Abstract
The mechanisms responsible for immunoglobulin G (IgG) immunity against allogeneic platelets are poorly understood. We studied the role that murine recipient CD8+ T and natural killer (NK) cells play in immunity against allogeneic platelets. BALB/c mice were depleted of the cells by cell-specific antibodies, transfused weekly with platelets from C57BL/6 mice, and serum IgG antidonor antibodies were measured by flow cytometry. While allogeneic platelet transfusions into wild-type recipients stimulated IgG antidonor antibodies in all mice by the fifth transfusion, CD8-depleted mice had significantly (P < .001) enhanced antibody production. Isotype analysis revealed that CD8+ T cells suppressed T-helper 2 (Th2)-associated IgG1 but enhanced Th1-associated IgG2a. Compared with wild-type mice, platelet transfusions into CD8-depleted mice stimulated enhanced intracellular interferon (IFN)-γ production by CD4- lymphocytes within 24 hours after the first transfusion. The early IFN-γ response correlated with nitric oxide-dependent splenic cytotoxicity (P < .001). In asialo ganglioside monosialic acid 1 (GM1)-depleted mice transfused with allogeneic platelets, the IFN-γ production, splenic cytotoxicity, and IgG antidonor antibody response were significantly suppressed. These results demonstrate that IgG antiplatelet immunity is dependent on an early NK cell-derived IFN-γ response that is negatively regulated by CD8+ T cells and suggest that targeting innate NK cell responses may significantly reduce platelet alloimmunization. (Blood. 2004;103:2705-2709)
Introduction
It is well established that leukoreduction of platelet concentrates significantly reduces alloimmunization and clinical refractoriness in patients with leukemia.1 However, approximately 20% of platelet recipients still become alloimmunized, which suggests that the platelets themselves have the ability to stimulate alloimmunization independently of leukocytes.2 Hence, a more platelet-specific immunotherapy may further reduce the incidence of alloimmunization. Understanding how alloantibodies are produced against leukoreduced platelets is fundamental to developing these modalities.
Using murine models of leukoreduced platelet alloimmunization, it was previously shown that leukoreduced donor platelets not only generate anti-major histocompatibility complex (MHC) class I-specific alloantibodies3 but do so via indirect allorecogniton where recipient CD4+ T cells recognize platelet-derived alloantigens on recipient antigen presenting cells.4-6 This indirect IgG response could be suppressed by aminoguanidine, a selective inhibitor of inducible nitric oxide synthase, suggesting that the recipient's innate immune mechanisms were critical for stimulating platelet antibodies.5 Related to this, it was found that recipient antigen processing pathways were critically involved in the regulation of the IgG alloantibody production against transfused donor platelets.7 Inhibition of the MHC class I antigen processing pathway responsible for the activation of CD8+ T cells enhanced Th1-associated IgG2a antidonor antibody production and suppressed Th2-associated IgG1 isotype production.7 However, the potential role of recipient CD8+ T cells in modulating allogeneic platelet immunity has not been studied.
For many years, innate immunity has been considered as a separate entity from the adaptive immune response and has been regarded to be of secondary importance in the hierarchy of immune functions.8-10 However, in recent years, evidence has been accumulating that the innate immune system may be the ultimate controller of adaptive responses.8 For example, cells of the innate immune system can significantly influence the type of adaptive immune response, by controlling the differentiation of naive T-helper (Th0) lymphocytes into effector cells of a particular type (Th1 or Th2).8 Recent evidence suggests that platelets themselves may bridge the innate and adaptive immune systems by expressing immunostimulatory molecules such as CD154 and help stimulate adaptive immunity such as antiviral CD8+ T-cell induction.11 Natural killer (NK) cells also play a critical role in bridging innate and adaptive immunity through the modulation of cytokine networks, and NK cells are one of the major sources of the Th1-type cytokine interferon (IFN)-γ.12,13 Although much progress has been made in understanding the role of NK cells in immunoregulation, little as yet is known about their function in IgG alloantibody production in recipients of platelet transfusions.
The aim of the present study was to define the role that recipient CD8+ and NK cells play in the regulation of IgG antibody responses produced in recipient mice against donor leukoreduced platelet transfusions. We investigated how depletion of CD8+ T and/or NK cells affected cytokine production and the IgG immune response against donor platelet transfusions. Our results suggest that IgG antiplatelet immunity is dependent on the early production of IFN-γ by recipient asialo GM1-positive NK cells and negatively regulated by recipient CD8+ T cells that inhibit the cytokine response.
Materials and methods
Animals
Inbred female BALB/c (H-2d) and C57BL/6 (H-2b) mice, 8 to 10 weeks of age, were used as the transfusion recipients and as platelet donors, respectively. The mice were purchased from Charles River Canada (Montreal, QC, Canada) and maintained in the research vivarium of St Michael's Hospital, Toronto, ON, Canada.
Cell lines
P815 (H-2d) DBA mastocytoma and EL-4 (H-2b) C57BL/6 thymoma cell lines were used as cytotoxic targets and were purchased from the American Type Culture Collection (ATCC; Rockville, MD). The cell lines were maintained with RPMI-1640 containing 10% fetal calf serum (FCS; Gibco BRL, Grand Island, NY), 100 μg/mL penicillin/streptomycin/fungizone, 100 mM l-glutamine (Gibco BRL) and 5 × 10-5 M 2-mercaptoethanol (cRPMI; Sigma Aldrich, St Louis, MO).
Platelet preparation
Donor mice were bled and leukoreduced platelets were prepared as previously described.14 Briefly, mice were bled via the tail vein into EDTA (ethylenediaminetetraacetic acid) microvettes (Sarstedt, St Laurent, QC, Canada), the blood was pooled and centrifuged at 250g, and the platelet-rich plasma (PRP) was collected. The PRP was centrifuged at 1000g for 18 minutes and platelets were adjusted to a concentration of 109/mL in phosphate-buffered saline (PBS) for transfusion. White blood cells (WBCs) were enumerated by flow cytometry as previously described.14 WBC levels in the stock platelets were found to be 1.5 ± 1.0 WBC/μL.
In vivo depletion of lymphocytes
Recipient CD8+ T cells were depleted using the 2.43 (rat antimouse CD8) monoclonal antibody produced from hybridomas obtained from the American Type Culture Collection. Recipient mice were injected intraperitoneally with 100 μg antibody/mouse one day prior to the first platelet transfusion and at weekly intervals. Within 24 hours of antibody infusion, no detectible CD8+ T cells were identified in the peripheral blood or spleen and the depletion lasted for at least 7 days. Depletion of NK cells was accomplished by intraperitoneal injections of 50 uL polyclonal rabbit anti-asialo GM1 (Cedarlane Laboratories, Hornby, ON, Canada) one day prior to the first platelet transfusion and at weekly intervals. Within 24 hours of antibody infusion, no detectible NK1.1+ NK cells were identified in the peripheral blood or spleen and depletion lasted for at least 7 days.
Transfusion protocol and blood preparation
In each transfusion protocol, all mice were bled 24 hours before the first transfusion and injected weekly with 100 uL stock platelets via the tail vein. Each week, blood was collected (24 hours before transfusion) into red-top microvettes (Sarstedt, Montreal, QC, Canada) and allowed to clot at 22°C for 1 hour. Fresh sera were used to determine IgG antidonor isotype titres.
Flow cytometric detection of antidonor antibodies
For the detection of IgG antidonor antibodies, 106 donor platelets were incubated with titrations of fresh recipient sera for 1 hour at 4°C. The cells were washed once and labeled with fluorescein isothiocyanate (FITC)-conjugated goat antimouse IgG (Fc specific; Cedarlane Laboratories) or FITC-conjugated goat antimouse IgG1, IgG2a, IgG2b, and IgG3 antibodies (Cedarlane Laboratories) for 45 minutes at 4°C in the dark. Cells were analyzed by a FACSort flow cytometer (Becton Dickinson, San Jose, CA) equipped with an Argon laser (Becton Dickinson) operating at 15 mW; 10 000 events were acquired using an electronic cellular (lymphocyte) gate based on forward and side scatter and were analyzed using CELLQUEST software (Becton Dickinson). Matched pretransfusion serum was used as the negative control in all experiments. Cross reactivity of the sera with donor leukocytes was also performed to confirm that the sera reacted primarily with donor MHC class I molecules.
Intracellular cytokine labeling
FITC-conjugated anti-IFN-γ and phycoerythrin (PE)-conjugated anti-interleukin 4 (IL-4) were purchased from Becton Dickinson (BD)-PharMingen (San Jose, CA) and used for intracellular cytokine staining. The final concentrations of the antibodies during the labeling procedures were 0.2 μg/2 × 106 cells. Ionomycin, phorbol 12-myristate acetate (PMA), and Brefeldin-A were obtained from Sigma Aldrich (Oakville, ON, Canada). Permeabilization, staining, and fixation buffers were obtained from BD-PharMingen. Anti-CD4 (both FITC- or PE-conjugated) antibodies for cell-surface staining were purchased from BD-PharMingen and used to differentiate CD4+ from CD4- cells in the CD8- and NK-depleted recipient mice. This analysis was optimal to determine cytokine levels in the NK-depleted mice.
Recipient cells were prepared from peripheral blood on a 1.077 g/mL Percoll cushion, washed and resuspended to 5 × 106 cells/mL in cRPMI in 24-well culture plates. Cells were stimulated with 1 μM ionomycin and 20 ng/mL PMA. Brefeldin-A (10 μg/mL) was also added at the start of culture to inhibit cytokine secretion (protein transport inhibitor). The 24-well culture plate was incubated at 37°C in a humidified CO2 incubator for 4 hours. Intracellular cytokine staining was performed according to the protocol recommended by BD-PharMingen. Briefly, cells were washed and the cell surface was stained with the indicated anti-CD4 antibody for 15 minutes at 22°C in staining buffer (PBS, 2% fetal bovine serum, and 0.09% sodium azide, pH 7.4). Cells were washed once in PBS and incubated with Cytofix/cytoperm buffer (BD-PharMingen) for 20 minutes at 22°C. The cells were then washed once with Perm/Wash buffer (BD-PharMingen) and incubated with either FITC-conjugated anti-IFN or PE-conjugated anti-IL-4 antibodies for 15 minutes at 22°C and analyzed by flow cytometry. Frequencies of cytokine-producing cells were enumerated after gating on lymphocytes; 10 000 events were acquired for each sample. The data were then analyzed using CELLQUEST software. Fluorescent markers were set using unlabeled control cells as a reference.
Cytotoxicity assay
Cell-mediated cytotoxicity was measured using recipient spleen cells as effector cells in 6-hour cytotoxic assays using sodium 51chromate (Na51Cr)-labeled target lines. Recipient spleen cells were titrated in triplicate in 96-well V-bottom plates (Fisher Scientific, Nepean, ON, Canada) to make final effector-to-target (E/T) ratios of 50:1 to 6.25:1. Target cells were labeled for 2 hours at 37°C with 100 μCi (3.7 MBq) Na51Cr (specific activity 2 mCi/mL [7.4 × 107 Bq/mL]; ICN Biomedicals, Costa Mesa, CA) per 106 cells, washed 5 times, and used at 5000 cells/well. Following centrifugation at 100g for 4 minutes, the plates were incubated at 37°C for 6 hours and 100 μL of supernatant from each well collected and counted in a Beckman (Fullerton, CA) gamma counter (gain, 190). Results were expressed as percent specific 51Cr release (percent specific lysis) and were calculated by the formula: cpmtest - cpmmin/cpmmax - cpmmin × 100. Spontaneous release (minimum cytolysis or cpmmin) was determined from target cells alone. Total release (cpmmax) was determined by addition of 75 μL of a 2% Triton X-100 (Sigma Aldrich) solution.
Statistics
Significant differences between means were calculated by Student t test for unpaired values.
Results
IgG antidonor immunity is enhanced in CD8-depleted recipient mice
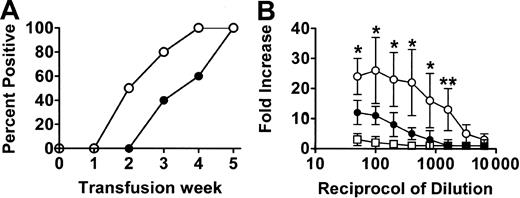
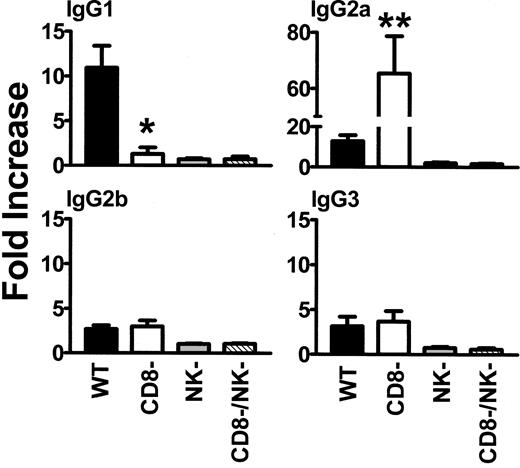
When wild-type (WT) BALB/c recipient mice were transfused weekly with 108 donor C57BL/6 platelets, all mice developed IgG antidonor antibodies by the fifth platelet transfusion (Figure 1) whereas CD8-depleted mice produced IgG antidonor antibodies earlier and in all mice by the fourth platelet transfusion (Figure 1A). By the fifth platelet transfusion, the CD8-depleted mice had significantly (P = .01) higher titres of IgG antidonor antibodies compared with WT mice (Figure 1B). The IgG isotypes produced were predominantly Th2-associated IgG1 and Th1-associated IgG2a isotypes, although detectable levels of IgG2b and IgG3 were also observed (Figure 2). However, compared with the WT mice, transfusion of platelets into the CD8-depleted BALB/c recipients significantly (P = .04) suppressed their ability to produce IgG1 isotypes but significantly (P = .009) enhanced the production of IgG2a antibodies. IgG2b and IgG3 isotype production was not changed by CD8 depletion in BALB/c recipients.
Antibody production in the platelet-transfused mice. (A) Percentage of WT (•) and CD8-depleted (○) mice positive for IgG antiplatelet antibodies during the transfusion protocol. Donor platelets were incubated with a 1:50 dilution of sera obtained from recipient mice after each transfusion, labeled with FITC-goat antimouse IgG antibody, and analyzed by flow cytometry. Results shown are the percent antidonor antibody-positive mice (n = 20) from each group. All the immune sera were also reactive with donor leukocytes. (B) Titres of IgG antidonor antibodies in the sera of WT (•), CD8-depleted (○), and asialo GM1-depleted (□) mice at the fifth platelet transfusion. Donor leukocytes were incubated with the indicated titrations of recipient sera obtained after the fifth platelet transfusion, labeled with FITC-goat antimouse IgG antibody, and analyzed by flow cytometry. Fold increase was calculated by the following formula: percentage of donor cells positive with test serum/percentage of donor cells positive with pretransfusion test serum. The results are expressed as the mean (± SD) of the fold increases in 20 mice from each group. No detectible antidonor antibodies were observed in any of the pretransfusion sera. Significance values between WT and CD8-depleted mice are indicated (*P < .01; **P < .001).
Antibody production in the platelet-transfused mice. (A) Percentage of WT (•) and CD8-depleted (○) mice positive for IgG antiplatelet antibodies during the transfusion protocol. Donor platelets were incubated with a 1:50 dilution of sera obtained from recipient mice after each transfusion, labeled with FITC-goat antimouse IgG antibody, and analyzed by flow cytometry. Results shown are the percent antidonor antibody-positive mice (n = 20) from each group. All the immune sera were also reactive with donor leukocytes. (B) Titres of IgG antidonor antibodies in the sera of WT (•), CD8-depleted (○), and asialo GM1-depleted (□) mice at the fifth platelet transfusion. Donor leukocytes were incubated with the indicated titrations of recipient sera obtained after the fifth platelet transfusion, labeled with FITC-goat antimouse IgG antibody, and analyzed by flow cytometry. Fold increase was calculated by the following formula: percentage of donor cells positive with test serum/percentage of donor cells positive with pretransfusion test serum. The results are expressed as the mean (± SD) of the fold increases in 20 mice from each group. No detectible antidonor antibodies were observed in any of the pretransfusion sera. Significance values between WT and CD8-depleted mice are indicated (*P < .01; **P < .001).
Analysis of IgG1, IgG2a, IgG2b, and IgG3 isotypes in WT, CD8-depleted, NK-depleted, and CD8/NK-depleted mice. Donor cells were incubated with a 1:50 dilution of recipient sera obtained after the fifth platelet transfusion, labeled with the indicated FITC-goat antimouse IgG isotype antibody, and analyzed by flow cytometry. Fold increase was calculated by the following formula: percentage of donor cells positive with test serum/percentage of donor cells positive with prebleed test serum. The results are expressed as the mean (± SD) of the fold increases in 20 mice from each group. • indicates WT; □, CD8-depleted; ▦, NK-depleted; ▧, CD8/NK-depleted. Significance values between WT and CD8-depleted mice are indicated (*P = .04; **P = .009).
Analysis of IgG1, IgG2a, IgG2b, and IgG3 isotypes in WT, CD8-depleted, NK-depleted, and CD8/NK-depleted mice. Donor cells were incubated with a 1:50 dilution of recipient sera obtained after the fifth platelet transfusion, labeled with the indicated FITC-goat antimouse IgG isotype antibody, and analyzed by flow cytometry. Fold increase was calculated by the following formula: percentage of donor cells positive with test serum/percentage of donor cells positive with prebleed test serum. The results are expressed as the mean (± SD) of the fold increases in 20 mice from each group. • indicates WT; □, CD8-depleted; ▦, NK-depleted; ▧, CD8/NK-depleted. Significance values between WT and CD8-depleted mice are indicated (*P = .04; **P = .009).
Enhanced IgG antiplatelet immunity in CD8-depleted mice correlated with elevated early IFN-γ production in CD4-lymphocytes
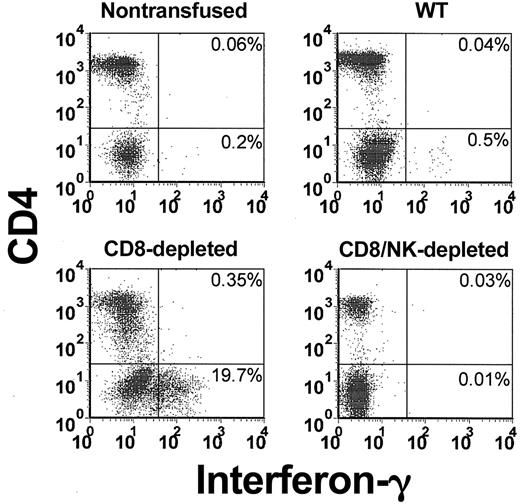
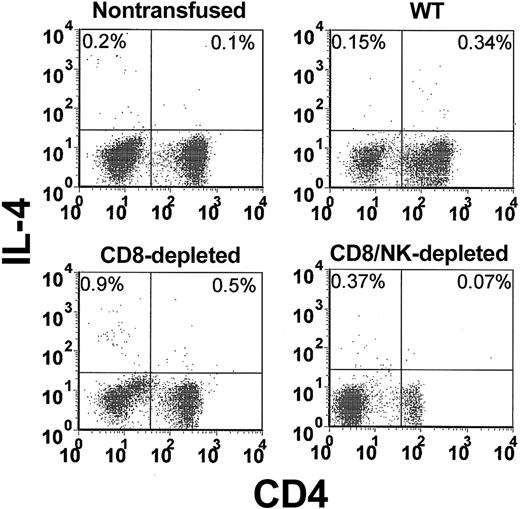
The levels of intracellular IFN-γ (Figure 3) and IL-4 (Figure 4) in peripheral blood lymphocytes were measured by flow cytometry. When WT mice were transfused with platelets, a significant (P = .01) elevation of the percentage of IFN-γ-producing cells in CD4 lymphocytes (5.4-fold higher than nontransfused controls) was observed within 24 hours (Figure 5A). In CD8-depleted mice, however, the increase in IFN-γ in CD4 lymphocytes was significantly (P < .001) higher (26.8 ± 7-fold higher than nontransfused controls, Figure 5A). Analysis of IL-4 production in CD4 lymphocytes showed that IL-4 production was significantly less than IFN-γ and not elevated in the transfused WT mice (Figure 5B). It was, however, significantly (P = .02) elevated in CD8-depleted recipients.
Flow cytometric analysis of intracellular IFN-γ in CD4-labeled T cells in the peripheral blood from nontransfused, WT, CD8-depleted, and CD8/NK-depleted recipient mice. The data shown are representative of all mice in each group.
Flow cytometric analysis of intracellular IFN-γ in CD4-labeled T cells in the peripheral blood from nontransfused, WT, CD8-depleted, and CD8/NK-depleted recipient mice. The data shown are representative of all mice in each group.
Flow cytometric analysis of intracellular IL-4 in CD4-labeled T cells in the peripheral blood from nontransfused, WT, CD8-depleted, and CD8/NK-depleted recipient mice. The data shown are representative of all mice in each group.
Flow cytometric analysis of intracellular IL-4 in CD4-labeled T cells in the peripheral blood from nontransfused, WT, CD8-depleted, and CD8/NK-depleted recipient mice. The data shown are representative of all mice in each group.
Change in the intracellular IFN-γ and IL-4 in CD4 peripheral blood lymphocytes from WT, CD8-depleted, NK-depleted, and CD8/NK-depleted mice. Fold increase was calculated by the following formula: percentage of cytokine-positive cells from transfused mouse/percentage of cytokine-positive cells from nontransfused mouse. The data are expressed as the mean (± SD) of the fold increases for 10 mice per group. • indicates WT; □, CD8-depleted; ▦, NK-depleted; ▧, CD8/NK-depleted. Significance values between WT and CD8-depleted mice are indicated (*P < .001; **P = .02).
Change in the intracellular IFN-γ and IL-4 in CD4 peripheral blood lymphocytes from WT, CD8-depleted, NK-depleted, and CD8/NK-depleted mice. Fold increase was calculated by the following formula: percentage of cytokine-positive cells from transfused mouse/percentage of cytokine-positive cells from nontransfused mouse. The data are expressed as the mean (± SD) of the fold increases for 10 mice per group. • indicates WT; □, CD8-depleted; ▦, NK-depleted; ▧, CD8/NK-depleted. Significance values between WT and CD8-depleted mice are indicated (*P < .001; **P = .02).
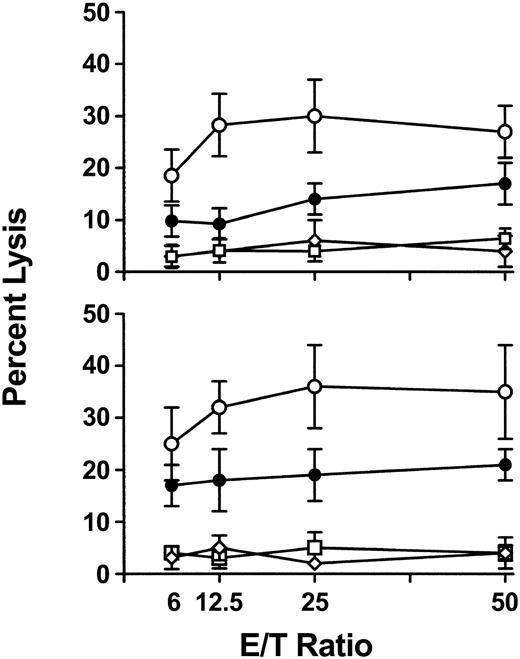
To further characterize the cytokine response, IFN-γ-dependent nitric oxide (NO)-mediated cytotoxicity against donor (Figure 6A) or recipient (Figure 6B) cell targets was measured in 6-hour chromium release assays. Compared with WT mice, CD8-depleted recipients had significantly (P < .01) elevated levels of cytotoxicity against both target cells at 24 hours after transfusion, indicating the nonspecific nature of NO-mediated killing (Figure 6). The NO dependence of the cytotoxicity was confirmed when addition of 100 μM aminoguanidine (AMG) to the assays completely inhibited the cytotoxic response (data not shown).
Splenic cytotoxicity in WT, CD8-depleted, NK-depleted, and NK/CD8-depleted mice. Spleen cells were prepared from mice 24 hours after initial transfusion and used as cytotoxic effectors against chromium-labeled (A) P815 and (B) EL-4 target cells in a 6-hour assay. Percent lysis at the indicated effector-to-target ratios was calculated by the following formula: cpmtest - cpmmin/cpmmax - cpmmin × 100. The results are expressed as the mean (± SD) percent lysis from 10 mice per group. • indicates WT; ○, CD8-depleted; □, NK-depleted; ⋄, NK/CD8-depleted. All cytotoxicities were inhibited by addition of 100 μM aminoguanidine (AMG) to the assays.
Splenic cytotoxicity in WT, CD8-depleted, NK-depleted, and NK/CD8-depleted mice. Spleen cells were prepared from mice 24 hours after initial transfusion and used as cytotoxic effectors against chromium-labeled (A) P815 and (B) EL-4 target cells in a 6-hour assay. Percent lysis at the indicated effector-to-target ratios was calculated by the following formula: cpmtest - cpmmin/cpmmax - cpmmin × 100. The results are expressed as the mean (± SD) percent lysis from 10 mice per group. • indicates WT; ○, CD8-depleted; □, NK-depleted; ⋄, NK/CD8-depleted. All cytotoxicities were inhibited by addition of 100 μM aminoguanidine (AMG) to the assays.
Depletion of asialo GM1-positive NK cells in recipient mice significantly suppressed the immune responses against donor platelet transfusions
Because the early IFN-γ response was contained within CD4 lymphocytes, the effect of depleting recipient NK cells was assessed. Compared with WT mice, platelet transfusions into either asialo GM1- or asialo GM1- and CD8-depleted recipients did not induce either any significant IgG antidonor antibody production (Figures 1 and 2), intracellular IFN-γ or IL-4 production (Figure 5), or any splenic NO-dependent cytotoxicity (Figure 6).
Discussion
To date, little is known of the recipient immune mechanisms responsible for IgG antiplatelet antibody production. Previous results of experiments have suggested that transfused platelets first interact with and stimulate innate immune responses such as NO production, which eventually culminates in the production of IgG antidonor antibodies by the adaptive immune system.5-7 On this basis, we further studied the nature of these innate responses and how they may be linked with adaptive immunity. Our results suggest that within 24 hours of the initial transfusion, donor platelets stimulate recipient asialo GM1-positive NK cells to secrete IFN-γ. This cytokine response correlated with in vitro splenic NO-mediated cytotoxicity and was negatively regulated by recipient CD8+ T cells that suppressed the IFN-γ production. This suggests that NK cells of the recipient's innate immune system can be targeted for manipulation of antiplatelet immunity.
Previous results indicated that the recipient's MHC class I antigen processing pathways play an important role in suppressing Th1-associated allogeneic platelet immunity.7 We tested this by depleting recipient mice of CD8+ T cells and then transfusing the mice with allogeneic platelets. There was a significant enhancement of Th1-associated IgG2a isotypes and suppressed Th2-associated IgG1 isotypes that correlated with an early and enhanced IFN-γ response in CD4 lymphocytes (Figure 2). These results suggest that recipient CD8+ T cells actively suppress Th1-associated IgG antiplatelet immunity by reducing IFN-γ production and are analogous to previous studies using whole blood transfusions.15-18 For example, Douillard et al17 have shown that CD8+ T cells play a role in the induction of allograft tolerance by donor-specific whole blood transfusion and this may be mediated by reducing Th1 cytokines, particularly IFN-γ.18 Taken together, these results suggest that even in different transfusion settings (eg, platelets versus whole blood), recipient CD8+ T cells are responsible for suppressing adaptive immune responses such as IgG antiplatelet immunity.
The mechanisms of how CD8+ T cells suppress IgG antiplatelet immunity are unknown. With respect to the recognition patterns of T cells, recipient CD8+ T cells could potentially be stimulated either indirectly via presentation of allogeneic platelet peptides on recipient MHC class I molecules or directly by recognizing intact MHC molecules on the donor platelet's surface. However, Gouttefangeas et al19 have shown that platelets cannot stimulate cytotoxic T lymphocyte allocytotoxicity by the direct pathway in vitro and our previous results suggest that recipient MHC class I processing pathways are important in regulating antiplatelet immunity.7 These results suggest that the CD8+ T-cell-mediated immunosuppression may be stimulated via the indirect pathway of T-cell recognition. We are currently studying this.
The early and enhanced IFN-γ response in CD4 lymphocytes in CD8-depleted recipients led us to examine the role that NK cells might play in IgG antiplatelet immunity. We found that an asialo GM1-positive NK cell population was responsible for the early IFN-γ production and this response was essential for the generation of NO-dependent cytotoxicity and the production of IgG antiplatelet antibodies (Figures 2, 5, and 6). These data suggest that antiplatelet adaptive immune responses are ultimately stimulated and regulated by signals from NK cells of the innate immune system. NK cells represent a key component of the innate immune response and have been shown to mediate graft rejection in mice following bone marrow20,21 or skin transplantation.22 Furthermore, NK cell-derived IFN-γ has been shown to be responsible for other immune mechanisms, such as the regulation of IgG isotype production during in vivo23 and in vitro24 immune responses. The effects of IFN-γ on IgG isotype selection may have an advantage in clearing allogeneic platelets because IgG2a antibodies can additionally fix complement.25 It is not, however, clear how recipient NK cells interact with transfused allogeneic platelets in order to stimulate IFN-γ production, although Nieswandt et al26 have demonstrated that platelets can inhibit NK-mediated cytolysis independently of platelet MHC class I molecules. Taken together, the present results suggest that methods to reduce NK cell-derived IFN-γ production may be effective in significantly suppressing platelet immunity.
In summary, IgG antidonor platelet immunity in BALB/c mice is dependent on an early recipient NK cell-derived IFN-γ response that is negatively regulated by CD8+ T cells. Our data reveal a novel mechanism by which early recipient innate NK cell responses significantly influence subsequent adaptive immune response directed against transfused donor platelet antigens. They suggest that recipient innate immune mechanisms represent a potential therapeutic target for reducing platelet immunogenicity.
Prepublished online as Blood First Edition Paper, December 4, 2003; DOI 10.1182/blood-2003-10-3552.
Supported by grants from the Canadian Blood Services R&D Fund and the Canadian Institutes of Health Research. E. Sayeh was the recipient of a Post Doctoral Fellowship from the Canadian Blood Services.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal