Abstract
Chronic joint disease from repeated bleeding into joints is a serious complication of hemophilia. To measure the extent of and to identify risk factors for deviations from normal in joint range of motion (ROM), we used cross-sectional data collected from 4343 males with hemophilia aged 2 to 19 years who received care at 136 US hemophilia treatment centers (HTCs). Factors examined included age, race/ethnicity, family history, insurance status, age at diagnosis and first HTC visit, frequency of HTC visits, hemophilia type, bleeding frequency, prophylaxis use, inhibitor status, body mass index (BMI), and recent orthopedic procedures. Trained personnel using a standard protocol obtained ROM measurements on 10 joints (hips, knees, shoulders, elbows, and ankles). Analyses used multiple linear regression to model overall ROM limitation separately by disease severity. For persons in all severity groups, joint ROM limitation was positively associated with older age, nonwhite race, and increased BMI. For those with severe disease, ROM limitation was also positively associated with number of bleeds and was greater for those with inhibitors or recent orthopedic procedures. We conclude that ROM limitations begin at an early age, especially for those with severe and moderate disease, and that BMI is an important, potentially modifiable risk factor.
Introduction
One of the most severe complications of hemophilia is the development of a characteristic chronic arthropathy that results from repeated bleeding into joints. While the natural history of the process leading to the joint damage and resultant limited joint mobility have been described,1 there is little available information about the prevalence and extent of joint disease among the hemophilia population or about risk factors for progression.
Since May 1998, the Hematologic Diseases Branch of the Centers for Disease Control and Prevention (CDC) has provided assistance to federally funded hemophilia treatment centers (HTCs) in the United States to collect a uniform set of clinical outcomes information, including joint range-of-motion (ROM) measurements, from participants in a voluntary surveillance program called the Universal Data Collection (UDC) project.2,3 To date, data have been collected from more than 12 000 people with bleeding disorders who receive care in these centers. This report focuses on 2- to 19-year-old males with hemophilia and uses data collected by this project to describe the cross-sectional prevalence of joint disease, as manifested by limitations in joint ROM, and to examine factors associated with ROM limitation.
Patients, materials, and methods
To participate in the UDC project, persons must receive care in 1 of the 136 federally funded HTCs and meet at least one of the following criteria: (1) aged 2 years or older with a bleeding disorder due to congenital deficiency or acquired inhibitors in which any of the coagulation proteins are missing, reduced, or defective and with a functional level of less than 50% or (2) aged 2 years or older with a diagnosis by a physician of von Willebrand disease.
Persons with bleeding disorders are enrolled in UDC by staff in each HTC. These centers provide multidisciplinary, specialized comprehensive care, and as part of the project, HTC care providers collect a uniform set of clinical data and a plasma specimen each year during a participant's annual comprehensive clinic visit. Participation is voluntary and patients (or parents of minor children) give informed consent. The study has received approval from the Human Investigation Review Boards of CDC and all participating HTCs. This report includes 2- to 19-year-old males with hemophilia only; for participants with multiple visits, data collected during the most recent comprehensive care visit were used.
Data collection
Information obtained from UDC participants during the annual comprehensive care visit is recorded on 1 of 2 standardized data collection forms. A registration form is completed one time for a new participant and includes month and year of birth, age at the time of hemophilia diagnosis, age at first HTC visit, hemophilia type, and the results of testing for clotting factor activity. Hemophilia severity level is categorized as mild if the factor activity is 6% to 30%, moderate if 1% to 5%, and severe if less than 1% of normal. Race and ethnicity is based on participant self-report and categorized in accordance with census reporting. A participant with at least one blood relative who has been diagnosed with a bleeding disorder is considered to have a positive family history.
HTC staff use the second data collection form, the annual visit form, to record current treatment and outcome data. HTC use is categorized as frequent (1 or more visits per year) or infrequent (< 1 visit per year) according to the frequency of HTC visits during the previous 2- to 3-year period. Clinic records are used to determine whether the participant has health insurance and to identify the treatment regimen used. If the person infuses clotting factor on a regular basis (eg, every other day) to prevent bleeding and the infusions are expected to continue indefinitely, he is considered to be on continuous prophylaxis treatment.
Persons are assessed regularly for the presence of neutralizing antibodies or inhibitors. Participants with an inhibitor titer of 0.5 Bethesda units or more at the time of the comprehensive care visit are classified as having an inhibitor. Clinic and hospital records are reviewed to determine whether or not orthopedic surgery has been performed on one or more joints during the previous year. Orthopedic surgical procedures include arthrodesis, synovectomy (surgical or radioisotopic), and joint replacement.
Persons are routinely instructed to keep a log of all bleeds that occur between clinic visits. Log entries record the severity and the location of every bleed as well as treatment applied. HTC staff review available logs and record the number of bleeds by site (joint, muscle, or other) during the 6-month period prior to the comprehensive care visit on the data form. For participants who do not complete a log (approximately 75% of participants), information about bleeding is based on patient and/or parent recall.
During the visit, participant height and weight are measured in light clothing without shoes, and ROM measurements on 10 joints (hips, knees, shoulders, elbows, and ankles) are obtained by a physical therapist or other trained health care provider according to detailed guidelines provided in a reference manual and training video supplied by CDC. Subject position is specified for each joint measurement as follows: sitting for ankle dorsiflexion and plantarflexion and for elbow pronation and supination; supine for knee flexion, extension, and hyperextension, for hip flexion, for shoulder flexion, and for elbow flexion, extension, and hyperextension; and sidelying for hip extension. Each joint is moved passively to its full extent, and end-point measurements are made to the nearest degree using a standard goniometer. Joints in which a bleed has occurred within 24 hours or whose motion is restricted for another medical reason (eg, recent surgery, presence of an immobilization device such as a splint or brace) are not measured. All health care providers performing these measurements were trained and certified by 1 of 12 regional physical therapists who had themselves received centralized training. Since this study began, 91% of the ROM data have been collected by licensed physical therapists.
Data analysis
We used a cross-sectional study design and limited the analysis to subjects 2 to 19 years old to minimize the effect of changes in risk factors over time. Age was calculated as of the date of the visit. Data collected on height and weight were used to calculate body mass index (BMI) as weight in kilograms divided by height in square meters.
An overall joint index was calculated for each participant that was the sum of all flexion and extension (including hyperextension) measurements taken on all 10 joints. Next, we summed normal adult ROM values—ankle dorsiflexion (20°), ankle plantarflexion (50°), knee flexion (135°), knee extension (0°), hip flexion (120°), hip extension (30°), elbow flexion (150°), elbow extension (0°), elbow pronation and supination (80°), and shoulder flexion (180°)4 —to obtain a normal overall joint index value. The total amount of joint ROM limitation in degrees was calculated as the difference between the normal overall joint index value (1690 degrees) and the participant's overall joint index. Negative values for overall joint ROM limitation meant that the participant had excess joint mobility relative to that of a healthy adult. The percent overall joint ROM limitation was determined for each person by dividing his total amount of joint ROM limitation by the normal overall joint index value and multiplying by 100.
Because we were interested in ROM limitations that were the result of bleeds, we compared each of the ROM measurements from the right and left joints for each subject. We defined an asymmetrically mobile joint as one whose right and left side measures differed by at least 20% of the normal value—a difference not likely due to measurement error. Since joint bleeding in hemophilia is rarely symmetrical, we reasoned that ROM limitations that occurred among persons with asymmetrically mobile joints would most likely be due to bleeds. Therefore, for patients with no asymmetrically mobile joints, we set the percentage of overall joint ROM limitation value to zero for the analyses. Average percentage of overall joint ROM limitation was calculated for groups of participants defined by demographic and clinical characteristics. Differences among the groups were evaluated for statistical significance using analysis of variance.
To model the patterns of joint ROM limitation across age levels and to determine factors associated with ROM limitation, we used multivariate linear regression. Overall percentage of ROM limitation was used as the outcome variable in the regression models, and risk factors were evaluated for significance using a backward elimination strategy. Using this strategy, all potential risk factors were entered into the model in the first step and those factors that did not contribute significantly to the model were removed one at a time until only statistically significant risk factors remained. In preliminary analyses, no colinearity between the studied risk factors was identified. We also performed diagnostics to identify influential outliers in the data. Data from persons with ROM limitation values that were more than 3 standard deviations from the mean in residual analyses were deleted from the regression analyses.
Separate regression models were used to evaluate patterns of ROM limitation and factors associated with ROM limitation among persons with severe, moderate, and mild hemophilia. Higher order terms were entered into the model to account for nonlinear patterns in ROM limitation. First-order interaction terms were created and evaluated for statistical significance in preliminary models. None of these terms contributed significantly to any of the models. Hypothesis tests were 2-tailed, and significance was based on achievement of a P value of .05 or less. All analyses were performed using SAS Version 8 statistical software (SAS Institute, Cary, NC).
Results
Between May 1998 and May 2002, 8950 males with hemophilia were asked to participate in the UDC project at the 136 HTCs; 8514 (95%) of them were enrolled. Of those enrolled, 4965 (58%) were 2 to 19 years of age and formed the eligible study population. We excluded 15 persons with incomplete data on hemophilia severity and 607 persons with incomplete joint ROM measurements, leaving 4343 subjects available for study.
Participant demographic and clinical characteristics by disease severity are shown in Table 1. The average age of study subjects was 10.6 years, 80% had hemophilia A, and the proportions of persons with mild, moderate, and severe hemophilia were 21%, 24%, and 55%, respectively. Two thirds of the subjects were white, 13% were black, 15% were of Hispanic ethnicity, and the rest were other races. The distributions of age, race, hemophilia type, insurance status, and BMI were relatively uniform across levels of severity. However, the greater the severity of hemophilia, the higher the proportion of participants who had been diagnosed at or before birth, had their first HTC visit within the first year of life, made frequent visits to an HTC, developed an inhibitor, used prophylaxis to prevent bleeds, reported 5 or more bleeding episodes in the previous 6 months, and had undergone an orthopedic procedure during the previous year. On the other hand, participants with severe disease were less likely to have a family history of a bleeding disorder than were those with mild or moderate hemophilia.
Population distribution and average percentage of overall joint range-of-motion limitation by hemophilia severity across levels of risk factors studied among 4343 young males with hemophilia who participated in UDC, May 1998-May 2002
. | Mild, n = 917 . | . | . | . | Moderate, n = 1048 . | . | . | . | Severe, n = 2378 . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics . | No. . | % . | % Limitation . | P* . | No. . | % . | % Limitation . | P* . | No. . | % . | % Limitation . | P* . | |||||||||
| Age, y | |||||||||||||||||||||
| 2 to 5 | 158 | 17.2 | –0.5 | <.001 | 211 | 20.1 | –0.3 | <.001 | 491 | 20.6 | –0.5 | <.001 | |||||||||
| 6 to 10 | 259 | 28.2 | –0.3 | 316 | 30.2 | 0.3 | 711 | 29.9 | 0.6 | ||||||||||||
| 11 to 15 | 312 | 34.0 | 0.5 | 317 | 30.2 | 1.4 | 700 | 29.4 | 3.1 | ||||||||||||
| 16 to 19 | 188 | 20.5 | 0.6 | 204 | 19.5 | 3.0 | 476 | 20.2 | 5.9 | ||||||||||||
| Race/ethnicity | |||||||||||||||||||||
| White, non-Hispanic | 631 | 68.8 | 0.1 | NS | 708 | 67.6 | 0.8 | .01 | 1492 | 62.7 | 1.7 | <.001 | |||||||||
| Other | 286 | 31.2 | 0.3 | 340 | 32.4 | 1.6 | 886 | 37.3 | 2.9 | ||||||||||||
| Hemophilia type | |||||||||||||||||||||
| A | 743 | 81.0 | 0.1 | NS | 737 | 70.3 | 1.2 | .02 | 2022 | 85.0 | 2.2 | NS | |||||||||
| B | 174 | 19.0 | 0.2 | 311 | 29.7 | 0.6 | 356 | 15.0 | 1.7 | ||||||||||||
| Family history | |||||||||||||||||||||
| Yes | 754 | 82.2 | 0.1 | NS | 773 | 73.8 | 0.9 | NS | 1367 | 57.5 | 2.1 | NS | |||||||||
| No | 163 | 17.8 | 0.1 | 275 | 26.2 | 1.3 | 1011 | 42.5 | 2.2 | ||||||||||||
| Age at diagnosis | |||||||||||||||||||||
| At/before birth | 370 | 40.4 | –0.1 | NS | 665 | 63.4 | 1.0 | NS | 2039 | 85.7 | 2.0 | NS | |||||||||
| After birth | 524 | 57.1 | 0.2 | 354 | 33.8 | 1.0 | 272 | 11.4 | 2.6 | ||||||||||||
| Age at first HTC visit, y | |||||||||||||||||||||
| Younger than 1 | 255 | 27.8 | –0.1 | .02 | 480 | 45.8 | 0.9 | NS | 1554 | 65.4 | 1.7 | <.001 | |||||||||
| 1 to 2 | 130 | 14.2 | –0.3 | 182 | 17.4 | 1.0 | 332 | 14.0 | 2.6 | ||||||||||||
| 2+ | 501 | 54.6 | 0.4 | 339 | 32.4 | 1.2 | 387 | 16.3 | 3.2 | ||||||||||||
| Insurance status | |||||||||||||||||||||
| Insured | 884 | 96.4 | 0.1 | NS | 1018 | 97.1 | 1.0 | NS | 2330 | 98.0 | 2.1 | .03 | |||||||||
| Uninsured | 33 | 3.6 | 0.1 | 30 | 2.9 | 1.2 | 48 | 2.0 | 3.8 | ||||||||||||
| HTC use | |||||||||||||||||||||
| Frequent | 705 | 76.9 | 0.3 | .01 | 924 | 88.2 | 1.2 | <.01 | 2248 | 94.5 | 2.1 | <.01 | |||||||||
| Infrequent | 212 | 23.1 | –0.3 | 124 | 11.8 | 0.1 | 130 | 5.5 | 3.4 | ||||||||||||
| Inhibitor status | |||||||||||||||||||||
| Yes | 6 | 0.6 | 0 | NS | 25 | 2.4 | 1.5 | NS | 186 | 7.8 | 4.8 | <.001 | |||||||||
| No | 911 | 99.4 | 0.1 | 1023 | 97.6 | 1.0 | 2192 | 92.2 | 1.9 | ||||||||||||
| Prophylaxis use | |||||||||||||||||||||
| Yes | 8 | 0.9 | –0.5 | NS | 159 | 15.2 | 1.6 | .04 | 1056 | 44.4 | 1.3 | <.001 | |||||||||
| No | 909 | 99.1 | 0.1 | 889 | 84.8 | 0.9 | 1322 | 55.6 | 2.8 | ||||||||||||
| Body mass index | |||||||||||||||||||||
| 13.0 to 17.0 | 271 | 29.6 | –0.6 | <.001 | 328 | 31.3 | –0.1 | <.001 | 816 | 34.3 | 0.4 | <.001 | |||||||||
| 17.1 to 21.1 | 319 | 34.8 | –0.2 | 352 | 33.6 | 0.6 | 742 | 31.2 | 2.1 | ||||||||||||
| 21.2 to 42.0 | 309 | 33.7 | 0.9 | 345 | 32.9 | 2.4 | 760 | 32.0 | 4.1 | ||||||||||||
| No. of bleeds, 6 mo | |||||||||||||||||||||
| 0 | 408 | 44.5 | 0.2 | NS | 261 | 24.9 | 0.6 | NS | 426 | 17.9 | 0.8 | <.001 | |||||||||
| 1 to 4 | 432 | 47.1 | 0.1 | 463 | 44.2 | 1.0 | 843 | 35.4 | 1.4 | ||||||||||||
| 5+ | 77 | 8.4 | 0.1 | 322 | 30.7 | 1.4 | 1105 | 46.5 | 3.3 | ||||||||||||
| Orthopedic procedure | |||||||||||||||||||||
| Yes | 10 | 1.1 | 0.6 | NS | 17 | 1.6 | 2.8 | NS | 103 | 4.3 | 5.6 | <.001 | |||||||||
| No | 907 | 98.9 | 0.1 | 1031 | 98.4 | 1.0 | 2275 | 95.7 | 2.0 | ||||||||||||
. | Mild, n = 917 . | . | . | . | Moderate, n = 1048 . | . | . | . | Severe, n = 2378 . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics . | No. . | % . | % Limitation . | P* . | No. . | % . | % Limitation . | P* . | No. . | % . | % Limitation . | P* . | |||||||||
| Age, y | |||||||||||||||||||||
| 2 to 5 | 158 | 17.2 | –0.5 | <.001 | 211 | 20.1 | –0.3 | <.001 | 491 | 20.6 | –0.5 | <.001 | |||||||||
| 6 to 10 | 259 | 28.2 | –0.3 | 316 | 30.2 | 0.3 | 711 | 29.9 | 0.6 | ||||||||||||
| 11 to 15 | 312 | 34.0 | 0.5 | 317 | 30.2 | 1.4 | 700 | 29.4 | 3.1 | ||||||||||||
| 16 to 19 | 188 | 20.5 | 0.6 | 204 | 19.5 | 3.0 | 476 | 20.2 | 5.9 | ||||||||||||
| Race/ethnicity | |||||||||||||||||||||
| White, non-Hispanic | 631 | 68.8 | 0.1 | NS | 708 | 67.6 | 0.8 | .01 | 1492 | 62.7 | 1.7 | <.001 | |||||||||
| Other | 286 | 31.2 | 0.3 | 340 | 32.4 | 1.6 | 886 | 37.3 | 2.9 | ||||||||||||
| Hemophilia type | |||||||||||||||||||||
| A | 743 | 81.0 | 0.1 | NS | 737 | 70.3 | 1.2 | .02 | 2022 | 85.0 | 2.2 | NS | |||||||||
| B | 174 | 19.0 | 0.2 | 311 | 29.7 | 0.6 | 356 | 15.0 | 1.7 | ||||||||||||
| Family history | |||||||||||||||||||||
| Yes | 754 | 82.2 | 0.1 | NS | 773 | 73.8 | 0.9 | NS | 1367 | 57.5 | 2.1 | NS | |||||||||
| No | 163 | 17.8 | 0.1 | 275 | 26.2 | 1.3 | 1011 | 42.5 | 2.2 | ||||||||||||
| Age at diagnosis | |||||||||||||||||||||
| At/before birth | 370 | 40.4 | –0.1 | NS | 665 | 63.4 | 1.0 | NS | 2039 | 85.7 | 2.0 | NS | |||||||||
| After birth | 524 | 57.1 | 0.2 | 354 | 33.8 | 1.0 | 272 | 11.4 | 2.6 | ||||||||||||
| Age at first HTC visit, y | |||||||||||||||||||||
| Younger than 1 | 255 | 27.8 | –0.1 | .02 | 480 | 45.8 | 0.9 | NS | 1554 | 65.4 | 1.7 | <.001 | |||||||||
| 1 to 2 | 130 | 14.2 | –0.3 | 182 | 17.4 | 1.0 | 332 | 14.0 | 2.6 | ||||||||||||
| 2+ | 501 | 54.6 | 0.4 | 339 | 32.4 | 1.2 | 387 | 16.3 | 3.2 | ||||||||||||
| Insurance status | |||||||||||||||||||||
| Insured | 884 | 96.4 | 0.1 | NS | 1018 | 97.1 | 1.0 | NS | 2330 | 98.0 | 2.1 | .03 | |||||||||
| Uninsured | 33 | 3.6 | 0.1 | 30 | 2.9 | 1.2 | 48 | 2.0 | 3.8 | ||||||||||||
| HTC use | |||||||||||||||||||||
| Frequent | 705 | 76.9 | 0.3 | .01 | 924 | 88.2 | 1.2 | <.01 | 2248 | 94.5 | 2.1 | <.01 | |||||||||
| Infrequent | 212 | 23.1 | –0.3 | 124 | 11.8 | 0.1 | 130 | 5.5 | 3.4 | ||||||||||||
| Inhibitor status | |||||||||||||||||||||
| Yes | 6 | 0.6 | 0 | NS | 25 | 2.4 | 1.5 | NS | 186 | 7.8 | 4.8 | <.001 | |||||||||
| No | 911 | 99.4 | 0.1 | 1023 | 97.6 | 1.0 | 2192 | 92.2 | 1.9 | ||||||||||||
| Prophylaxis use | |||||||||||||||||||||
| Yes | 8 | 0.9 | –0.5 | NS | 159 | 15.2 | 1.6 | .04 | 1056 | 44.4 | 1.3 | <.001 | |||||||||
| No | 909 | 99.1 | 0.1 | 889 | 84.8 | 0.9 | 1322 | 55.6 | 2.8 | ||||||||||||
| Body mass index | |||||||||||||||||||||
| 13.0 to 17.0 | 271 | 29.6 | –0.6 | <.001 | 328 | 31.3 | –0.1 | <.001 | 816 | 34.3 | 0.4 | <.001 | |||||||||
| 17.1 to 21.1 | 319 | 34.8 | –0.2 | 352 | 33.6 | 0.6 | 742 | 31.2 | 2.1 | ||||||||||||
| 21.2 to 42.0 | 309 | 33.7 | 0.9 | 345 | 32.9 | 2.4 | 760 | 32.0 | 4.1 | ||||||||||||
| No. of bleeds, 6 mo | |||||||||||||||||||||
| 0 | 408 | 44.5 | 0.2 | NS | 261 | 24.9 | 0.6 | NS | 426 | 17.9 | 0.8 | <.001 | |||||||||
| 1 to 4 | 432 | 47.1 | 0.1 | 463 | 44.2 | 1.0 | 843 | 35.4 | 1.4 | ||||||||||||
| 5+ | 77 | 8.4 | 0.1 | 322 | 30.7 | 1.4 | 1105 | 46.5 | 3.3 | ||||||||||||
| Orthopedic procedure | |||||||||||||||||||||
| Yes | 10 | 1.1 | 0.6 | NS | 17 | 1.6 | 2.8 | NS | 103 | 4.3 | 5.6 | <.001 | |||||||||
| No | 907 | 98.9 | 0.1 | 1031 | 98.4 | 1.0 | 2275 | 95.7 | 2.0 | ||||||||||||
Totals for characteristics do not all sum to 100% because of missing data.
NS indicates not significant.
P values of .05 or less indicate that average joint ROM limitation differs significantly across levels of the characteristic based on analysis of variance
The numbers and percents of patients with asymmetrically mobile joints by severity were: mild, 305 (33.3%); moderate, 396 (37.8%); and severe, 1180 (49.6%). The average values of overall percentage of ROM limitation for participants in each category of patient characteristic by severity level are also shown in Table 1. For all severity groups, ROM limitation increased with age and BMI. For persons with moderate or severe disease, ROM limitation was greater among persons whose race/ethnicity was other than non-Hispanic white. Frequent HTC users with mild or moderate disease had more ROM limitation than infrequent users. On the other hand, frequent HTC users with severe disease had less ROM limitation than infrequent users. In addition, among persons with mild hemophilia, ROM limitation was greater among those whose age at first HTC visit was 2 years or older. Among persons with moderate disease, ROM limitation was greater among participants with hemophilia A. For persons with severe hemophilia, ROM limitation increased significantly with more frequent bleeding episodes and with older age at the first HTC visit; ROM limitation for these persons was greater among those who were uninsured, those with an inhibitor, those not on prophylaxis, and those who had had a recent orthopedic procedure.
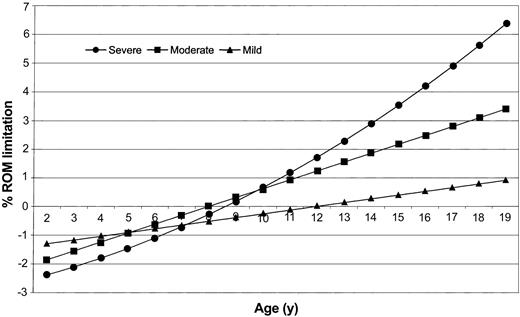
The results of 3 separate linear regression analyses are shown in Table 2. The coefficient, standard error, and P value are given for those factors that were independently associated with the overall percentage of ROM limitation for persons with severe, moderate, and mild hemophilia. For those with severe disease, ROM limitation increased with age, BMI, and number of bleeding episodes and was greater among persons who were nonwhite, had an inhibitor, and had undergone an orthopedic procedure. The age-squared term, significant only in the severe hemophilia regression model, implied that ROM limitation was not linear across age groups but was curvilinear instead (Figure 1).
Factors independently associated with overall percentage of joint range-of-motion limitation among 2- to 19-year-old males with hemophilia based on separate linear regression models for disease severity
. | Severe, n = 2282 . | . | . | Moderate, n = 1010 . | . | . | Mild, n = 895 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor . | β . | SE . | P . | β . | SE . | P . | β . | SE . | P . | ||||||
| Intercept | —3.37 | 0.455 | < .001 | —2.71 | 0.418 | < .001 | —2.12 | 0.323 | < .001 | ||||||
| Age | —0.03 | 0.075 | .65 | 0.15 | 0.023 | < .001 | 0.02 | 0.018 | .2 | ||||||
| Age-squared | 0.02 | 0.003 | < .001 | — | — | — | — | — | — | ||||||
| Nonwhite race | 0.88 | 0.170 | < .001 | — | — | — | — | — | — | ||||||
| Body mass index | 0.13 | 0.018 | < .001 | 0.08 | 0.022 | < .001 | 0.09 | 0.018 | < .001 | ||||||
| Inhibitor | 1.71 | 0.317 | < .001 | — | — | — | — | — | — | ||||||
| Number of bleeds | 0.04 | 0.006 | < .001 | — | — | — | — | — | — | ||||||
| Orthopedic procedure | 0.99 | 0.416 | .02 | — | — | — | — | — | — | ||||||
| Infrequent HTC use | — | — | — | —0.66 | 0.294 | .02 | —0.42 | 0.169 | .01 | ||||||
| Hemophilia A (vs B) | — | — | — | 0.50 | 0.208 | .02 | — | — | — | ||||||
. | Severe, n = 2282 . | . | . | Moderate, n = 1010 . | . | . | Mild, n = 895 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor . | β . | SE . | P . | β . | SE . | P . | β . | SE . | P . | ||||||
| Intercept | —3.37 | 0.455 | < .001 | —2.71 | 0.418 | < .001 | —2.12 | 0.323 | < .001 | ||||||
| Age | —0.03 | 0.075 | .65 | 0.15 | 0.023 | < .001 | 0.02 | 0.018 | .2 | ||||||
| Age-squared | 0.02 | 0.003 | < .001 | — | — | — | — | — | — | ||||||
| Nonwhite race | 0.88 | 0.170 | < .001 | — | — | — | — | — | — | ||||||
| Body mass index | 0.13 | 0.018 | < .001 | 0.08 | 0.022 | < .001 | 0.09 | 0.018 | < .001 | ||||||
| Inhibitor | 1.71 | 0.317 | < .001 | — | — | — | — | — | — | ||||||
| Number of bleeds | 0.04 | 0.006 | < .001 | — | — | — | — | — | — | ||||||
| Orthopedic procedure | 0.99 | 0.416 | .02 | — | — | — | — | — | — | ||||||
| Infrequent HTC use | — | — | — | —0.66 | 0.294 | .02 | —0.42 | 0.169 | .01 | ||||||
| Hemophilia A (vs B) | — | — | — | 0.50 | 0.208 | .02 | — | — | — | ||||||
Total numbers of persons with complete data for all variables and included in each regression analysis.
β indicates parameter estimate; SE, standard error; and HTC, hemophilia treatment center. — indicates factor not included in the regression model.
Predicted percentage of overall range-of-motion (ROM) limitation by age and severity, for 2- to 19-year-old males with hemophilia. Graph was created using separate regression models for severe, moderate, and mild hemophilia (models are presented in Table 2). Percentage of ROM limitation was calculated by setting categoric variables equal to the reference value and by using the severity group mean value for continuous variables such as body mass index and number of bleeds.
Predicted percentage of overall range-of-motion (ROM) limitation by age and severity, for 2- to 19-year-old males with hemophilia. Graph was created using separate regression models for severe, moderate, and mild hemophilia (models are presented in Table 2). Percentage of ROM limitation was calculated by setting categoric variables equal to the reference value and by using the severity group mean value for continuous variables such as body mass index and number of bleeds.
Increasing age and BMI were also positively associated with percentage of ROM limitation among persons with moderate hemophilia (Table 2). In addition, those with moderate hemophilia A had more ROM limitation than persons with moderate hemophilia B. Increasing BMI was also associated with ROM limitation among persons with mild hemophilia. None of the other factors associated with ROM limitation among persons with severe disease were related to ROM limitation among those more mildly affected. However, unlike for those with severe disease, infrequent HTC use by participants with mild and moderate disease was associated with a lesser degree of ROM limitation than was frequent use, as indicated by the negative parameter values (Table 2).
The parameter values from the regression analyses are an estimate of the amount of ROM limitation attributable to each of the studied risk factors. For example, a person with severe hemophilia and an inhibitor was predicted by the model to have an average 1.71% more overall ROM limitation than an otherwise identical patient without an inhibitor (Table 2).
Because we used normal adult ROM values as the standard, ROM limitation was a negative value for the youngest children since their joints were more flexible than those of adults (Figure 1). We used the regression models to predict the average age at which children lost this excess flexibility (the age at which percentage of ROM limitation was equal to zero). The models predicted that the ages were 8, 8.6, and 12 years for participants with severe, moderate, and mild hemophilia, respectively.
Discussion
The basis for the ROM limitation among persons with hemophilia is chronic bleeding into the joint that produces an inflammatory response that leads over time to a characteristic hemophilic arthropathy.1 Our analyses focused on ROM limitation that was accompanied by asymmetric joint mobility. We used a conservative definition for asymmetry to limit the effects of measurement errors. When we examined joint data collected by our surveillance on 817 males older than the age of 30 with severe hemophilia A, we found that less than 5% had bilateral ROM limitations. These data provide evidence that asymmetric ROM limitations are more likely to be hemophilia related.
Our finding that the number of reported bleeds was significantly associated with ROM limitation only among participants with severe disease was unexpected. We used the reported number of bleeds that occurred in the 6 months prior to the visit at which the joint measurements were taken as an estimate of bleeding frequency. The results were unchanged when we repeated the analysis using the number of joint bleeds. One possible explanation for the lack of association that we found between ROM limitation and this measure among participants with mild or moderate hemophilia involves the fact that bleeding episodes occur less frequently and in irregular patterns over time among more mildly affected patients.5 Therefore, our estimate may not accurately reflect the long-term bleeding frequency for these persons. Another possibility is that patients with milder disease were less likely to accurately report their bleeding frequency either because of poor record keeping or perhaps because bleeding episodes went unnoticed. Unrecognized bleeding in these patients (because of its infrequency or subclinical presentation) could be an important cause of joint damage. A lack of correlation between bleeding history and both clinical and radiologic evidence of arthritis was also found by Steven et al,6 who speculated that perhaps subclinical bleeding that did not cross the synovial lining layer might be arthritogenic.
The inverse relationship that we found between ROM limitation and the frequency of HTC use among persons with moderate and mild hemophilia is also probably related to variations in the frequency of bleeding among less severely affected patients. Persons who experience few bleeding episodes not only are less likely to make frequent visits to an HTC but also should have less ROM limitation as a result. Our measure of bleeding frequency, because it was based on bleeding during a very short period of time, was probably too insensitive a measure to adequately account for these variations. More study of bleeding patterns among mildly affected patients is needed to adequately address these issues.
The lesser degree of ROM limitation that we observed among prophylaxis users with moderate or severe hemophilia compared with nonusers was no longer statistically significant after adjustment for the other factors that we examined. One reason is that the relationship between prophylaxis use and ROM limitation was probably confounded by age since prophylaxis use was more prevalent among younger participants. An additional complicating factor is that some participants currently using prophylaxis may have received this treatment from an early age to prevent any bleeds, while others may have begun prophylaxis only after repeated bleeds in an attempt to prevent further joint damage. Since we collected only the current treatment type and had no information about duration of use, we were unable to account for this important distinction in the analysis. The cross-sectional design of this study limited our ability to adequately assess whether prophylaxis use influences ROM limitation.
Among persons with moderate hemophilia, those deficient in factor VIII (FVIII) had a greater degree of ROM limitation than those who were factor IX (FIX) deficient. This finding provides evidence against the commonly held belief that the hemophilia types behave similarly. The distributions of baseline factor activity levels within the moderate category were nearly identical for both hemophilia types. A possible explanation may lie with differences in the pharmacokinetics of the 2 infused plasma proteins. FVIII resides exclusively in the plasma fraction of the intravascular space, and its residence time is determined exclusively by plasma clearance. In contrast, FIX distributes extravascularly as well as in the plasma volume of the circulation. In addition, the half-life of FIX is longer than that of FVIII (19-24 vs 9-13 hours). As a result, better sustained circulating levels of FIX than FVIII after an infusion would tend to reduce the number of recurrent bleeding episodes.
In addition, a substantial proportion of patients has been shown to have mutations of the hemophilia B gene (founders effects) that are associated with a mild phenotype despite a measured factor activity level in the moderate range.7 An examination of data collected on all UDC participants regardless of age or severity revealed that, overall, persons with hemophilia B consistently reported fewer bleeds and had less ROM limitation than those with hemophilia A. Further study of this issue is needed.
When we adjusted for differences in all of the studied risk factors using our regression models, persons of other races or ethnicities with severe hemophilia had nearly 1% more overall ROM limitation than did non-Hispanic whites. One possible reason for this finding is less access to care among minorities than among non-Hispanic whites. We have previously reported that minority males with hemophilia are more likely to be hospitalized for a bleeding complication than are their white, non-Hispanic counterparts.8 It is likely that lack of prompt access to and use of factor to treat bleeds would lead not only to higher rates of hospitalization to treat resultant bleeding complications but also, in the case of joint bleeding, to greater ROM limitation over time.
Another possible explanation involves the hypercoagulable mutations, such as factor V Leiden and prothrombin, which have been suggested to provide a protective effect in people with hemophilia,9-11 although some investigators have not seen this association.12 The prevalence of these hypercoagulable mutations is greater in whites13,14 than in African Americans.15,16 Therefore, this could be one mechanism by which ethnicity (via genetics) influences the impact that recurrent hemarthroses have on ROM limitation.
The increased ROM limitation that we saw among minority males may also reflect a propensity for these boys to participate in physical activities that expose joints to severe stress and trauma. One recent consumer panel survey17 that included more than 1500 nonhemophilic boys aged 9 to 18 years found that, compared with whites, a higher proportion of African-American boys reported playing basketball and football, while a lower proportion reported swimming on a regular basis (Porter Novelli International, Washington, DC, unpublished data, November 2002). Unfortunately, we had no information about physical activity among participants of our study with which to examine this possibility. The influence of any of these possible reasons for the racial/ethnic differences in ROM limitation that we observed is likely to be strongest among those with severe disease.
BMI is a potentially modifiable risk factor that was strongly associated with ROM limitation regardless of hemophilia severity. Moreover, the effect of greater BMI on ROM limitation varied greatly depending on the level of severity. For example, a 14-year-old boy with severe hemophilia in the 50th percentile of BMI for his age is predicted by the regression model to have a 2.9% overall limitation in ROM. If the same boy is in the 95th percentile of BMI, his predicted ROM limitation is 3.8%—an increase of 30%. A similar analysis for a 14-year-old boy with mild hemophilia is predicted to have a 0.01% and 0.7% overall ROM limitation for the 2 BMI percentile levels, which represents a 70-fold difference. In the general population, a much higher proportion of persons who are overweight report arthritis and chronic joint problems compared with those who are not overweight.18 For persons with hemophilia who are overweight, an additional health benefit of weight loss may be a substantial reduction in ROM limitation over time.
In the general population, very young children have greater flexibility in their joints than adults, a characteristic that gradually disappears with age. Exactly when this increased flexibility relative to adults is lost is difficult to determine because ROM data in the general population are scarce. A British study of 3354 school children found excessive joint laxity in 45% of 2-year-olds, which rapidly declined in prevalence to about 5% of 6-year-olds and to less than 1% of 12-year-olds.19 If we assume a similar pattern for the loss of hyperflexibility in children, our estimate of 12 years of age for this loss among persons with mild hemophilia seems appropriate. However, our models predict that excess flexibility loss occurs about 4 years earlier for those with moderate or severe hemophilia. These findings suggest that ROM limitation due to bleeding may begin at a very early age for persons with moderate or severe disease and that more aggressive treatment for bleeds that occur early in life may be required to prevent early onset of joint ROM limitation.
Several limitations should be considered when interpreting the results of this study. First, because we lacked sufficient follow-up data, we used a cross-sectional study design in which ROM limitation was examined among different persons over a range of ages rather than a prospective design in which ROM loss in individuals was measured over time. Use of this design has at least 3 important implications. First, our estimates of the strength of the associations between the risk factors that we studied and ROM limitation may be influenced by misclassification bias. This occurs when study subjects are incorrectly classified with respect to the studied risk factors. For example, a person who may have had an inhibitor in the past but was successfully immune tolerized by the time of the study would be classified as not currently having an inhibitor. However, he might have more ROM limitation than a person who had never had an inhibitor. In general, this type of study bias results in underestimates of the strength of the association.20 Because we included persons younger than 20 years in our study, the risk factors that we studied were less likely to have changed over time, thus minimizing this bias. Nonetheless, our estimates of the strength of some of the associations that we found may be conservative.
Second, with regard to our findings concerning BMI, we cannot determine from cross-sectional data whether increases in BMI were a cause or an effect of the joint ROM limitation that we observed. It is possible that children who have greater ROM limitation due to bleeding are more likely to become sedentary and, therefore, more at risk of becoming overweight. However, the fact that overweight people in the general population are more likely to experience joint problems and arthritis supports our contention that increased BMI is likely a cause.
Third, predictions based on our regression models are also influenced by study design. Our models assume that a 5-year-old boy with severe hemophilia in our cohort will have the same amount of ROM limitation after a 14-year period that a similarly affected 19-year-old boy in our cohort has today. Although this is a reasonable assumption for purposes of prediction, future changes in treatment or other aspects of hemophilia care may result in better outcomes than those predicted by our models.
Another limitation of our study is that since ROM data on the general population are scarce, we relied on published values for normal ROM that were based on small studies and that were unadjusted for BMI.4 It is hard to predict what effect this may have had on our results. To the extent that the values we used truly represent the general population, we may have either over- or underestimated the actual amount of ROM limitation due to the risk factors that we studied. However, our choice of standard would not have affected the relative quantitative effects among risk factors (eg, the similar extent of ROM limitation associated with nonwhite race and orthopedic procedures in persons with severe hemophilia) that we observed.
Finally, our cohort comprised persons who received care in an HTC and who volunteered to participate in our surveillance— factors that may affect the generalizability of our results. However, a comprehensive study of all persons with hemophilia in 6 US states indicated that nearly 70% of affected individuals receive care in an HTC.21 Furthermore, since the inception of the UDC project, HTC staff have consistently enrolled more than 90% of all eligible patients invited to participate.2 Nonetheless, it is important to recognize that our results reflect ROM limitations that occur among persons who are receiving high-quality care and may represent the lower end of the spectrum of joint damage that occurs in this population. Even so, a 1% overall ROM loss translates to nearly 17 degrees of ROM limitation; if concentrated in one “target” joint, which is a common pattern in this disorder, this loss may represent a substantial decrease in joint mobility.
In summary, we found that both the extent of and the risk factors for ROM limitation among children with hemophilia varied with disease severity. BMI, a potentially modifiable risk factor, was strongly associated with ROM loss across all severity levels, but especially among those with mild hemophilia. Current public health recommendations for persons with hemophilia include regular exercise using low-impact activities such as swimming or calisthenics to increase muscle tone and strengthen joints. An additional component that may be considered is routine monitoring of BMI, shown to be a better measure of adiposity than weight alone, using recently developed BMI growth charts.22 Dietary interventions designed to reduce weight among persons with bleeding disorders in the upper percentiles of BMI for age and sex may be the most effective prevention for ROM loss besides avoidance of joint bleeds.
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2003-05-1457.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank the staffs of the 136 federally funded hemophilia treatment centers for enrolling their patients in the UDC project and for collecting the data, without which this study could not have been done. We appreciate project coordination assistance from the Federal Hemophilia Treatment Center Program Regional Coordinators: Ann Forsberg, Worcester, MA; Mariam Voutsis, New York, NY; Sue Cutter, Philadelphia, PA; Richard Atwood, Winston-Salem, NC; Karen Droze, Atlanta, GA; Tamara Wood-Lively, Ann Arbor, MI; Mary Anne Schall, Wauwatosa, WI; John Drake, Houston, TX; Becky Dudley, Kansas City, MO; Mary Lou Damiano, Tucson, AZ; Judith Baker, Los Angeles, CA; and Robina Ingram-Rich, Portland, OR. We also wish to thank the Physical Therapy Working Group of the National Hemophilia Foundation for training and certification of HTC staff for UDC range-of-motion measurements: Kristin Keenaghan, Providence, RI; Irene Vlaskamp, New York, NY; Angela Forsyth, Philadelphia, PA; Michele Audet, Atlanta, GA; Sherry Herman-Hilker, Ann Arbor, MI; Kimberly Baumann, Minneapolis, MN; Alice Anderson, Dallas, TX; Julie Hart Musick, Kansas City, MO; Kris Albrecht, Phoenix, AZ; Grace Hernandez, Orange, CA; and David McFarland-Smith, Boise, ID. Finally, we acknowledge the kind assistance of Michelle Yore in the Physical Activities Branch of CDC with the analysis of the consumer panel survey data.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal