Abstract
Hematopoietic stem cells (HSCs) undergo dramatic expansion during fetal liver development, but attempts to expand their numbers ex vivo have failed. We hypothesized that unidentified fetal liver cells produce growth factors that support HSC proliferation. Here we describe a novel population of CD3+ and Ter119- day-15 fetal liver cells that support HSC expansion in culture, as determined by limiting dilution mouse reconstitution analyses. DNA array experiments showed that, among other proteins, insulin-like growth factor 2 (IGF-2) is specifically expressed in fetal liver CD3+ cells but not in several cells that do not support HSCs. Treatment of fetal liver CD3+Ter119- cells with anti–IGF-2 abrogated their HSC supportive activity, suggesting that IGF-2 is the key molecule produced by these cells that stimulates HSC expansion. All mouse fetal liver and adult bone marrow HSCs express receptors for IGF-2. Indeed, when combined with other growth factors, IGF-2 supports a 2-fold expansion of day-15 fetal liver Lin-Sca-1+c-Kit+ long-term (LT)–HSC numbers. Thus, fetal liver CD3+Ter119- cells are a novel stromal population that is capable of supporting HSC expansion, and IGF-2, produced by these cells, is an important growth factor for fetal liver and, as we show, adult bone marrow HSCs.
Introduction
In vivo stromal cells form a complex microenvironment for hematopoietic stem cells (HSCs) that controls their multiple fates, including apoptosis and migration as well as the cell divisions that lead to formation either of daughter HSCs or of lineage-committed progenitors that are capable of limited proliferation.1,2 Culture of HSCs in vitro, away from their natural microenvironment, leads to apoptosis or differentiation but not HSC self-renewal.
Genetic studies in mice, together with studies in which purified proteins were added to cultures of partially purified HSC populations, have led to the identification of secreted proteins that, in combination with others, maintain or expand the number of bone marrow HSCs. In mice, mutations either in stem cell factor (SCF) or its receptor, the protein tyrosine kinase Kit, lead to a profound reduction of HSC activities and numbers.3,4 In vitro, SCF can prevent apoptosis of HSCs and is a growth factor for primitive lymphoid and myeloid hematopoietic progenitor or stem cells.5,6 Both TPO-/- mice and mice with mutations in its receptor, mpl, have a marked reduction in HSC activity,7 and thrombopoietin (TPO) can promote survival8 and expansion9,10 of bone marrow HSCs in culture. Addition of interleukin-11 (IL-11) together with SCF or Flt-3 ligand (FL) maintains and expands bone marrow HSCs.11 Flt-3 ligand (FL) receptor-deficient mice have many deficiencies in hematopoiesis,6 but elimination of the IL-11 receptor does not affect HSC numbers.12 FL has been included in numerous culture systems for HSCs,6 and several papers suggested that the FL receptor is expressed in some fetal liver long-term (LT)–HSCs but not in bone marrow LT-HSCs.13-15
Addition of fibroblast growth factor-1 (FGF-1) resulted in robust expansion of repopulating HSCs when total bone marrow cells were cultured in serum-free medium.16 Because purified HSCs cannot be expanded using FGF-1, the exact nature of the FGF-1 target cell population remains to be determined.16 Recently, purified and lipid-modified Wnt3a protein, when added in combination with fetal bovine serum and SCF, enhanced ex vivo expansion of purified bone marrow c-Kit+Thy-1.1loSca-1+Lin- hematopoietic progenitor cells.17,18 However, mouse repopulation was studied only at 4 or 6 weeks after transplantation. Thus, it is not known whether Wnt3a supports expansion of long-term repopulating stem cells.
Although fetal liver HSCs have a greater proliferative potential than adult bone marrow HSCs and undergo significant expansion in vivo,19-21 attempts to expand fetal liver HSCs ex vivo have failed.11 We hypothesized that unknown growth proteins are produced by as yet unidentified populations of fetal liver cells that stimulate the expansion of fetal liver HSCs. Here we studied the effects of differentiated fetal liver hematopoietic cells on HSCs cultured ex vivo and identified a novel cell population, day-15 fetal liver CD3+ cells that support expansion of HSC numbers in culture. We show that insulin-like growth factor 2 (IGF-2) is specifically produced by fetal liver CD3+ cells and is the key molecule produced by these cells that stimulates HSC expansion. Furthermore, when combined with other growth factors, IGF-2 is capable of markedly enhancing ex vivo expansion of long-term repopulating fetal liver and adult bone marrow HSCs. Thus, IGF-2 is a novel growth factor for HSCs.
Materials and methods
Cell culture
Lin-Sca-1+Kit+ or side population (SP) cells were cultured alone or cocultured with the same number of fetal liver CD3+ cells as indicated, at a density of 50 Lin-Sca-1+Kit+ or SP cells per 30 μL in Iscove modified Dulbecco medium (IMDM) with 10% fetal bovine serum (FBS) (StemCell Technologies, Vancouver, BC), 1% bovine serum albumin (BSA) (Sigma, St Louis, MO), and 50 μM β-mercaptoethanol (Invitrogen, Carlsbad, CA) in a well of a U-bottom 96-well plate. Murine IGF-2 (R&D Systems, Minneapolis, MN), 20 ng/mL SCF (R&D Systems), 30 ng/mL Flt-3 ligand (R&D Systems), and 10 ng/mL IL-6 (R&D Systems), or 50 ng/mL SCF and 100 ng/mL TPO (R&D Systems), as well as anti–IGF-2 neutralizing antibody (R&D Systems) were used as indicated.
FACS and analysis
Donor fetal liver or bone marrow cells were isolated from C57BL/6 CD45.2 mice. To sort Lin-Sca-1+Kit+ cells, nucleated cells were incubated with rat serum for 15 minutes at 4°C at a density of 5 × 107/mL. Cells were then stained with a biotinylated Lin+ antibody cocktail (for fetal liver cells, anti-CD3, anti-CD5, anti-B220, anti–Gr-1, anti-Ter119, and anti–7-4; for bone marrow cells, anti–Mac-1 was also included; StemCell Technologies and BD Pharmingen, San Diego, CA) for 15 minutes at 4°C. Cells were washed and incubated for 20 minutes with streptavidin-allophycocyanin (streptavidin-APC), anti-c-Kit–phycoerythrin (anti-c-Kit–PE), and anti-Sca-1–fluorescein isothiocyanate (anti-Sca-1–FITC). After 2 washes the cells were isolated by fluorescence-activated cell sorting (FACS) in a MoFlo cell sorter. Fetal liver CD3+ cells were sorted after staining with the anti-CD3–PE antibody. Adult mouse bone marrow SP cells were isolated essentially as described.22
To sort IGF-2–hFc+ and IGF-2–hFc- cells, 107 cells were resuspended in 1 mL conditioned medium (with about 1 μg/mL IGF-2–hFc determined by Western blotting) at 4°C for 30 minutes, followed by staining with anti-human immunoglobulin G1 (IgG1) Fc-PE (Jackson ImmunoResearch, West Grove, PA). The conditioned medium from mock-transfected cells was used as control. When necessary, the costaining of biotinylated Lin+ antibody cocktail followed by streptavidin-APC and anti-Sca-1–FITC was performed before sorting Lin-Sca-1+IGF-2–hFc+ and Lin-Sca-1+IGF-2–hFc- cells.
For reconstitution analysis, peripheral blood cells were collected by retro-orbital bleeding, followed by lysis of red blood cells and staining with anti-CD45.2–FITC, anti-CD45.1–PE, anti-Thy1.2–PE, anti-B220–PE, anti-Mac-1–PE, anti-Gr-1–PE, or anti-Ter119–PE monoclonal antibodies (BD Pharmingen). When FACS analysis was used for characterizing CD3+ cells, day 15 fetal liver cells or adult splenic cells were stained with isotype control, anti-CD3–FITC, anti-CD4–PE, anti-CD8–PE, anti-Thy1.2–PE, and anti-Ter119–PE monoclonal antibodies (BD Pharmingen). FACS analyses were performed on a FACSCalibur instrument.
Competitive reconstitution analysis
Both activity assays and limiting dilution assays were performed using the competitive reconstitution protocol. Indicated numbers of CD45.2 donor cells were mixed with 2 × 105 (1 × 105 used in Figures 4A and 6B) freshly isolated CD45.1 competitor bone marrow cells, which were then injected intravenously via the retro-orbital route into a group of CD45.1 mice previously irradiated with a total dose of 10 Gy. To measure reconstitution of mice undergoing transplantation, peripheral blood was collected by retro-orbital bleeding at the indicated times after transplantation, and the presence of CD45.1+ and CD45.2+ cells in lymphoid and myeloid compartments was measured.
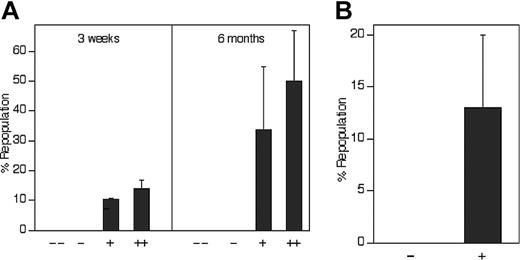
The level of expression of IGF-2 receptors positively correlates with repopulating HSC activity. (A) All repopulating HSCs in the total population of fetal liver cells bind IGF-2–hFc. Of the 36.2% of total CD45.2 fetal liver cells that bind the IGF-2–hFc fusion protein (Figure 3Bv), the top 30% and the lower 70% were arbitrarily sorted into the ++ and + fractions, respectively. Similarly, of the 63.8% that cannot bind the IGF-2–hFc fusion protein, the top 70% and the lower 30% were arbitrarily sorted into the - and - - fractions, respectively. As in Figure 3C, reanalysis of sorted cells indicted that the purity of IGF-2–hFc+ and IGF-2–hFc- cells was higher than 80% (not shown). From each group of total fetal liver cells, 104 cells were transplanted together with 105 CD45.1 competitor cells into lethally irradiated CD45.1 mice (n = 4 to 5). Peripheral blood cells were analyzed for the presence of CD45.2+ cells in lymphoid and myeloid compartments at 3 weeks and 6 months after transplantation. (B) All repopulating HSCs in the Lin-Sca-1+ subpopulation of fetal liver cells bind IGF-2–hFc. Fifty CD45.2 fetal liver Lin-Sca-1+IGF-2–hFc+ and 100 Lin-Sca-1+IGF-2–hFc- cells sorted as depicted in Figure 3Bv were transplanted together with 2 × 105 CD45.1 competitor cells into lethally irradiated CD45.1 mice (n = 4 to 5). Peripheral blood cells were analyzed for the presence of CD45.2+ cells in lymphoid and myeloid compartments at 6 months after transplantation. Error bars indicate SEM.
The level of expression of IGF-2 receptors positively correlates with repopulating HSC activity. (A) All repopulating HSCs in the total population of fetal liver cells bind IGF-2–hFc. Of the 36.2% of total CD45.2 fetal liver cells that bind the IGF-2–hFc fusion protein (Figure 3Bv), the top 30% and the lower 70% were arbitrarily sorted into the ++ and + fractions, respectively. Similarly, of the 63.8% that cannot bind the IGF-2–hFc fusion protein, the top 70% and the lower 30% were arbitrarily sorted into the - and - - fractions, respectively. As in Figure 3C, reanalysis of sorted cells indicted that the purity of IGF-2–hFc+ and IGF-2–hFc- cells was higher than 80% (not shown). From each group of total fetal liver cells, 104 cells were transplanted together with 105 CD45.1 competitor cells into lethally irradiated CD45.1 mice (n = 4 to 5). Peripheral blood cells were analyzed for the presence of CD45.2+ cells in lymphoid and myeloid compartments at 3 weeks and 6 months after transplantation. (B) All repopulating HSCs in the Lin-Sca-1+ subpopulation of fetal liver cells bind IGF-2–hFc. Fifty CD45.2 fetal liver Lin-Sca-1+IGF-2–hFc+ and 100 Lin-Sca-1+IGF-2–hFc- cells sorted as depicted in Figure 3Bv were transplanted together with 2 × 105 CD45.1 competitor cells into lethally irradiated CD45.1 mice (n = 4 to 5). Peripheral blood cells were analyzed for the presence of CD45.2+ cells in lymphoid and myeloid compartments at 6 months after transplantation. Error bars indicate SEM.
Culture with IGF-2 increases in vivo repopulating activity of adult bone marrow HSCs. (A) About 50% of adult bone marrow Lin-Sca-1+ cells specifically bind IGF-2–hFc. Adult bone marrow cells were incubated at 4°C for 30 minutes with conditioned medium from control transfected 293T cells (iii) or cells transfected with the IGF-2–hFc expression vector (iv,v); the latter medium contained about 1 μg/mL IGF-2–hFc. Cells were then stained with antihuman IgG1-PE, followed by the biotinylated Lin+ antibody cocktail, streptavidin-APC, and anti-Sca-1–FITC. Total bone marrow cells and Lin- cells were then stained, gated (i,ii), and analyzed as in Figure 3B to quantify the binding to IGF-2–hFc and the level of Sca-1. (B) All repopulating HSCs in the total population of bone marrow cells bind IGF-2–hFc. Of the 42.0% of total CD45.2 bone marrow cells that bind the IGF-2–hFc fusion protein (panel Aiv), the top 30% and the lower 70% were arbitrarily sorted into the ++ and + fractions, respectively. Similarly, of the 58.0% that cannot bind the IGF-2–hFc fusion protein (as shown in panel A), the top 70% and the lower 30% were arbitrarily sorted into the - and - - fractions, respectively. From each group of total bone marrow cells, 104 cells were transplanted together with 105 CD45.1 competitor cells into lethally irradiated CD45.1 mice (n = 4 to 5). Peripheral blood cells were analyzed for the presence of CD45.2+ cells in lymphoid and myeloid compartments at 3 weeks and 6 months after transplantation. (C) All repopulating HSCs in the Lin-Sca-1+ subpopulation of bone marrow cells bind IGF-2–hFc. Fifty CD45.2 bone marrow Lin-Sca-1+IGF-2–hFc+ and 100 Lin-Sca-1+IGF-2–hFc- cells, sorted as depicted in panel Av, were transplanted together with 2 × 105 CD45.1 competitor cells into lethally irradiated CD45.1 mice (n = 4 to 5). Peripheral blood cells were analyzed for the presence of CD45.2+ cells in lymphoid and myeloid compartments at 6 months after transplantation. Error bars represent SEM. (D) Culture of adult bone marrow SP cells with IGF-2 enhances in vivo repopulating stem cell activity. Fifty freshly isolated adult CD45.2 bone marrow SP cells were transplanted directly (with 2 × 105 CD45.1 competitor bone marrow cells per mouse, n = 9) into CD45.1 congenic mice (i). From the same isolation 50 SP cells were cultured 3 days in medium alone (ii) or in medium with 1 μg/mL IGF-2 (iii). Serum-containing medium supplemented with SCF, FL, and IL-6 was used. Together with 2 × 105 competitor CD45.1 bone marrow cells, the entire culture derived from these initial 50 SP cells was coinjected into CD45.1 recipients (n = 8 to 9). Peripheral blood cells from mice undergoing transplantation were analyzed for the presence of CD45.2+ cells in lymphoid and myeloid compartments at 3.5 months after transplantation. *Significantly different from panel i and ii values; P < .05.
Culture with IGF-2 increases in vivo repopulating activity of adult bone marrow HSCs. (A) About 50% of adult bone marrow Lin-Sca-1+ cells specifically bind IGF-2–hFc. Adult bone marrow cells were incubated at 4°C for 30 minutes with conditioned medium from control transfected 293T cells (iii) or cells transfected with the IGF-2–hFc expression vector (iv,v); the latter medium contained about 1 μg/mL IGF-2–hFc. Cells were then stained with antihuman IgG1-PE, followed by the biotinylated Lin+ antibody cocktail, streptavidin-APC, and anti-Sca-1–FITC. Total bone marrow cells and Lin- cells were then stained, gated (i,ii), and analyzed as in Figure 3B to quantify the binding to IGF-2–hFc and the level of Sca-1. (B) All repopulating HSCs in the total population of bone marrow cells bind IGF-2–hFc. Of the 42.0% of total CD45.2 bone marrow cells that bind the IGF-2–hFc fusion protein (panel Aiv), the top 30% and the lower 70% were arbitrarily sorted into the ++ and + fractions, respectively. Similarly, of the 58.0% that cannot bind the IGF-2–hFc fusion protein (as shown in panel A), the top 70% and the lower 30% were arbitrarily sorted into the - and - - fractions, respectively. From each group of total bone marrow cells, 104 cells were transplanted together with 105 CD45.1 competitor cells into lethally irradiated CD45.1 mice (n = 4 to 5). Peripheral blood cells were analyzed for the presence of CD45.2+ cells in lymphoid and myeloid compartments at 3 weeks and 6 months after transplantation. (C) All repopulating HSCs in the Lin-Sca-1+ subpopulation of bone marrow cells bind IGF-2–hFc. Fifty CD45.2 bone marrow Lin-Sca-1+IGF-2–hFc+ and 100 Lin-Sca-1+IGF-2–hFc- cells, sorted as depicted in panel Av, were transplanted together with 2 × 105 CD45.1 competitor cells into lethally irradiated CD45.1 mice (n = 4 to 5). Peripheral blood cells were analyzed for the presence of CD45.2+ cells in lymphoid and myeloid compartments at 6 months after transplantation. Error bars represent SEM. (D) Culture of adult bone marrow SP cells with IGF-2 enhances in vivo repopulating stem cell activity. Fifty freshly isolated adult CD45.2 bone marrow SP cells were transplanted directly (with 2 × 105 CD45.1 competitor bone marrow cells per mouse, n = 9) into CD45.1 congenic mice (i). From the same isolation 50 SP cells were cultured 3 days in medium alone (ii) or in medium with 1 μg/mL IGF-2 (iii). Serum-containing medium supplemented with SCF, FL, and IL-6 was used. Together with 2 × 105 competitor CD45.1 bone marrow cells, the entire culture derived from these initial 50 SP cells was coinjected into CD45.1 recipients (n = 8 to 9). Peripheral blood cells from mice undergoing transplantation were analyzed for the presence of CD45.2+ cells in lymphoid and myeloid compartments at 3.5 months after transplantation. *Significantly different from panel i and ii values; P < .05.
Calculation of stem cell frequency
In limiting dilution assays, the frequency of repopulating cells (competitive repopulating units [CRUs]) was estimated on the basis of Poisson statistics as described11 ; the ranges of CRUs are given as 95% confidence intervals. For the experiment in Figure 6D, because the numbers of injected cells were insufficient for limiting dilution assays, the frequencies of HSCs were calculated as follows. If n = unknown frequency of HSCs in the population and m = injected cell number, the probability of a mouse not repopulated by the donor cells is P = (1 -n)m. For a group of mice in Figure 6D, the P value, which is the ratio of the number of nonchimeric mice to the total number of mice, and m, which is the number of injected cells, are both known and n can be calculated. When all the recipient mice were repopulated, we could not calculate the actual n value; instead we calculated a mock n value by assuming that 1 of the recipient mice was not repopulated. The actual 1/n value was represented as less than 1 per mock n value.
DNA array experiments and analyses
Fifteen micrograms of total RNA and cRNA were prepared for hybridization to Affymetrix U74Av2 mouse chips according to manufacturer's instructions. The analysis was performed as detailed in our recent paper.23
Measuring the expression of IGF-2 and its receptors
Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed on cDNAs isolated from FACS-sorted murine day 15 fetal liver Lin-Sca-1+Kit+ cells, CD3+ cells, CD3+Ter119+ cells, CD3+Ter119- cells, IGF-2–hFc+ cells, and IGF-2–hFc- cells to check expression of IGF-2, IGF type 1 receptor (IGF1R), IGF type 2 receptor (IGF2R), insulin receptor (IR), and glyceraldehydes-3-phosphate dehydrogenase (GAPDH). Forward primer for IGF-2: GTCGATGTTGGTGCTTCTCA; reverse primer for IGF-2: AAGCAGCACTCTTCCACGAT. Forward primer for IGF1R: CAAGCTGTGTGTCTCCGAAA; reverse primer for IGF1R: TGATGAGATCCCGGTAGTCC. 5′ primer for IGF2R: GCACCAAGATGAAGCAGTCA; reverse primer for IGF2R: ACATCCGGTAGCTGTTGGTC. Forward primer for IR: AAAGTTTGCCCAACCATCTG; reverse primer for IR: GTGAAGGTCTTGGCAGAAGC. Forward primer for GAPDH: AACTTTGGCATTGTGGAAGG; reverse primer for GAPDH: ACACATTGGGGGTAGGAACA. The level of murine IGF-2 in the medium of cultured cells was measured by enzyme-linked immunosorbent assay (ELISA) as described in the DuoSet ELISA Development Kit for mouse IGF-2 (R&D Systems).
Expression of IGF-2–hFc
The DNAs encoding Met1-Glu91 of human prepro–IGF-2 and Pro100-Lys330 of human IgG1 Fc sequences were linked by a DNA sequence encoding IEGRMD linker peptide. The whole 1.1-kilobase (kb) fragment was inserted into between KpnI and XhoI sites of pcDNA3.1, downstream of cytomegalovirus (CMV) promoter. The plasmid was transfected into 293T cells using lipofectamine 2000 (Invitrogen), and conditioned medium at 48 hours after transfection was collected. The conditioned medium with about 1 μg/mL IGF-2–hFc determined by Western blotting was used in the subsequent staining of fetal liver or bone marrow cells.
Results
Coculture of fetal liver HSCs with fetal liver CD3+ cells increases the number of long-term repopulating stem cells
Because fetal liver HSCs undergo significant expansion during fetal development, we hypothesized that certain lineage-positive (Lin+) cells might produce protein(s) that support HSCs. A medium containing fetal calf serum, SCF, FL, and IL-6 cannot in itself support the maintenance of purified fetal liver HSCs,24 presumably because it is missing some essential growth factor(s). In preliminary studies we confirmed this by showing that culture of day-15 fetal liver Lin- cells in this medium for 3 or 6 days resulted in loss of HSC activity. However, adding day-15 fetal liver Lin+ cells in this culture system for 3 days supported a net 3-fold expansion in the number of in vivo repopulating HSCs (data not shown), suggesting that some Lin+ cells are expressing HSC supportive growth factor(s).
To identify the Lin+ cell population(s) that support HSC ex vivo expansion, we fractionated day-15 fetal liver cells by flow cytometry into 4 populations: CD3+, B220+, Gr-1+, and Ter119+, which were then individually cocultured with Lin- cells. Only fetal liver CD3+ cells supported increases in HSC numbers and activity during a 3- or 6-day culture; the activity of HSCs was maintained from day 3 to day 6 and dropped after 6 days of culture (data not shown and Figure 1). Controls showed, as expected, no hematopoietic reconstitution ability in either freshly isolated or 3-day–cultured day 15 fetal liver CD3+ cells (104 cells tested) 1 to 8 months after transplantation.
Coculture of fetal liver HSCs with fetal liver CD3+ cells increases in vivo repopulating stem cells. (A) In vivo limiting dilution analysis of the repopulating ability of day-15 fetal liver Lin-Sca-1+Kit+ cells before and after in vitro culture with day-15 fetal liver CD3+ cells. Freshly isolated day-15 fetal liver Lin-Sca-1+Kit+ cells were mixed with the same number of freshly isolated day-15 fetal liver CD3+ cells. Half of the mixed cells were used for direct transplantation (* and solid line). The other half were cultured for 3 days in a volume of 30 μL followed by transplantation of the entire culture (▪ and dashed line). In addition, 100 freshly isolated day-15 fetal liver Lin-Sca-1+Kit+ cells were cultured alone without CD3+ cells for 3 days followed by transplantation (▴). The medium contained fetal calf serum supplemented with SCF, FL, and IL-6. Irradiated CD45.1 congenic mice were injected with 2 × 105 CD45.1 bone marrow cells and the indicated numbers of freshly isolated CD45.2 Lin-Sca-1+Kit+ fetal liver cells or their progeny after culture. Plotted is the percentage of recipient mice containing less than 1% CD45.2 lymphoid (B220+) and myeloid (Gr-1+/Mac-1+) subpopulations in nucleated peripheral blood cells 4 months after transplantation versus the number of initial Lin-Sca-1+Kit+ cells; note that the abscissa is presented as the number of LSK cells initially added to the culture. The curve was anchored by the 0 cells/100% negative mice point. Injection of the culture of 50 or 100 initial Lin-Sca-1+Kit+ cells with CD3+ cells resulted in repopulation of 100% of the mice. A data point of 0% negative mice cannot be plotted on a logarithmic axis; so to be conservative we assume and plot that only 90% of the mice injected with the culture derived from these 50 input LSK cells are positive, or 10% negative. Dotted lines show the determination of the CRU frequency values by the method of maximum likelihood (at 37% negative mice).11 (B) Details of a typical competitive repopulation by 100 CD45.2 fetal liver Lin-Sca-1+Kit+ cells cultured alone or with fetal liver CD3+ cells (as shown in panel A) 4 months after transplantation into lethally irradiated CD45.1 recipient mice. Data shown are representative FACS plots of peripheral blood mononuclear cells from 1 mouse in each group. To assess the repopulation frequencies of the donor cells, cell isolates were double stained with antibodies against CD45.2 (donor) and CD45.1 (recipient), Thy1.2 (T cells), B220 (B cells), Mac-1 (macrophages), and Gr-1 (granulocytes). As illustrated in the top panels, Lin-Sca-1+Kit+ cells cultured in the absence of CD3+ cells yielded no reconstitution of any of the lineages analyzed.
Coculture of fetal liver HSCs with fetal liver CD3+ cells increases in vivo repopulating stem cells. (A) In vivo limiting dilution analysis of the repopulating ability of day-15 fetal liver Lin-Sca-1+Kit+ cells before and after in vitro culture with day-15 fetal liver CD3+ cells. Freshly isolated day-15 fetal liver Lin-Sca-1+Kit+ cells were mixed with the same number of freshly isolated day-15 fetal liver CD3+ cells. Half of the mixed cells were used for direct transplantation (* and solid line). The other half were cultured for 3 days in a volume of 30 μL followed by transplantation of the entire culture (▪ and dashed line). In addition, 100 freshly isolated day-15 fetal liver Lin-Sca-1+Kit+ cells were cultured alone without CD3+ cells for 3 days followed by transplantation (▴). The medium contained fetal calf serum supplemented with SCF, FL, and IL-6. Irradiated CD45.1 congenic mice were injected with 2 × 105 CD45.1 bone marrow cells and the indicated numbers of freshly isolated CD45.2 Lin-Sca-1+Kit+ fetal liver cells or their progeny after culture. Plotted is the percentage of recipient mice containing less than 1% CD45.2 lymphoid (B220+) and myeloid (Gr-1+/Mac-1+) subpopulations in nucleated peripheral blood cells 4 months after transplantation versus the number of initial Lin-Sca-1+Kit+ cells; note that the abscissa is presented as the number of LSK cells initially added to the culture. The curve was anchored by the 0 cells/100% negative mice point. Injection of the culture of 50 or 100 initial Lin-Sca-1+Kit+ cells with CD3+ cells resulted in repopulation of 100% of the mice. A data point of 0% negative mice cannot be plotted on a logarithmic axis; so to be conservative we assume and plot that only 90% of the mice injected with the culture derived from these 50 input LSK cells are positive, or 10% negative. Dotted lines show the determination of the CRU frequency values by the method of maximum likelihood (at 37% negative mice).11 (B) Details of a typical competitive repopulation by 100 CD45.2 fetal liver Lin-Sca-1+Kit+ cells cultured alone or with fetal liver CD3+ cells (as shown in panel A) 4 months after transplantation into lethally irradiated CD45.1 recipient mice. Data shown are representative FACS plots of peripheral blood mononuclear cells from 1 mouse in each group. To assess the repopulation frequencies of the donor cells, cell isolates were double stained with antibodies against CD45.2 (donor) and CD45.1 (recipient), Thy1.2 (T cells), B220 (B cells), Mac-1 (macrophages), and Gr-1 (granulocytes). As illustrated in the top panels, Lin-Sca-1+Kit+ cells cultured in the absence of CD3+ cells yielded no reconstitution of any of the lineages analyzed.
The limiting dilution experiment in Figure 1A shows that day-15 fetal liver CD3+ cells directly stimulate ex vivo expansion of purified LT-HSCs during a 3-day coculture. The CRU frequency for freshly isolated fetal liver Lin-Sca-1+Kit+ (LSK) cells is 1 per 50 (95% confidence interval for mean, 1/36 to 1/57; n = 41). That is, as calculated from Poisson statistics, injection on average of 50 freshly isolated LSK cells is sufficient to repopulate 63% (1 -1/e) of mice undergoing transplantation. After culture in medium containing serum, SCF, FL, and IL-6, with or without CD3+ cells, the number of cells was too few to be counted reliably. However, after a 3-day culture without CD3+ cells, all HSC activity was lost, as judged by the fact (“▴” data point) that all 10 mice undergoing transplantation with the progeny of 100 input LSK cells failed to reconstitute (less than 1% donor contribution to nucleated blood cells; Figure 1).
After a 3-day culture of LSK cells with an equivalent number of day-15 fetal liver CD3+ cells, the number of functional LT-HSCs had increased about 2.5-fold (P < .05, Student t test), as evidenced by the fact that 63% of the mice undergoing transplantation with the progeny of, on average, only 22 initial LSK cells became reconstituted. In other words, the CRU frequency was 1 per 22 input equivalent LSK cells (95% confidence interval for mean, 1/13 to 1/26; n = 35). Figure 1B shows that fetal liver LSK cells cocultured with CD3+ cells repopulated, 4 months after transplantation, all blood lineages we tested (Figure 1B), attesting to net expansion of LT-HSCs.
IGF-2 is a key factor expressed in day-15 fetal liver CD3+ cells to support HSCs
At day 15, CD3+ cells represent 2% to 3% of total fetal liver cells. Although they are nonadherent to plastic and remain in suspension during culture, they are very different from CD3+ cells found in adult spleen or bone marrow (Figure 2A and data not shown). CD3+ cells isolated from adult spleen are mature T cells; they are Thy1.2+ (Figure 2Axi) and either CD4+ or CD8+ (Figure 2Aix-x). Most of them are Ter119- (Figure 2Axii). Fetal liver CD3+ cells, in contrast, are Thy1.2-CD4-CD8- (Figure 2Aiii-v), and half are Ter119+ (Figure 2Avi). In addition, the level of CD3 expression on fetal liver CD3+ cells is less than that on adult splenic CD3+ cells (Figure 2A, compare plots ii and viii). Fetal liver HSCs cocultured with CD3+ cells isolated from adult spleen or bone marrow lose most if not all repopulation ability (data not shown).
IGF-2 produced by the fetal liver CD3+Ter119- cells is the key factor that supports cultured HSCs. (A) FACS analysis of surface expression of CD3, CD4, CD8, Thy1.2, and Ter119 in murine fetal liver and adult spleen cells. Cells freshly isolated from day-15 fetal liver (i-vi) or from adult spleen (vii-xii) were stained with the isotype control (I, vii), anti-CD3–FITC (ii, viii), anti-CD3–FITC and anti-CD4–PE (iii, ix), anti-CD3–FITC and anti-CD8–PE (iv, x), anti-CD3–FITC and anti-Thy1.2–PE (v, xi), and anti-CD3–FITC and anti-Ter119–PE (vi, xii), respectively. (B) RT-PCR analyses of expression of IGF-2 and IGF-2 receptors in fetal liver cells. On the left, RT-PCR was performed on cDNAs isolated from FACS-sorted murine day-15 fetal liver Lin-Sca-1+Kit+ (FL LSK) cells and CD3+ cells to determine expression of IGF-2, IGF type 1 receptor (IGF1R), IGF type 2 receptor (IGF2R), and insulin receptor (IR) as indicated. On the right, RT-PCR was performed on cDNAs isolated from FACS-sorted murine day-15 fetal liver CD3+Ter119+ and CD3+Ter119- cells to determine expression of IGF-2. Expression of glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was measured as an indicator of the amount of RNA. (C) Fetal liver CD3+Ter119- cells secrete more IGF-2 than do CD3+Ter119+ cells when cocultured with LSK cells. FACS-sorted fetal liver CD3+Ter119- cells, CD3+Ter119+ cells, and LSK cells were cultured for 2 days in 100 μL serum containing medium supplemented with SCF, FL, and IL-6 as follows: 7 × 104 CD3+Ter119- cells (i), 7 × 104 CD3+Ter119+ cells (ii), 2 × 103 LSK cells (iii), 7 × 104 CD3+Ter119- cells and 2 × 103 LSK cells (iv), or 7 × 104 CD3+Ter119+ cells and 2 × 103 LSK cells (v). ELISA was then performed to determine the level of IGF-2 in the medium. Error bars represent SEM. (D) Fetal liver CD3+Ter119- cells supported cultured HSCs by producing IGF-2. Fifty freshly isolated day 15 CD45.2 fetal liver Lin-Sca-1+Kit+ cells were cultured 3 days in 30 μL medium alone (i), with 50 fetal liver CD3+Ter119+ cells (ii), with 50 fetal liver CD3+Ter119- cells (iii), or with 50 fetal liver CD3+Ter119- cells and 20 μg/mL anti–IGF-2 antibody (iv), respectively. The medium contained fetal calf serum supplemented with SCF, FL, and IL-6. Together with 2 × 105 competitor CD45.1 bone marrow cells, the total culture, derived from 50 initial Lin-Sca-1+Kit+ cells, was coinjected into CD45.1 recipients (n = 4 to 5). Peripheral blood cells from mice undergoing transplantation were analyzed for the presence of CD45.2+ cells in lymphoid and myeloid compartments at 4 months after transplantation. Each ▴ represents the percentage of repopulation from a single recipient mouse. Bars represent the average percentage of repopulation for each group; data points below zero represent no detectable repopulation. *Significantly different from lane 1 and 2 values; P < .05. **Significantly different from lane 3 value; P < .005.
IGF-2 produced by the fetal liver CD3+Ter119- cells is the key factor that supports cultured HSCs. (A) FACS analysis of surface expression of CD3, CD4, CD8, Thy1.2, and Ter119 in murine fetal liver and adult spleen cells. Cells freshly isolated from day-15 fetal liver (i-vi) or from adult spleen (vii-xii) were stained with the isotype control (I, vii), anti-CD3–FITC (ii, viii), anti-CD3–FITC and anti-CD4–PE (iii, ix), anti-CD3–FITC and anti-CD8–PE (iv, x), anti-CD3–FITC and anti-Thy1.2–PE (v, xi), and anti-CD3–FITC and anti-Ter119–PE (vi, xii), respectively. (B) RT-PCR analyses of expression of IGF-2 and IGF-2 receptors in fetal liver cells. On the left, RT-PCR was performed on cDNAs isolated from FACS-sorted murine day-15 fetal liver Lin-Sca-1+Kit+ (FL LSK) cells and CD3+ cells to determine expression of IGF-2, IGF type 1 receptor (IGF1R), IGF type 2 receptor (IGF2R), and insulin receptor (IR) as indicated. On the right, RT-PCR was performed on cDNAs isolated from FACS-sorted murine day-15 fetal liver CD3+Ter119+ and CD3+Ter119- cells to determine expression of IGF-2. Expression of glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was measured as an indicator of the amount of RNA. (C) Fetal liver CD3+Ter119- cells secrete more IGF-2 than do CD3+Ter119+ cells when cocultured with LSK cells. FACS-sorted fetal liver CD3+Ter119- cells, CD3+Ter119+ cells, and LSK cells were cultured for 2 days in 100 μL serum containing medium supplemented with SCF, FL, and IL-6 as follows: 7 × 104 CD3+Ter119- cells (i), 7 × 104 CD3+Ter119+ cells (ii), 2 × 103 LSK cells (iii), 7 × 104 CD3+Ter119- cells and 2 × 103 LSK cells (iv), or 7 × 104 CD3+Ter119+ cells and 2 × 103 LSK cells (v). ELISA was then performed to determine the level of IGF-2 in the medium. Error bars represent SEM. (D) Fetal liver CD3+Ter119- cells supported cultured HSCs by producing IGF-2. Fifty freshly isolated day 15 CD45.2 fetal liver Lin-Sca-1+Kit+ cells were cultured 3 days in 30 μL medium alone (i), with 50 fetal liver CD3+Ter119+ cells (ii), with 50 fetal liver CD3+Ter119- cells (iii), or with 50 fetal liver CD3+Ter119- cells and 20 μg/mL anti–IGF-2 antibody (iv), respectively. The medium contained fetal calf serum supplemented with SCF, FL, and IL-6. Together with 2 × 105 competitor CD45.1 bone marrow cells, the total culture, derived from 50 initial Lin-Sca-1+Kit+ cells, was coinjected into CD45.1 recipients (n = 4 to 5). Peripheral blood cells from mice undergoing transplantation were analyzed for the presence of CD45.2+ cells in lymphoid and myeloid compartments at 4 months after transplantation. Each ▴ represents the percentage of repopulation from a single recipient mouse. Bars represent the average percentage of repopulation for each group; data points below zero represent no detectable repopulation. *Significantly different from lane 1 and 2 values; P < .05. **Significantly different from lane 3 value; P < .005.
In an attempt to determine what factor(s) are made by day-15 fetal liver CD3+ cells that support HSC expansion, we compared the transcriptional profile of day-15 fetal liver CD3+ cells with those of 2 negative cell populations: adult splenic CD3+ cells and day-15 fetal liver Gr-1+ cells. Table 1 shows that several potentially important secreted and membrane proteins are differentially expressed, including IGF-2, Dlk1-like, CD59, and PRNP. Dlk1-like was previously shown to support maintenance of fetal liver HSCs in long-term culture.25 Because IGF-2 was highly expressed in fetal liver CD3+ cells and not by adult CD3+ cells or fetal liver Gr-1+ cells, we hypothesized that this protein, not previously implicated in HSC biology, might mediate expansion of HSCs.
Genes known to encode secreted and membrane proteins that are abundant in fetal liver CD3+ cells and low in adult splenic CD3+ cells and fetal liver Gr-1+ cells
. | . | FEtal liver CD3+ cells . | . | Adult spleen CD3+ cells . | . | Fetal liver Gr-1+ cells . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| GenBank accession no. . | Gene description . | Raw . | Normalized . | Raw . | Normalized . | Raw . | Normalized . | |||
| X71922 | IGF-2 | 3128 | 15.01 | 107 | 0.67 | 384 | 2.39 | |||
| M22998 | Solute carrier family 2 | 1116 | 5.36 | 50 | 0.31 | 349 | 2.17 | |||
| X57349 | Transferrin receptor | 893 | 4.28 | 50 | 0.31 | 131 | 0.82 | |||
| X53081 | Erythropoietin receptor | 680 | 3.26 | 50 | 0.31 | 159 | 0.98 | |||
| M26385 | Glycophorin A | 3364 | 16.14 | 246 | 1.54 | 995 | 6.19 | |||
| J04766 | Plasminogen | 538 | 2.58 | 50 | 0.31 | 50 | 0.31 | |||
| Z12171 | Dlkl-like homolog | 858 | 4.12 | 82 | 0.51 | 149 | 0.92 | |||
| U60473 | CD59 | 517 | 2.48 | 50 | 0.31 | 87 | 0.54 | |||
| J03398 | p glycoprotein 2 | 644 | 3.09 | 69 | 0.43 | 148 | 0.91 | |||
| M58661 | CD24a | 5677 | 27.25 | 856 | 5.35 | 2841 | 17.67 | |||
| AB017189 | CD98 light chain | 1109 | 5.32 | 239 | 1.49 | 368 | 2.28 | |||
| M18070 | PRNP | 447 | 2.15 | 116 | 0.72 | 114 | 0.71 | |||
. | . | FEtal liver CD3+ cells . | . | Adult spleen CD3+ cells . | . | Fetal liver Gr-1+ cells . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| GenBank accession no. . | Gene description . | Raw . | Normalized . | Raw . | Normalized . | Raw . | Normalized . | |||
| X71922 | IGF-2 | 3128 | 15.01 | 107 | 0.67 | 384 | 2.39 | |||
| M22998 | Solute carrier family 2 | 1116 | 5.36 | 50 | 0.31 | 349 | 2.17 | |||
| X57349 | Transferrin receptor | 893 | 4.28 | 50 | 0.31 | 131 | 0.82 | |||
| X53081 | Erythropoietin receptor | 680 | 3.26 | 50 | 0.31 | 159 | 0.98 | |||
| M26385 | Glycophorin A | 3364 | 16.14 | 246 | 1.54 | 995 | 6.19 | |||
| J04766 | Plasminogen | 538 | 2.58 | 50 | 0.31 | 50 | 0.31 | |||
| Z12171 | Dlkl-like homolog | 858 | 4.12 | 82 | 0.51 | 149 | 0.92 | |||
| U60473 | CD59 | 517 | 2.48 | 50 | 0.31 | 87 | 0.54 | |||
| J03398 | p glycoprotein 2 | 644 | 3.09 | 69 | 0.43 | 148 | 0.91 | |||
| M58661 | CD24a | 5677 | 27.25 | 856 | 5.35 | 2841 | 17.67 | |||
| AB017189 | CD98 light chain | 1109 | 5.32 | 239 | 1.49 | 368 | 2.28 | |||
| M18070 | PRNP | 447 | 2.15 | 116 | 0.72 | 114 | 0.71 | |||
Listed genes have normalized value more than 2.0 in fetal liver CD3+ cells and are at least 3-fold greater than adult CD3+ and 2-fold greater than fetal liver Gr-1+ normalized values. Total RNA and cRNA was prepared for hybridization to Affymetrix U74Av2 mouse chips according to manufacturer's instructions, and the analysis was performed as in our recent paper.23
Figure 2B shows that IGF-2 as well as the 3 known IGF-2 receptors26 —IGF type 1 receptor (IGF1R), IGF type 2 receptor (IGF2R), and insulin receptor (IR)—are expressed in fetal liver CD3+ cells. In contrast, fetal liver LSK cells express all 3 IGF-2 receptors but do not contain detectable amounts of IGF-2 mRNA.
About half of the day-15 fetal liver CD3+ cells are Ter119+ (Figure 2A, plot 6), which is a marker for terminally differentiating erythroblasts and mature erythrocytes. Figure 2D shows that the CD3+Ter119- cells showed a much better ability than their CD3+Ter119+ counterparts to support the in vivo repopulating activity of LSK cells cocultured for 3 days (Figure 2D, compares lanes 3 and 2, P < .05). Thus, the HSC supportive activity of day-15 fetal liver CD3+ cells lies within the Ter119- subfraction.
Addition of neutralizing anti–IGF-2 antibody to the coculture of LSK and CD3+Ter119- cells completely blocked expansion of HSC activity by the cocultured CD3+Ter119- cells (Figure 2D, compare lanes 4 and 3, P < .005), suggesting that IGF-2 is a key factor produced by fetal liver CD3+Ter119- cells to support HSCs in culture.
Interestingly, within the fetal liver CD3+ cell population, both CD3+Ter119+ and CD3+Ter119- cells express IGF-2 mRNA (Figure 2B, right) whereas only the latter population supports HSC expansion. Thus, we measured the level of IGF-2 secreted by cultured fetal liver CD3+Ter119+ and CD3+Ter119- cells using ELISA (Figure 2C). When cultured alone, both populations secreted moderate levels (25 to 30 ng/mL) of IGF-2 (Figure 2C, lanes 1 and 2). When cocultured with fetal liver LSK cells, which in themselves produce little IGF-2 (Figure 2C, lane 3), CD3+Ter119- cells express significantly more IGF-2 (250 ng/mL) than their Ter119+ counterparts (40 ng/mL IGF-2) (Figure 2C, compare lanes 5 and 4). Taken together, these results strongly support the notion that IGF-2, produced by fetal liver CD3+Ter119- cells, is a key factor that supports the growth of HSCs ex vivo and that exposure to HSCs increases IGF-2 production by these supportive cells.
All fetal liver HSCs express receptors for IGF-2
While fetal liver LSK cells express receptors for IGF-2 (Figure 2B), only a minority of these cells actually are HSCs. Existing antibodies against IGF1R and IGF2R are suitable for applications such as Western blotting but not for flow cytometry. Therefore, we generated a fusion protein of prepro–IGF-2 and a human IgG1 Fc fragment (IGF-2–hFc) that could be used in flow cytometry to detect and isolate cells expressing receptors for IGF-2. To this end, a plasmid expressing the IGF-2–hFc was constructed (Figure 3A, top) and transfected into 293T cells. The IGF-2–hFc fusion protein was secreted and could be detected by antibodies against either human IGF-2 or human IgG1 Fc (Figure 3A, bottom panels). The 2 bands observed on the Western blots suggest that some of the IGF-2–hFc has undergone partial protease degradation.
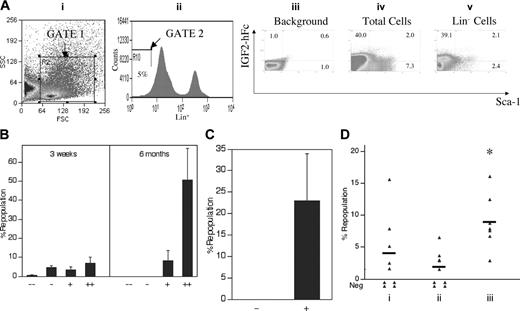
Detection by flow cytometry of IGF-2 receptor expression in fetal liver cells. (A) Production and secretion of IGF-2–hFc in transfected 293T cells. The upper panel shows a schematic of the plasmid expressing the human prepro–IGF-2 protein fused to a human IgG1 Fc fragment. The bottom panels show Western blots of the 48-hour conditioned medium of 293T cells transfected either by control or IGF-2–hFc–expressing vectors probed with antibodies against human IGF-2 (left) or human IgG1 (right). (B) Most fetal liver Lin-Sca-1+ cells specifically bind IGF-2–hFc. Total day 15 fetal liver cells were incubated at 4°C for 30 minutes with conditioned medium from control transfected 293T cells (iii) or cells transfected with the IGF-2–hFc expression vector (iv, v); the latter medium contained about 1 μg/mL IGF-2–hFc. Cells were then stained with anti-human IgG-PE, followed by the biotinylated Lin+ antibody cocktail, streptavidin-APC, anti-Sca-1–FITC, and propidium iodide (PI). For FACS analysis of total fetal liver, single cells that excluded PI were further gated by forward scatter/side scatter (i) to include only nucleated cells. Lin- cells were gated as the lowest 5% of APC-stained cells (ii). The gated cells were then analyzed for the binding of IGF-2–hFc and the level of Sca-1 (iii-v). (C) Western blot of total fetal liver cells sorted based on binding of IGF-2–hFc. (i, ii) The purity of IGF-2–hFc+ and IGF-2–hFc- fetal liver cells sorted after staining with IGF-2–hFc and anti-hIgG1–PE; panel i is the reanalysis of the sorted IGF-2–hFc- cells and panel ii, the reanalysis of the sorted IGF-2–hFc+ cells. In panel iii, antibodies against IGF1R (Cell Signaling Technology, Beverly, MA) and IGF2R (a gift from Dr Stuart Kornfeld) were used to detect the expression of these IGF-2 receptors in IGF-2–hFc+ and IGF-2–hFc- fetal liver cells. These results confirmed the specificity of IGF-2–hFc binding because cells unable to bind the IGF-2–hFc fusion protein did not express these 2 principal IGF-2 receptors. Blotting by anti–β-tubulin (Sigma) served as the loading control. (D) RT-PCR analysis of cDNA isolated from total fetal liver cells sorted based on binding of IGF-2–hFc. RT-PCR was used to detect the mRNA levels of IGF1R, IGF2R, and insulin receptor in IGF-2–hFc+ and IGF-2–hFc- fetal liver cells sorted as in panel C. Expression of GAPDH was measured as an indicator of the amount of RNA.
Detection by flow cytometry of IGF-2 receptor expression in fetal liver cells. (A) Production and secretion of IGF-2–hFc in transfected 293T cells. The upper panel shows a schematic of the plasmid expressing the human prepro–IGF-2 protein fused to a human IgG1 Fc fragment. The bottom panels show Western blots of the 48-hour conditioned medium of 293T cells transfected either by control or IGF-2–hFc–expressing vectors probed with antibodies against human IGF-2 (left) or human IgG1 (right). (B) Most fetal liver Lin-Sca-1+ cells specifically bind IGF-2–hFc. Total day 15 fetal liver cells were incubated at 4°C for 30 minutes with conditioned medium from control transfected 293T cells (iii) or cells transfected with the IGF-2–hFc expression vector (iv, v); the latter medium contained about 1 μg/mL IGF-2–hFc. Cells were then stained with anti-human IgG-PE, followed by the biotinylated Lin+ antibody cocktail, streptavidin-APC, anti-Sca-1–FITC, and propidium iodide (PI). For FACS analysis of total fetal liver, single cells that excluded PI were further gated by forward scatter/side scatter (i) to include only nucleated cells. Lin- cells were gated as the lowest 5% of APC-stained cells (ii). The gated cells were then analyzed for the binding of IGF-2–hFc and the level of Sca-1 (iii-v). (C) Western blot of total fetal liver cells sorted based on binding of IGF-2–hFc. (i, ii) The purity of IGF-2–hFc+ and IGF-2–hFc- fetal liver cells sorted after staining with IGF-2–hFc and anti-hIgG1–PE; panel i is the reanalysis of the sorted IGF-2–hFc- cells and panel ii, the reanalysis of the sorted IGF-2–hFc+ cells. In panel iii, antibodies against IGF1R (Cell Signaling Technology, Beverly, MA) and IGF2R (a gift from Dr Stuart Kornfeld) were used to detect the expression of these IGF-2 receptors in IGF-2–hFc+ and IGF-2–hFc- fetal liver cells. These results confirmed the specificity of IGF-2–hFc binding because cells unable to bind the IGF-2–hFc fusion protein did not express these 2 principal IGF-2 receptors. Blotting by anti–β-tubulin (Sigma) served as the loading control. (D) RT-PCR analysis of cDNA isolated from total fetal liver cells sorted based on binding of IGF-2–hFc. RT-PCR was used to detect the mRNA levels of IGF1R, IGF2R, and insulin receptor in IGF-2–hFc+ and IGF-2–hFc- fetal liver cells sorted as in panel C. Expression of GAPDH was measured as an indicator of the amount of RNA.
As judged by Western blotting, we estimate that medium from transfected 293T cells contains about 1 μg/mL IGF-2–hFc. Figure 3B shows that, after incubation in this conditioned medium, about 36% of total fetal liver cells stain positive for IGF-2–hFc (Figure 3Biv), as do 76% (67.0% + 9.3%) of Lin- fetal liver cells and 83% (9.3 of 11.2) of Lin-Sca-1+ fetal liver cells (Figure 3Bv). As expected, there is no IGF-2 staining of total fetal liver cells incubated in control-conditioned medium (Figure 3Biii). Both Western blot and RT-PCR analyses showed that fetal liver cells unable to bind the IGF-2–hFc fusion protein express little or undetectable IGF1R or IGF2R while those that bind the IGF-2–hFc fusion protein express both (Figure 3C-D). The insulin receptor, which binds IGF-2 weakly, is expressed by both cell populations (Figure 3D). Therefore, IGF-2–hFc specifically binds to IGF1R and IGF2R expressed on the surface of fetal liver cells.
Figure 4 demonstrates that all fetal liver HSCs express receptors for IGF-2. In Figure 4A, total fetal liver cells were sorted according to their ability to bind the IGF-2–hFc fusion protein. Those that did not bind the fusion protein were unable to reconstitute these mice, either at 3 weeks (measuring primarily short-term [ST]–HSCs) or 6 months after transplantation, primarily detecting LT-HSCs. In contrast, total fetal liver cells arbitrarily sorted according to their weak (+) or strong (++) ability to bind the IGF-2–hFc fusion protein supported both strong short-term and long-term engraftment (Figure 4A). Similarly, 100 sorted fetal liver Lin-Sca-1+ cells unable to bind the IGF-2–hFc fusion protein were unable to reconstitute irradiated mice at 6 months after transplantation while 50 Lin-Sca-1+IGF-2–hFc+ cells supported significant long-term reconstitution (Figure 4B). Further experiments (data not shown) showed that even 1000 fetal liver Lin-Sca-1+IGF-2–hFc- cells contain no HSC activity. Thus, all fetal liver HSC activity resides in the Lin-Sca-1+IGF-2–hFc+ but not in the Lin-Sca-1+IGF-2–hFc- population, confirming the results of Figure 4A that all long-term repopulating HSCs bind IGF-2.
IGF-2 stimulates ex vivo expansion of purified fetal liver HSCs
When 50 fetal liver LSK cells were cultured in the presence of serum supplemented with SCF, FL, and IL-6 for 3 days, all HSC activity was lost, similar to the results in Figure 1 (Figure 5Ai). After culture in serum supplemented with SCF and TPO, some HSC activity was retained (Figure 5Aii), but only half of the animals were repopulated by the progeny of 50 LSK cells. However, when 500 ng/mL IGF-2 was added to either medium, significant increases of HSC activities were observed, and all mice undergoing transplantation exhibited chimerism (Figure 5A, compare lanes iii versus i, P < .05; lanes iv versus ii, P < .005). Impressively, the combination of SCF, TPO, and IGF-2 substantially increased HSC activity, and each of 4 recipient mice was strongly reconstituted by the cultured progeny of only 50 original LSK cells (Figure 5Aiv). This suggests that IGF-2 is capable of strongly stimulating expansion HSCs in culture. Surprisingly, IGF-1, which only binds to IGF1R but not the IGF2R, does not support HSC maintenance or expansion in our culture system (data not shown).
Culture with IGF-2 increases in vivo repopulating activity of fetal liver HSCs. (A) Culture of day-15 fetal liver Lin-Sca-1+Kit+ cells with 500 ng/mL IGF-2 enhances in vivo repopulating stem cell activity. A representative experiment of the 3 performed is illustrated. Fifty freshly isolated day-15 CD45.2 fetal liver Lin-Sca-1+Kit+ cells were cultured for 3 days in medium containing FBS and supplemented with SCF, FL, and IL-6 (i); in medium containing FBS and supplemented with SCF and TPO (ii); in medium containing FBS and supplemented with SCF, FL, IL-6, and 500 ng/mL IGF-2 (iii); and in medium containing FBS and supplemented with SCF, TPO, and 500 ng/mL IGF-2 (iv). Together with 2 × 105 competitor CD45.1 bone marrow cells, the entire culture derived from these initial 50 Lin-Sca-1+Kit+ cells were coinjected into CD45.1 recipients (n = 4 to 6). Peripheral blood cells from mice undergoing transplantation were analyzed for the presence of CD45.2+ cells in lymphoid and myeloid compartments at 3 months after transplantation. Each ▴ represents the percentage of population from a single recipient mouse. Horizontal bars represent the average of repopulation percentage in each group. Note that data points below zero represent no detectable population. *Significantly different from panel i value; P < .05. **Significantly different from lane ii value; P < .005. (B) In vivo limiting dilution analysis of the repopulating ability of day 15 fetal liver Lin-Sca-1+Kit+ cells before and after in vivo culture with IGF-2. Freshly isolated day 15 CD45.2 fetal liver Lin-Sca-1+Kit+ cells were transplanted directly (* and solid line) or cultured in medium containing FBS together with SCF, TPO, and 500 ng/mL IGF-2 (▪ and dashed line) followed by transplantation (with 2 × 105 CD45.1 competitor bone marrow cells per mouse) into CD45.1 congenic recipients. Plotted is the percentage of recipient mice containing less than 1% CD45.2 lymphoid (B220+) and myeloid (Gr-1+/Mac-1+) subpopulations in nucleated peripheral blood cells 4 months after transplantation versus the number of fetal liver Lin-Sca-1+Kit+ cells initially injected or cultured. The curve is anchored by the 0 cells/100% negative mice point. Dotted lines show the determination of the CRU frequency values by the method of maximum likelihood (at 37% negative mice).11
Culture with IGF-2 increases in vivo repopulating activity of fetal liver HSCs. (A) Culture of day-15 fetal liver Lin-Sca-1+Kit+ cells with 500 ng/mL IGF-2 enhances in vivo repopulating stem cell activity. A representative experiment of the 3 performed is illustrated. Fifty freshly isolated day-15 CD45.2 fetal liver Lin-Sca-1+Kit+ cells were cultured for 3 days in medium containing FBS and supplemented with SCF, FL, and IL-6 (i); in medium containing FBS and supplemented with SCF and TPO (ii); in medium containing FBS and supplemented with SCF, FL, IL-6, and 500 ng/mL IGF-2 (iii); and in medium containing FBS and supplemented with SCF, TPO, and 500 ng/mL IGF-2 (iv). Together with 2 × 105 competitor CD45.1 bone marrow cells, the entire culture derived from these initial 50 Lin-Sca-1+Kit+ cells were coinjected into CD45.1 recipients (n = 4 to 6). Peripheral blood cells from mice undergoing transplantation were analyzed for the presence of CD45.2+ cells in lymphoid and myeloid compartments at 3 months after transplantation. Each ▴ represents the percentage of population from a single recipient mouse. Horizontal bars represent the average of repopulation percentage in each group. Note that data points below zero represent no detectable population. *Significantly different from panel i value; P < .05. **Significantly different from lane ii value; P < .005. (B) In vivo limiting dilution analysis of the repopulating ability of day 15 fetal liver Lin-Sca-1+Kit+ cells before and after in vivo culture with IGF-2. Freshly isolated day 15 CD45.2 fetal liver Lin-Sca-1+Kit+ cells were transplanted directly (* and solid line) or cultured in medium containing FBS together with SCF, TPO, and 500 ng/mL IGF-2 (▪ and dashed line) followed by transplantation (with 2 × 105 CD45.1 competitor bone marrow cells per mouse) into CD45.1 congenic recipients. Plotted is the percentage of recipient mice containing less than 1% CD45.2 lymphoid (B220+) and myeloid (Gr-1+/Mac-1+) subpopulations in nucleated peripheral blood cells 4 months after transplantation versus the number of fetal liver Lin-Sca-1+Kit+ cells initially injected or cultured. The curve is anchored by the 0 cells/100% negative mice point. Dotted lines show the determination of the CRU frequency values by the method of maximum likelihood (at 37% negative mice).11
Figure 5B definitely shows that IGF-2 increases the number of fetal liver stem cells. Freshly isolated fetal liver LSK cells were directly transplanted or were cultured for 3 days in serum-containing medium supplemented with SCF, TPO, and IGF-2. The CRU frequency for freshly isolated LSK cells was 1 per 50 (95% confidence interval for mean, 1/40 to 1/57; n = 31). After culture in medium containing serum, 500 ng/mL IGF-2, SCF, and TPO, the number of cells was again too few to be counted reliably. However, the number of functional LT-HSCs had increased about 2-fold (50 vs 25; P < .05, Student t test), as evidenced by the fact that 63% of the mice undergoing transplantation with the progeny of, on average, only 25 initial LSK cells became reconstituted. In other words, the CRU frequency was 1 per 25 input equivalent LSK cells (95% confidence interval for mean, 1/9 to 1/50; n = 26). To our knowledge, this is the first report showing the expansion of purified fetal liver HSCs ex vivo.
IGF-2 stimulates ex vivo expansion of purified adult bone marrow HSCs
IGF-2 is thought not to be ubiquitously expressed in adult mice, but Figure 6A shows that about 42% of total adult bone marrow cells do bind significant amounts of the IGF-2–hFc fusion protein (Figure 6A, plot 4), as do 41% (39.1% + 2.1%) of Lin- bone marrow cells and 47% (2.1 of 4.5) of Lin-Sca-1+ bone marrow cells (Figure 6Av). Total bone marrow cells that did not bind the IGF-2–hFc fusion protein reconstituted mice at 3 weeks but not at 6 months after transplantation. In contrast, total bone marrow cells arbitrarily sorted according to their weak (+) or strong (++) ability to bind the IGF-2–hFc fusion protein supported both strong short-term and long-term engraftment (Figure 6B). Similarly, 100 sorted bone marrow Lin-Sca-1+ cells unable to bind the IGF-2–hFc fusion protein were unable to reconstitute irradiated mice 6 months after transplantation. In contrast, 50 Lin-Sca-1+IGF-2–hFc+ cells supported significant long-term reconstitution (Figure 6C). Thus, like their fetal counterparts, all long-term repopulating bone marrow HSCs bind IGF-2.
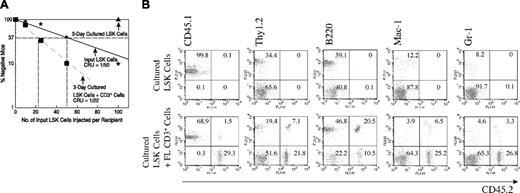
Compared with freshly isolated bone marrow SP cells (Figure 6Di), SP cells cultured for 3 days in serum-containing medium with SCF, FL, and IL-6 but without IGF-2 maintained some stem cell activity (Figure 6Dii). Importantly, addition of 1 μg/mL IGF-2 increased the repopulating activity and the stem cell frequency (Figure 6D, compare lanes iii versus i and ii, P < .05). Based on the finding that 5 of the 9 mice injected with 50 freshly isolated SP cells exhibited significant chimerism (Figure 6Di), we estimate that the HSC frequency in fresh bone marrow is 1 per 62 SP cells. After culture with IGF-2 the frequency increases to more than 1 per 26 input equivalent cultured SP cells, representing at least a 2-fold increase in HSC numbers.
Discussion
Here we describe a new population of nonadherent fetal liver Ter119-CD3+ cells that are capable of supporting a 2.5-fold expansion of LT-HSCs in culture and that are different from the other known population of CD3+ cells, adult T cells. We further showed that fetal liver CD3+ cells specifically express high levels of IGF-2 and that IGF-2 is the key factor in these cells that supports HSCs in culture. Importantly, all fetal liver and adult bone marrow HSCs express IGF-2 receptors, and culture of day 15 fetal liver LSK cells with IGF-2 increased HSC numbers and activities. IGF-2 also supported ex vivo expansion of adult bone marrow HSCs. Thus, fetal liver CD3+ cells are a novel population that is capable of supporting HSC expansion, and IGF-2, produced by these cells, is a growth factor for fetal liver and adult bone marrow HSCs.
Fetal liver CD3+ cells represent a new type of hematopoietic stromal cells
In vivo, stromal cells form a complex microenvironment for HSCs that controls their multiple fates, including apoptosis, migration, and the cell divisions that lead to formation either of daughter HSCs or of lineage-committed progenitors that are capable of limited proliferation.1,2 Different types of stromal and other cell types can have either positive or negative effects on fates of HSCs.24 Certain differentiated hematopoietic cells negatively regulate the ability of transplanted HSCs to repopulate the hematopoietic system.27,28 Consistent with these findings, in a preliminary study we observed that fetal liver B220+, Gr-1+, and Ter119+ cells did not support maintenance of fetal liver HSCs in culture while CD3+Ter119- cells did. Thus, differentiated hematopoietic cells are similar to stromal cells in that they provide both positive and negative signals to cocultured HSCs.
Fetal liver CD3+Ter119+ cells are mainly erythroid precursors (C.C.Z., M. Socolovsky, H.F.L., unpublished observations, April 2003), while the nature of the CD3+Ter119- population is unknown. Although both CD3+ fractions express IGF-2, only CD3+Ter119- cells are capable of supporting HSC activity in culture. Because both CD3+Ter119- and CD3+Ter119+ populations express IGF-2, it is likely that CD3+Ter119- cells also produce other factor(s) that facilitate IGF-2's function in supporting HSC activity. Alternatively, CD3+Ter119- but not CD3+Ter119+ cells might physically interact with HSCs; this hypothesis is supported by our observation that the level of IGF-2 secreted by CD3+Ter119- cells increases when cultured in the presence of HSCs. In addition, fetal liver CD3+Ter119+ cells might express negatively acting factors that counteract the positive effects of IGF-2.
Fetal liver CD3+Ter119- cells are different from a CD3+ “facilitating” cell population in adult bone marrow that enhances allografts.29 Fetal liver CD3+Ter119- cells do not enhance engraftment when freshly cotransplanted with donor cells (data not shown). They lack expression of the classical CD8 and Thy-1 T-cell markers while facilitating cells are CD8+Thy-1+.29 Furthermore, the function of the facilitating cells was mediated by the CD3 T-cell receptor (TCR) β-Fcp33 complex30 while HSC supportive activity of fetal liver CD3+ cells is mediated by IGF-2.
IGF-2 is the principal growth factor produced by fetal liver CD3+ cells that supports expansion of HSCs
Normally maternally imprinted, IGF-2 has been suggested to play important roles in embryonic but not adult growth and development.26,31 Targeted disruption of IGF-2 leads to significant fetal growth retardation,32,33 partially as a result of decreased placenta growth.34 Although no role in HSC biology was previously identified, IGF-2 has been used to establish pluripotential cell lines35 and maintain undifferentiated stem cells from the inner cell mass in culture.35,36 IGF-2 stimulates growth and/or differentiation and inhibits apoptosis of a number of cell types including hematopoietic progenitors. While stimulating the growth of human bone marrow CD34+ cells,37 IGF-2 also increases the formation of myeloid and erythroid progenitors such as granulocyte macrophage colony-forming units (CFU-GMs), erythroid burst-forming units (BFU-Es), and erythroid colony-forming units (CFU-Es)37,38 and regulates T-cell development and stimulates T-cell proliferation.39,40 However, this is the first report showing that IGF-2 is a growth factor for HSCs.
Different IGF-2 receptors have either a positive or a negative effect on cell proliferation. Two receptor protein tyrosine kinases, IGF1R and IR, mediate the mitogenic activities of IGF-2, while IGF2R mediates degradation of IGF-2. Lack of IGF1R results in fetal growth retardation, and IGF1R-deficient mice invariably die within minutes of birth.41 In contrast, mice genetically deficient in the negatively acting IGF2R show increased serum and tissue levels of IGF-2 that result in cell overgrowth, developmental abnormalities, and prenatal death.42,43
Multiple IGF-2 receptors could explain the fact that we need to add 500 ng/mL (67 nM) IGF-2 to support HSC expansion during culture of LSK cells; preliminary experiments (not shown) indicated that neither 25 ng/mL nor 50 ng/mL IGF-2 support cultured HSCs when combined with SCF, FL, and IL-6. Given the low nanomolar values of the dissociation constants for binding of IGF-2 to both the IGF1R and IGF2R,44 it is possible that, at low concentrations, the balance of binding of IGF-2 to its 3 types of receptors triggers the HSC differentiation; hypothetically, only higher concentrations of hormone could effectively prevent apoptosis and promote self-renewal. This is consistent with the “threshold” model45 positing that a low concentration of a growth factor stimulates stem cell differentiation but that high levels of the factor lead to a net expansion of stem cells.
The situation may be different when fetal liver CD3+Ter119- cells are cocultured with HSCs. Because cells tend to closely interact in the U-bottomed wells we used, it is possible that CD3+Ter119- cells physically contact HSCs and thus that the local concentration of IGF-2 around an HSC is much higher than in the rest of the medium. On the other hand, IGF2R+ cells might degrade IGF-2, and this would mandate a high initial concentration of hormone to support expansion of HSCs over a 3-day culture period.
Both SCF and TPO are required to maintain normal HSC levels, mainly by preventing their apoptosis.5,8 IGFs prevent apoptosis and trigger proliferation of different types of cells, principally through both the mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3 kinase (PI-3K) pathways.46 Here we showed that the combination of IGF-2 with SCF and TPO supports a more than 2-fold increase in HSC numbers. Most likely, when combined with SCF and TPO, IGF-2 is both antiapoptotic and mitogenic for HSCs. We are trying to determine whether IGF-2 affects apoptosis of HSCs or whether it acts by accelerating the rate at which HSCs enter the cell cycle. IGF-2 may also increase the proportion of the daughter cells that become HSCs.
In adult rodents, IGF-2 is reportedly not expressed and its functions are suggested to be replaced by IGF-1. However, we found that some non-HSC and stromal bone marrow cells express high levels of IGF-2 (C.C.Z., B. Luo, H.F.L., unpublished observations, July 2003). Therefore, IGF-2 likely is functionally important in the adult bone marrow to maintain homeostasis of adult stem cells, consistent with our observations that all adult bone marrow HSCs express receptors for IGF-2 and that IGF-2 supports expansion of bone marrow HSCs in culture.
In summary, we identified IGF-2 as a novel stem cell growth factor and for the first time achieved a 2- to 3-fold net expansion of fetal liver HSCs in the presence of IGF-2. Although rodent IGF-2 is thought to play important roles only in fetal development, we showed that IGF-2 also stimulated the ex vivo expansion of adult bone marrow HSCs. Because IGF-2 has pleiotropic effects, the expression of receptors for IGF-2 may define pan–stem cell properties, and IGF-2 may have the potential to act on a broader spectrum of stem cells, both ex vivo and in vivo.
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2003-08-2955.
Supported by the Engineering Research Centers Program of the National Science Foundation under NSF award no. EEC 9843342 through the Biotechnology Process Engineering Center at the Massachusetts Institute of Technology (H.F.L.). C.C.Z. is a Leukemia and Lymphoma Society Fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Nir Hacohen for advice and insightful discussions on the project and Drs Biao Luo, Chang-Zheng Chen, Xiao-Wu Zhang, Wei Tong, Alec Gross, and Christopher Hug for reading of the manuscript and help in experiments. We are grateful for help with FACS provided by Glenn Paradis and Michael Doire at the Massachusetts Institute of Technology (MIT) flow cytometry core facility and the technical assistance by Sonali Rudra. We appreciate the kind gift of anti-IGF2R by Dr Stuart Kornfeld at Washington University.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal