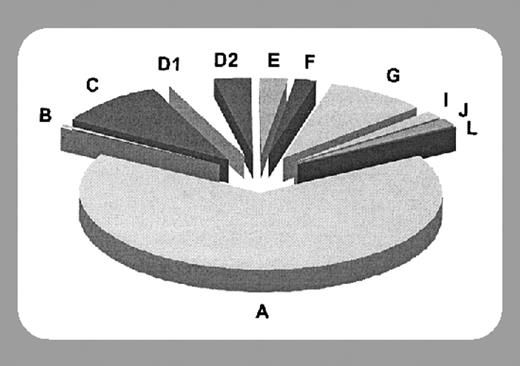
Fanconi anemia (FA) is a rare genetic syndrome characterized by a variable range of developmental anomalies, progressive bone marrow failure, and susceptibility to a number of different types of cancers.1 It may also hold the key to an improved understanding of fundamental processes in DNA repair that are applicable to a number of disease states—most tantalizingly breast cancer and myeloid malignancies. To date, 9 distinct subtypes of FA have been identified (A, B, C, D1, D2, E, F, G, and L), all of which except B have been mapped to specific gene products. Mutations in any of these gene products are sufficient to interfere with DNA repair, as manifested by hypersensitivity to cross-linking agents such as diepoxybutane (DEB), and to contribute to the FA phenotype. The FANCA, -C, -E, -F, -G, and -L proteins associate to form a nuclear “core complex” that serves to activate FANCD2 to its active form FANCD2-L by mono-ubiquitination, probably mediated by the FANCL ubiquitin ligase. FANCD2-L interacts and colocalizes with the breast cancer susceptibility gene product BRCA2, which is probably identical to FANCD1, in DNA-damage–induced subnuclear foci.2 Thus, the FA/BRCA pathway seems likely to play an integral role in the maintenance of genomic integrity, although the number of different components, their functions, and their precise relationships remain largely unknown. FANCG appears identical to XRCC9, while FANCD2 and NBS1 (defective in Nijmegen breakage syndrome) have also been shown to interact in DNA repair.3
In this issue, Levitus and colleagues (page 2498) define 2 additional subtypes of FA, which they designate FA-I and FA-J. They characterized 8 previously nontypable FA cell lines and showed that they divided into 2 groups based on noncomplementation in vitro and analysis of linked polymorphic genetic markers. Thus, the 4 FA-I cell lines failed to complement each other, but did complement the FA-J cell lines; conversely, the FA-J cell lines failed to mutually complement, but did complement the FA-I cell lines. Both FA-I and FA-J cell lines were able to form the FA nuclear core complex; however, FA-I cell lines did not ubiquitinate FANCD2, indicating a defect proximal to FANCD2, while FA-J cell lines did ubiquitinate FANCD2, indicating a downstream lesion in the pathway.
This study raises a number of important questions as well as answers. First, are these truly 2 additional new complementation groups? There is precedent for this: FANCD was initially thought to be homogeneous and only subsequently divided into FANCD2 and FANCD1/BRCA2. Similarly, the putative FA-H cell line EUFA173 was shown to belong to FA-A, after it was shown to be the product of a genetic reversion event.4 The major point of possible confusion is with FA-B for which a specific gene has not been identified to date; however, both FA-I and FA-J cell lines are able to form the nuclear core complex, unlike FA-B cell lines. Second, how do FA-I and FA-J relate to disease phenotype and to other syndromes characterized by DNA repair defects? At first glance, neither of the new complementation groups appears to correlate with mild or severe disease, or to associate with the Nijmegen breakage syndrome gene NBS1. is significant that the putative FANCJ protein is the only one (other than FANCD1/BRCA2) to act downstream of FANCD2 ubiquitination. However, further studies, including identification of the genes and their associated proteins, will be necessary to resolve these points.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal