figure 2B), the PCNA-positive and PNA-positive zones clearly coincided (compare Figure 2A-B). Thus, as in the small germinal centers of healthy mice, cells within the florid germinal centers of VavP-Bcl2 mice are in cycle.
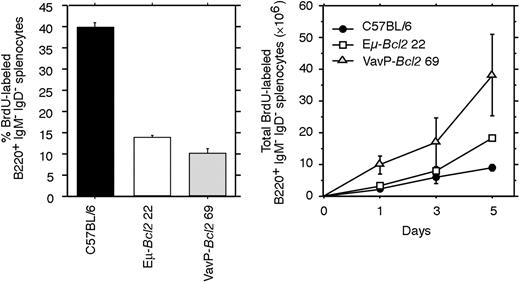
To quantify the proliferation, we measured BrdU-labeled B220+ IgM- IgD- spleen cells from mice fed BrdU in their drinking water for 1 to 3 days (Figure 6). The proportion of labeled cells was actually lower in both types of Bcl2 transgenic mice than in healthy littermates (Figure 6, left panel) presumably because, as we have reported previously, high levels of Bcl2 retard entry into cell cycle.17,18,27 Nevertheless, the total number of BrdU-labeled switched cells per spleen was higher at each time point in the VavP-Bcl2 mice than in wild-type or Eμ-Bcl2 22 mice (Figure 6, right panel). Thus, there is a larger pool of cycling B cells that have a germinal center phenotype in VavP-Bcl2 mice.
Proliferation of immunoglobulin class–switched B cells. Flow cytometric analysis of BrdU-labeled B220+ IgM- IgD- spleen cells from mice fed with BrdU in their drinking water continuously for 1, 3, or 5 days. Three mice of each indicated genotype were analyzed at each time point. The left panel shows the proportion of B220+ IgM- IgD- cells labeled with BrdU after 3 days. The right panel shows the total number of BrdU-labeled B220+ IgM- IgD- cells per spleen after 1, 3, and 5 days.
Proliferation of immunoglobulin class–switched B cells. Flow cytometric analysis of BrdU-labeled B220+ IgM- IgD- spleen cells from mice fed with BrdU in their drinking water continuously for 1, 3, or 5 days. Three mice of each indicated genotype were analyzed at each time point. The left panel shows the proportion of B220+ IgM- IgD- cells labeled with BrdU after 3 days. The right panel shows the total number of BrdU-labeled B220+ IgM- IgD- cells per spleen after 1, 3, and 5 days.
Class-switched B cells show hypermutation
Somatic hypermutation of immunoglobulin V genes is a salient feature of a germinal center reaction. To assess whether the expanded pool of switched cells in VavP-Bcl2 mice had undergone somatic mutation, we analyzed the well-studied S107 VH gene, which is a member of a very small gene family expressed early during ontogeny. S107 VH genes rearranged to the JH1 joining region were cloned from sorted IgM- IgD- κ+ B cells, using a proofreading enzyme to ensure high fidelity, and sequenced. The genomic approach minimized the contribution from any rare plasma cells in the sorted material and allowed analysis of nonproductive rearrangements as an unselected control.
Five S107 clones from control IgM-bearing B cells sorted from C57BL/6 spleen showed no mutations relative to the germ line sequence. In contrast, 22 of 23 clones isolated from the class-switched cells of VavP-Bcl2 mice each harbored an average of about 6 somatic mutations, many of which were located in “hotspots” known to be targeted by the hypermutation machinery. Figure 7 shows 4 representative mutated sequences compared with the germ line sequence, and Table 2 summarizes all the data.
S107 VH gene sequences from class-switched B cells contain somatic mutations. The S107 VH germ line sequence is shown aligned with 4 S107 sequences cloned from the PCR product derived from sorted B220+ IgM- IgD- κ+ spleen cells pooled from 2 VavP-Bcl2 mice. Boxes indicate CDR regions 1 and 2. Bracketed bases in the consensus represent mutational hot spots. Boxed residues indicate mutations: those in black boxes encode amino acid changes (replacement mutations); those in gray boxes represent silent mutations.
S107 VH gene sequences from class-switched B cells contain somatic mutations. The S107 VH germ line sequence is shown aligned with 4 S107 sequences cloned from the PCR product derived from sorted B220+ IgM- IgD- κ+ spleen cells pooled from 2 VavP-Bcl2 mice. Boxes indicate CDR regions 1 and 2. Bracketed bases in the consensus represent mutational hot spots. Boxed residues indicate mutations: those in black boxes encode amino acid changes (replacement mutations); those in gray boxes represent silent mutations.
Somatic mutations in S107 VH sequences cloned from class-switched B cells of VavP-Bcl2 transgenic mice
Parameter . | Productive sequences . | Nonproductive sequences . | P (productive vs nonproductive) . | P (targeted productive vs random)* . |
|---|---|---|---|---|
| Mutations | 94 | 63 | .93 | |
| Ratio of hot spot to nonhot spot | 41:53 | 25:38 | .62 | <.0001 |
| Ratio of R to S† | 58:36 | 52:11 | .0052 | |
| R (ratio of C to F)‡ | 26:32 | 19:33 | .38 | .0011 |
| Mutations (ratio of C to F) | 40:54 | 21:42 | .25 | .0005 |
| F (ratio of R to S)§ | 32:22∥ | 33:9∥ | .045 | |
| C (ratio R to S)§ | 26:14∥ | 19:2∥ | .032 |
Parameter . | Productive sequences . | Nonproductive sequences . | P (productive vs nonproductive) . | P (targeted productive vs random)* . |
|---|---|---|---|---|
| Mutations | 94 | 63 | .93 | |
| Ratio of hot spot to nonhot spot | 41:53 | 25:38 | .62 | <.0001 |
| Ratio of R to S† | 58:36 | 52:11 | .0052 | |
| R (ratio of C to F)‡ | 26:32 | 19:33 | .38 | .0011 |
| Mutations (ratio of C to F) | 40:54 | 21:42 | .25 | .0005 |
| F (ratio of R to S)§ | 32:22∥ | 33:9∥ | .045 | |
| C (ratio R to S)§ | 26:14∥ | 19:2∥ | .032 |
S107 sequences were cloned from the PCR product derived from sorted B220+ IgM− IgD− κ+ spleen cells pooled from 2 VavP-Bcl2 mice. For productive sequences, n = 14; for nonproductive sequences, n = 9.
Tests of whether mutations in productive sequences were significantly concentrated in the 36-base hot spots and 72-base C regions of the 300-base length analyzed
Replacement (R) vs silent (S) mutations
Replacement mutations in complementarity-determining (C) vs framework (F) regions
Replacement vs silent mutations in F and C regions, respectively
Ratios of replacement to silent mutations were not significantly different between C and F regions in productive (P = .57) or nonproductive (P = .24) sequences
When the sequences from productive (in-frame) gene rearrangements were compared with those from nonproductive (out-of-frame or stop-codon blocked) rearrangements, it was apparent that replacement mutations in framework regions were less frequent in the former (32 of 54, 59% versus 33 of 42, 79%), indicating ongoing selection to preserve the structural integrity of the antigen receptor. Unexpectedly, this “suppression” of sequence diversity was also apparent in complementarity-determining regions (26 of 40, 65% versus 19 of 21, 90%), seemingly indicative of selection against affinity maturation (see “Discussion”).
In summary, our results suggest that the formation of numerous large germinal centers in VavP-Bcl2 mice involves proliferative expansion of B cells that have undergone immunoglobulin class switching and hypermutation. Because these cells accumulate in such large numbers in the spleen, we infer that they do not exit the germinal center reaction efficiently, presumably because of overexpression of Bcl2, and therefore spend a much-extended time in this state. Cells within this population would presumably have an increased probability of acquiring additional somatic mutations, including oncogenic mutations. They, therefore, represent a highly plausible precursor population for follicular lymphoma.
Discussion
Overexpression of Bcl2 is a hallmark of follicular lymphoma and is widely accepted as the disease-defining oncogenic change. Nevertheless, the t(14;18) Bcl2-IGH translocation is present in rare cells from many healthy individuals,10,11 suggesting that other events are also obligatory. In support of this view, previous attempts to engender follicular lymphoma in mice, by mimicking the translocation and overexpressing Bcl2 in B cells, did not reproduce this disease,6-9 although they did produce some follicular hyperplasia and a low incidence of high-grade B-lymphoid malignancies.
We generated VavP-Bcl2 transgenic mice to explore the effect of panhematopoietic overexpression of Bcl2.13 Somewhat unexpectedly, all 3 independent strains of these mice proved to be highly predisposed to late-onset (> 10 months) follicular lymphoma, preceded by a striking hyperplasia of germinal centers in lymphoid tissues. The number of B cells that had undergone immunoglobulin class switching was elevated 50-fold in the spleen of healthy adult mice, and many were in cycle and bore evidence of immunoglobulin V-gene somatic mutation. We infer that this population is the seed bed which ultimately generates the monoclonal follicular lymphoma.
Seeking to understand why VavP-Bcl2 mice develop follicular lymphoma while Eμ-Bcl2 transgenic strains do not, we compared transgene expression levels and cellular distribution in the 2 types of mice. Neither the level of Bcl2 in B cells nor the size of the total B-cell population provided a satisfactory explanation. This led us to ask whether other cell populations expressing the VavP-Bcl2 transgene played a role in conferring susceptibility. Although dendritic cells expressed the transgene, they did not seem to be increased in number. In contrast, the peripheral T-cell compartment was significantly enlarged in the VavP-Bcl2 mice compared with the Eμ-Bcl2 strains, consistent with the much higher expression of the transgenic Bcl2 in the T cells of the former. In particular, the number of CD4 T cells was 5-fold higher than normal, similar to the elevation in B cells (Table 1). These data suggested that T-cell help might be of importance for the development of the enlarged germinal centers and the greatly increased pool of immunoglobulin class–switched B cells observed in VavP-Bcl2 mice. To test this hypothesis, we deleted CD4 cells from VavP-Bcl2 69 mice by an antibody targeting strategy and observed a loss of class-switched cells (Figure 5) and germinal centers.
Our results suggest that CD4 T cells make a critical input into the exaggerated germinal center reactions in VavP-Bcl2 mice and, eventually, the onset of follicular lymphoma. Does the increased germinal center activity reflect prolonged antigen responsiveness, because of enhanced survival of Bcl2-expressing activated T cells in the face of limiting cytokine levels?28 Or could it instead result from increased autoreactivity? Although Bcl2 overexpression can inhibit deletion of autoreactive thymocytes,26,29 peripheral T-cell tolerance appears to be largely intact in VavP-Bcl2 mice.29 Nevertheless, the moderately frequent appearance of autoimmune-type glomerulonephritis in these mice is certainly suggestive of enhanced autoreactivity, at least at the B-cell level. Interestingly, the cellular composition of human follicular lymphoma includes T cells,30 and a number of studies have suggested the involvement of antigenic stimulation in the etiology of the disease.31-36
Increased CD4 T-cell help would be expected to stimulate proliferation and immunoglobulin class switching. Indeed the germinal centers in VavP-Bcl2 mice were PCNA-positive (Figure 2B), and the spleen contained greatly increased numbers of class-switched B cells (Figure 3B), many of which were in cycle (Figure 6). In contrast, despite also having a much larger total B-cell population than wild-type mice, the number of cycling IgM- IgD- κ+ cells was elevated only modestly above normal in Eμ-Bcl2 mice (Figure 6B), consistent with the largely normal size of their germinal centers and CD4 T-cell population (Figure 2; Table 1). Presumably T-cell help remains limiting in these mice.
The majority (22 of 23) of S107 Igh V genes randomly cloned from the IgM- IgD- κ+ population in VavP-Bcl2 mice were mutated relative to the germ line sequence. Significantly, of the 94 mutations in productive rearrangements, 41 (43%) were found at the “hotspots” targeted in normal germinal center cells (Figure 7; Table 2). A previous study of S107 sequences from phosphorylcholine-immunized Eμ-Bcl2 22 mice, which analyzed 33 mutations,37 failed to detect such targeting. The apparent disparity may reflect experimental differences in the latter study—the B-cell–specific expression of the Eμ-Bcl2 transgene, the different genetic background (BALB/c rather than C57BL/6), and prior immunization.
More than 100 × 106 B cells in the spleen of a healthy adult VavP-Bcl2 mouse have undergone immunoglobulin class switching (Figure 3B). Assuming the high frequency of mutant S107 VH sequences in switched cells is representative of all rearranged VH sequences in all such cells, then more than 96 × 106 cells in each mouse have been subjected to hypermutation. The hypermutation machinery acts not only on immunoglobulin genes but also on other transcribed genes; notably, BCL6 and FAS/APO1 genes have been reported as targets of mutation in human germinal center cells.38,39 It is possible, therefore, that sustained hypermutation in a greatly expanded population of germinal center cells that are refractory to apoptosis would eventually produce a transformed tumor cell.
Analysis of the distribution of the 157 mutations in S107 VH genes and of the ratio of replacement to silent mutations may provide some insight into the selection processes acting in the germinal centers of VavP-Bcl2 mice. As expected, replacement mutations were suppressed throughout the framework regions of productively rearranged S107 V-gene sequences, consistent with selection for a structurally intact receptor. Interestingly, however, replacement mutations were also suppressed in the complementarity-determining regions (Table 2), which implies selection against any change of affinity for the primary antigen.
In conclusion, VavP-Bcl2 mice represent the first animal model for Bcl2-induced human follicular lymphoma. These mice make it possible to investigate hypotheses for the critical early stages of pathogenesis that are not amenable to analysis in humans. They also provide a new avenue by which to study the molecular and cellular biology of germinal center reactions and, in particular, the mechanisms governing positive and negative selection of B cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
An Inside Blood analysis of this article appears in the front of this issue.
Supported by grants from the National Health and Medical Research Council (NHMRC; Canberra) (block grant 222099 and program grant 257502), the National Cancer Institute (grant CA43540), the Leukemia and Lymphoma Society Specialized Center of Research (SCOR; grant 7015), and the Austrian Science Fund (FWF; grants J2129 and J1921) (A.E.).
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-07-2469.
We thank D. Tarlinton and A. Strasser for helpful discussions, A. Lew and Y. Zhan for their kind gift of GK5 mice, K. Shortman and D. Vremec for assistance with dendritic cell analysis, A. Wiegmans and V. Marshall for expert technical assistance, and C. Tilbrook and H. Millar for skilled animal husbandry.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal