Abstract
In this study, we investigated the role of hepatocyte growth factor (HGF) in blood formation during Xenopus development. First, we examined the gene expression of HGF and its receptor, c-met, by whole-mount in situ hybridization during development. Strong signals of HGF as well as c-met were detected early in the developing ventral mesoderm, which later gives rise to the ventral blood island. Furthermore, to study the role of HGF, we blocked the HGF signaling pathway in Xenopus embryos by using truncated c-met lacking the tyrosine kinase domain. Injection of truncated c-met mRNA resulted in a marked decrease in the number of circulating blood cells. Similar results were obtained using morpholino antisense HGF oligonucleotides. Moreover, we also analyzed the expression of several early primitive blood markers in the blood island of these embryos. RNA in situ analysis revealed a significant reduction (or absence) of stem cell leukemia (SCL), α-globin, and GATA-1 expression, but not GATA-2 expression. In contrast, no significant difference was observed in the levels of expression of early definitive blood markers, SCL, GATA-2, and GATA-3 in the dorsolateral plate, as analyzed by in situ hybridization. Overall, the present study demonstrated that HGF is necessary for primitive hematopoiesis by regulating the expression of SCL. (Blood. 2004;103:3320-3325)
Introduction
In all vertebrate development, blood cell formation occurs in 2 successive waves. These are termed primitive and definitive hematopoiesis, based on the time of initiation, site of development, cell morphology, globin content, and potential to differentiate.1 Primitive hematopoiesis occurs first and gives rise to predominantly erythrocytes (primitive red blood cells). Primitive red blood cells are produced exclusively in the ventral blood island (VBI, a functional equivalent of the extraembryonic yolk sac of mammals) of Xenopus. This phenomenon is then followed by definitive hematopoiesis, which leads to the production of all the blood lineages that are required throughout the life span of a vertebrate. Definitive blood cells arise predominantly from the dorsolateral plate (DLP, equivalent to the intraembryonic aorta-gonadsmesonephros region).2 Stem cell leukemia (SCL), a basic helix-loophelix transcription factor, and GATA-1, GATA-2, and GATA-3, zinc-finger transcription factors, are required for normal blood cell development. Targeted disruption of each of these genes in mice resulted in a failure of hematopoiesis and death due to anemia.3-7 In Xenopus, these are early markers of the hematopoietic mesoderm. GATA-1 was detected by in situ hybridization in the VBI in the late neurula, and expression was increased in the VBI as the blood island forms.8 GATA-2 and SCL were expressed in both the VBI and DLP,2 while GATA-3 was expressed in the DLP, but not the VBI.9 In addition, it was shown that overexpression of SCL, GATA-1, or GATA-2 leads to increased globin production.10-12
Hepatocyte growth factor (HGF) was originally identified as a mitogen for hepatocytes and a motility enhancing factor for epithelial cells.13,14 During mouse development, HGF and c-met were expressed in a wide variety of developing organs,15 and were shown to play multiple roles as a mitogen, a motogen, and a morphogen in the organization of multicellular structures.16 In Xenopus, the HGF/c-met system was also shown to play a crucial role in liver development and structures of the pronephros at the late stage of development.17 In addition, it was also reported that HGF was expressed in the early mesoderm region, especially in the ventral mesoderm, where primitive hematopoiesis occurs.18 Although these data suggested that the HGF signaling pathway might also play a role in regulating hematopoiesis, none of our data revealed a function of HGF or c-met in blood differentiation. The present study demonstrated that HGF and c-met were expressed in the ventral mesoderm region, which contains primitive blood cell precursors. Of importance, blockade of the HGF signaling pathway by dominant-negative c-met inhibited primitive hematopoiesis, but not definitive hematopoiesis. Our results clearly indicate that the HGF signaling pathway regulated primitive blood formation by regulating the expression of SCL.
Materials and methods
Embryo generation and RNA microinjection
Xenopus laevis embryos were generated using standard techniques and staged according to Nieuwkoop and Faber.19 For blastomere injections, regularly cleaving embryos were selected, and 2000 pg synthetic mRNA encoding truncated c-met or LacZ was microinjected per blastomere at the 8-cell stage. For lineage tracing, RNA encoding green fluorescent protein (GFP) was coinjected at 50 pg per blastomere. Injected embryos were cultured in 3% ficoll/100% Steinberg solution for 12 hours and then in 80% Steinberg solution until death.
In vitro transcription
For mRNA production, truncated c-met lacking the tyrosine kinase domain,17 full-length c-met, LacZ, and GFP cDNAs were cloned into pCS2+ or pSP64TS.20 In vitro-transcribed RNA was prepared from lineaged plasmid DNA using a Megascript Kit (Ambion, Austin, TX). Concentration and integrity of the RNA were checked by optical density 260 and agarose gel electrophoresis in the presence of formaldehyde.
Morpholino oligonucleotides
HGF morpholino antisense oligonucleotides (HGF-MOs) and HGF-5misMO as control were purchased from Gene Tools (Corvallis, OR). The HGF-MO is 5′-TGAGAGATGTTGCCTGGTGGTT-3′. The HGF-5misMO is 5′-TGtGAaATGTTcCCTGcTGcTT-3′, where lower cases indicate mismatch mutations. At the 2-cell stage, 4 ng was microinjected per blastomere.
Whole embryo RNA in situ hybridization and o-dianisidine staining
The protocol of Harland21 was used with slight modifications, including the use of BM purple (Roche Diagnostics GmbH, Mannheim, Germany) as a substrate and without protease K and RNase treatment. Some stained embryos were embedded in paraffin and sectioned at 15 μm. Then, o-dianisidine staining was performed as previously described.22 The number was counted in a blinded manner.
Results
Developmental expression pattern of HGF and c-met
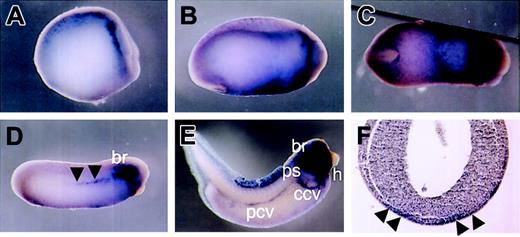
Although HGF and its receptor, c-met, were isolated in Xenopus,18,23 detailed analysis of their spatio-temporal expression patterns during Xenopus development has not been performed. Therefore, we decided to examine the HGF and c-met expression patterns in detail during development by whole-mount in situ hybridization. We found that HGF transcripts were first detected at neurula stage 14, in the head mesenchyme and a subset of neuroblasts present in the lateral stripe of the neural plate (Figure 1A). By stage 18, HGF was expressed in the branchial arches and ventral region (Figure 1B). Ventral expression of HGF formed a V-shaped pattern characteristic of the developing VBI (Figure 1C). Transverse sections of the embryo revealed HGF staining in the mesoderm layer beneath the ectoderm (Figure 1F). By the early tailbud stage, HGF expression was also detected in the DLP. As development continued, HGF expression decreased in the ventral region, and by the late tailbud stage, strong signals were detected in the branchial arches, embryonic vasculature, and heart (Figure 1E).
Localization of HGF expression by whole-mount in situ hybridization. (A) Lateral view of stage-14 embryo. HGF is detected in the head mesenchyme and neuroblasts. (B and C) Lateral and ventral view, respectively, of stage-20 embryo. HGF is expressed in the branchial arches and ventral region. Ventral expression of HGF forms a V-shaped pattern. (D) Lateral view of stage-24 embryo. Ventral expression of HGF is decreased. Staining is evident in the branchial arches and dorsolateral plate (DLP) mesoderm (black arrowheads). (E) Lateral view of stage-36 embryo. HGF is detected in the branchial arches, embryonic vasculature (common cardinal vein, posterior cardinal vein, and pronephric sinus), and heart. (F) Transverse section of stage-20 embryo. HGF staining is present in the mesoderm layer beneath the ectoderm (black arrowheads). br indicates branchial arches; ccv, common cardinal vein; h, heart; pcv, posterior cardinal vein; and ps, pronephric sinus.
Localization of HGF expression by whole-mount in situ hybridization. (A) Lateral view of stage-14 embryo. HGF is detected in the head mesenchyme and neuroblasts. (B and C) Lateral and ventral view, respectively, of stage-20 embryo. HGF is expressed in the branchial arches and ventral region. Ventral expression of HGF forms a V-shaped pattern. (D) Lateral view of stage-24 embryo. Ventral expression of HGF is decreased. Staining is evident in the branchial arches and dorsolateral plate (DLP) mesoderm (black arrowheads). (E) Lateral view of stage-36 embryo. HGF is detected in the branchial arches, embryonic vasculature (common cardinal vein, posterior cardinal vein, and pronephric sinus), and heart. (F) Transverse section of stage-20 embryo. HGF staining is present in the mesoderm layer beneath the ectoderm (black arrowheads). br indicates branchial arches; ccv, common cardinal vein; h, heart; pcv, posterior cardinal vein; and ps, pronephric sinus.
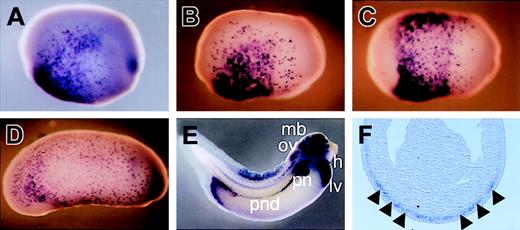
In contrast, c-met expression was first detected by whole embryo in situ analysis at stage 11 in the posteroventral region of the embryo (data not shown). c-met expression expanded laterally as the embryo developed. By neurula stage 17, c-met expression was detectable in the ventral region in a salt-and-pepper fashion, except for the region behind the prospective cement gland and ectoderm where it was in a mosaic fashion (Figure 2B-C). The ectodermal expression pattern remained at least until stage 36. Closer inspection of the expression domain in sectioned material showed c-met-positive cells in the outer layer of the endomesoderm (Figure 2F). This includes the region that later gives rise to the VBI. Then, c-met expression in the endomesoderm gradually decreased. By stage 23, c-met expression was restricted to almost only the ectodermal cells (Figure 2D). As development continued, c-met expression was again detectable in the presumptive pronephric tubules and duct, liver, heart, and central nervous system (Figure 2E).
Localization of c-met expression by whole-mount in situ hybridization. (A) Lateral view of stage-16 embryo. c-met expression is detected in the ventral region (with the exception of the region behind the prospective cement gland) in a salt-and-pepper fashion and in the ectoderm in a mosaic fashion. (B and C) Lateral and ventral view, respectively, of stage-17 embryo. c-met staining increases as development proceeds. (D) Lateral view of stage-23 embryo. c-met expression is maintained in the ectoderm but decreased in the dorsal mesoderm. (E) Lateral view of stage-36 embryo. c-met is detected in the liver, midbrain, otic vesicle, pronephric tubules, pronephric duct, heart, and posterior mesoderm near the anus. ccv indicates common cardinal vein; h, heart; lv, liver; mb, midbrain; ov, otic vesicle; pn, pronephric tubules; and pnd, pronephric duct. (F) Transverse section of stage-17 embryo. c-met staining is present in the mesoderm layer beneath the ectoderm (black arrowheads).
Localization of c-met expression by whole-mount in situ hybridization. (A) Lateral view of stage-16 embryo. c-met expression is detected in the ventral region (with the exception of the region behind the prospective cement gland) in a salt-and-pepper fashion and in the ectoderm in a mosaic fashion. (B and C) Lateral and ventral view, respectively, of stage-17 embryo. c-met staining increases as development proceeds. (D) Lateral view of stage-23 embryo. c-met expression is maintained in the ectoderm but decreased in the dorsal mesoderm. (E) Lateral view of stage-36 embryo. c-met is detected in the liver, midbrain, otic vesicle, pronephric tubules, pronephric duct, heart, and posterior mesoderm near the anus. ccv indicates common cardinal vein; h, heart; lv, liver; mb, midbrain; ov, otic vesicle; pn, pronephric tubules; and pnd, pronephric duct. (F) Transverse section of stage-17 embryo. c-met staining is present in the mesoderm layer beneath the ectoderm (black arrowheads).
Inhibition of circulating mature red blood cell formation by blocking of HGF signaling
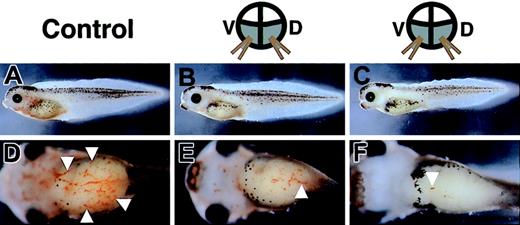
In situ hybridization analysis revealed that both HGF and its receptor, c-met, were expressed in the early developing ventral mesoderm that gives rise to primitive red blood cells. Thus, the HGF signaling pathway may play an important role in primitive blood formation. In an effort to investigate this possibility, we analyzed the blood formation in embryos in which the HGF signaling pathway was blocked by injection of RNA encoding a dominant-negative truncated c-met.17 Truncated c-met mRNA was injected into 4 vegetable blastomeres of 8-cell embryos, with coinjection of GFP mRNA as a lineage tracer. After injection, these embryos were allowed to develop into tadpoles, and were then arrested for examination at the stage when red blood cells can be observed easily in the heart and blood vessels. In control tadpoles coinjected with LacZ and GFP mRNAs, numerous GFP-expressing cells were found circulating throughout the vasculature, as previously reported (data not shown).24,25 In contrast, in tadpoles coinjected with truncated c-met and GFP mRNAs, a few GFP-positive cells were found in the circulation, although no marked difference in the localization of GFP activity was observed except in circulating blood cells (data not shown). To assess their hemoglobin levels, these tadpoles were stained with o-dianisidine, a marker for mature red blood cells. The tadpoles injected with truncated c-met mRNA showed a marked reduction in o-dianisidine staining (Figure 3B-C,E-F), compared with control tadpoles injected with LacZ mRNA (Figure 3A,D). Injection of LacZ mRNA resulted in a marked decrease in red blood cells in only 20% of embryos (n = 40), whereas injection of truncated c-met mRNA resulted in a marked decrease in red blood cells in 84% of embryos (n = 44). These data revealed that injection of truncated c-met mRNA led to a reduction in the number of circulating mature, hemoglobin-positive red blood cells. Consistent with a previous report,17 injection of truncated c-met mRNA also interfered with liver development and pronephros formation in the later stage of development (data not shown). As the development of red blood cells did not affect their viability in Xenopus,26 these embryos were viable for at least a week.
Decrease in red blood cells induced by overexpression of truncated c-met mRNA. Tadpoles are stained with o-dianisidine, which demonstrates cells containing hemoglobin (white arrowheads). (A-C) Lateral views. (D-F) Ventral views. (A,D) Stained cells are detected in the heart and vitelline veins of a control tadpole injected with LacZ mRNA, but are rare in tadpoles injected with truncated c-met mRNA (B,C,E-F).
Decrease in red blood cells induced by overexpression of truncated c-met mRNA. Tadpoles are stained with o-dianisidine, which demonstrates cells containing hemoglobin (white arrowheads). (A-C) Lateral views. (D-F) Ventral views. (A,D) Stained cells are detected in the heart and vitelline veins of a control tadpole injected with LacZ mRNA, but are rare in tadpoles injected with truncated c-met mRNA (B,C,E-F).
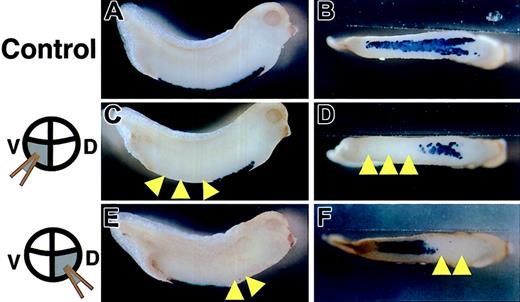
Since in the above experiments all blood cells were still primitive blood cells derived from the VBI, we examined the expression pattern of larval α-globin in the VBI of embryos injected with truncated c-met mRNA into 2 dorsal-vegetable or ventral-vegetable blastomeres at the 8-cell stage. The anterior and posterior portions of the VBI are derived from dorsal-vegetable and ventral-vegetable blastomeres of 8-cell embryos, respectively.24,27 α-globin was expressed throughout the VBI in LacZ mRNA injected embryos, as analyzed by whole-mount in situ hybridization (Figure 4A-B). In contrast, expression of α-globin was markedly reduced or absent in truncated c-met mRNA-injected embryos (Figure 4C-D). Importantly, the embryos injected with truncated c-met mRNA into ventral-vegetable blastomeres showed a decrease or absence of α-globin expression only in the posterior portion of the VBI (Figure 4C-D), while the embryos injected with truncated c-met mRNA into dorsal-vegetable blastomeres showed a decrease or absence of α-globin expression only in the anterior portion of the VBI (Figure 4E-F). These regions overlapped with the location of cells that received the injected mRNA, as summarized in Table 1. Approximately 70% of embryos displayed a decrease or absence of α-globin expression in the cells that received truncated c-met mRNA. When full-length c-met mRNA was coinjected into dorsal-vegetable blastomeres, rescue of the decrease or absence of α-globin expression, caused by injection of the truncated c-met, was observed (Table 2). The injection of 2000 pg truncated c-met mRNA into dorsal-vegetable blastomeres caused a decrease or absence of α-globin expression in 71% of the embryos. Coinjection of 4000 pg full-length c-met mRNA resulted in 46% normal embryos, and only 38% embryos with a decrease or absence of α-globin expression (Table 2).
Inhibition of expression of α-globin by overexpression of truncated c-met mRNA. Stage 33/34 embryos were stained for α-globin RNA by in situ hybridization. (A,C,E) Lateral views. (B,D,F) Ventral views. Embryos were injected at the 8-cell stage with either (A-B) LacZ mRNA into 2 ventral-vegetable blastomeres (control), (C-D) truncated c-met mRNA into 2 ventral-vegetable blastomeres, or (E-F) truncated c-met mRNA into 2 dorsal-vegetable blastomeres. Yellow arrowheads indicate the absence of α-globin expression, compared with control.
Inhibition of expression of α-globin by overexpression of truncated c-met mRNA. Stage 33/34 embryos were stained for α-globin RNA by in situ hybridization. (A,C,E) Lateral views. (B,D,F) Ventral views. Embryos were injected at the 8-cell stage with either (A-B) LacZ mRNA into 2 ventral-vegetable blastomeres (control), (C-D) truncated c-met mRNA into 2 ventral-vegetable blastomeres, or (E-F) truncated c-met mRNA into 2 dorsal-vegetable blastomeres. Yellow arrowheads indicate the absence of α-globin expression, compared with control.
Gene expression pattern in response to injected truncated c-met mRNA
. | . | Gene expression in ventral-vegetable injections, % . | . | . | . | Gene expression in dorsal-vegetable injections, % . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Decreased or absent . | . | . | . | Decreased or absent . | . | ||||||
| RNA . | In situ . | n . | Normal . | Posterior VBI . | Throughout VBI . | n . | Normal . | Anterior VBI . | Throughout VBI . | ||||||
| tc-met | α-globin | 42 | 33 | 67 | 0 | 46 | 9 | 71 | 20 | ||||||
| LacZ | α-globin | 36 | 86 | 14 | 0 | 28 | 74 | 15 | 11 | ||||||
| tc-met | GATA-1 | 34 | 39 | 61 | 0 | 39 | 23 | 67 | 10 | ||||||
| LacZ | GATA-1 | 37 | 78 | 19 | 3 | 31 | 81 | 6 | 13 | ||||||
| tc-met | SCL | 39 | 49 | 51 | 0 | 41 | 20 | 78 | 2 | ||||||
| LacZ | SCL | 25 | 92 | 8 | 0 | 32 | 91 | 9 | 0 | ||||||
| tc-met | GATA-2 | 33 | 100 | 0 | 0 | 29 | 82 | 14 | 4 | ||||||
| LacZ | GATA-2 | 31 | 97 | 0 | 3 | 40 | 82 | 5 | 13 | ||||||
. | . | Gene expression in ventral-vegetable injections, % . | . | . | . | Gene expression in dorsal-vegetable injections, % . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Decreased or absent . | . | . | . | Decreased or absent . | . | ||||||
| RNA . | In situ . | n . | Normal . | Posterior VBI . | Throughout VBI . | n . | Normal . | Anterior VBI . | Throughout VBI . | ||||||
| tc-met | α-globin | 42 | 33 | 67 | 0 | 46 | 9 | 71 | 20 | ||||||
| LacZ | α-globin | 36 | 86 | 14 | 0 | 28 | 74 | 15 | 11 | ||||||
| tc-met | GATA-1 | 34 | 39 | 61 | 0 | 39 | 23 | 67 | 10 | ||||||
| LacZ | GATA-1 | 37 | 78 | 19 | 3 | 31 | 81 | 6 | 13 | ||||||
| tc-met | SCL | 39 | 49 | 51 | 0 | 41 | 20 | 78 | 2 | ||||||
| LacZ | SCL | 25 | 92 | 8 | 0 | 32 | 91 | 9 | 0 | ||||||
| tc-met | GATA-2 | 33 | 100 | 0 | 0 | 29 | 82 | 14 | 4 | ||||||
| LacZ | GATA-2 | 31 | 97 | 0 | 3 | 40 | 82 | 5 | 13 | ||||||
Summary of gene expression pattern obtained in injection experiments. Frequency of gene expression pattern is given as a percentage. n represents the number of embryos scored from independent experiments. Expressions of genes were analyzed by in situ hybridization at stage 33/34 (α-globin), stage 32/33 (GATA-1), and stage 30/31 (SCL and GATA-2). Embryos were scored for decreased or absent staining throughout the VBI or the posterior portion of the VBI in embryos injected with mRNAs to ventral-vegetable blastomeres, and throughout the VBI or the anterior portion of the VBI in embryos injected to dorsal-vegetable blastomeres.
tc-met indicates truncated c-met.
Rescue of α-globin expression induced by truncated c-met mRNA injection by c-injection of full length of c-met mRNA
. | . | . | Decreased or absent . | . | |
|---|---|---|---|---|---|
| Dose full-length c-met* . | n . | Normal, % . | Anterior VBI, % . | Throughout VBI, % . | |
| 0 pg | 46 | 9 | 71 | 20 | |
| 2000 pg | 62 | 23 | 66 | 11 | |
| 4000 pg | 45 | 46 | 38 | 16 | |
. | . | . | Decreased or absent . | . | |
|---|---|---|---|---|---|
| Dose full-length c-met* . | n . | Normal, % . | Anterior VBI, % . | Throughout VBI, % . | |
| 0 pg | 46 | 9 | 71 | 20 | |
| 2000 pg | 62 | 23 | 66 | 11 | |
| 4000 pg | 45 | 46 | 38 | 16 | |
Frequency of globin expression pattern is given as a percentage. Embryos were scored for either the decrease of or absence in staining throughout the VBI, or the anterior portion of the VBI in embryos injected with mRNAs to dorsal-vegetable blastomeres.
The dose of truncated c-met used in these injections was 2000 pg.
These results were also confirmed by antisense strategy. Recently, morpholino technology has been used to knock down endogenous gene expression in Xenopus by blocking translation of specific genes.28 To further confirm that the HGF signaling pathway is required for primitive blood formation, we used the morpholino technology. The embryos injected with antisense HGF oligonucleotides showed the suppression of expression of α-globin throughout VBI compared with embryos injected with control oligonucleotides (Figure 5A-B). Injection of control oligonucleotides (HGF-5misMO) resulted in the suppression of α-globin expression in only 25% of embryos (n = 25), while injection of antisense HGF oligonucleotides suppressed α-globin expression in 87% of embryos (n = 39).
Suppression of α-globin expression by injection of morpholino antisense HGF oligonucleotides (HGF-MO). Embryos were injected with either (A) HGF-MO or (B) HGF-5misMO (control) into 2 blastomeres at the 2-cell stage. Blue arrowheads indicate the heterogeneous α-globin expression in the VBI.
Suppression of α-globin expression by injection of morpholino antisense HGF oligonucleotides (HGF-MO). Embryos were injected with either (A) HGF-MO or (B) HGF-5misMO (control) into 2 blastomeres at the 2-cell stage. Blue arrowheads indicate the heterogeneous α-globin expression in the VBI.
Analysis of expression pattern of transcriptional factors GATA-1, GATA-2, and SCL in the VBI
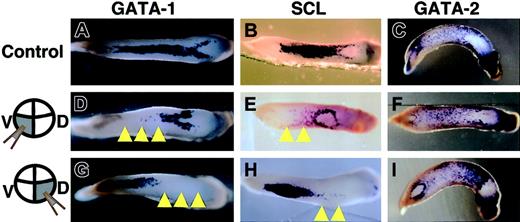
Next, we examined the expression patterns of 3 other blood markers, namely GATA-1, GATA-2, and SCL,8,11,29 since these transcriptional factors are thought to control the differentiation of blood cells.10-12 A significant reduction in the expression of GATA-1 and SCL was observed only in the cells injected with truncated c-met mRNA (Figure 6). Unexpectedly, no marked decrease in GATA-2 expression was observed in the cells injected with truncated c-met mRNA, albeit the expression was slightly reduced throughout the VBI compared with the control (Figure 6; Table 1).
Expression pattern of GATA-1, SCL, and GATA-2 shown by in situ hybridization. (A-C) Control embryos injected with LacZ mRNA into 2 ventral-vegetable blastomeres. Embryos injected with truncated c-met mRNA into (D-F) 2 ventral-vegetable blastomeres or (G-I) dorsal-vegetable blastomeres. (A,D,G) Expression of GATA-1 at stage 32/33. (B,E,H) Expression of SCL at stage 30/31. (C,F,I) Expression of GATA-2 at stage 30/31. Yellow arrowheads indicate absence of expression of these genes. Anterior is right for all embryos.
Expression pattern of GATA-1, SCL, and GATA-2 shown by in situ hybridization. (A-C) Control embryos injected with LacZ mRNA into 2 ventral-vegetable blastomeres. Embryos injected with truncated c-met mRNA into (D-F) 2 ventral-vegetable blastomeres or (G-I) dorsal-vegetable blastomeres. (A,D,G) Expression of GATA-1 at stage 32/33. (B,E,H) Expression of SCL at stage 30/31. (C,F,I) Expression of GATA-2 at stage 30/31. Yellow arrowheads indicate absence of expression of these genes. Anterior is right for all embryos.
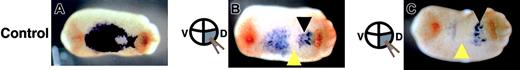
As the injection of truncated c-met mRNA caused a reduction in α-globin, GATA-1, and SCL expression at the late stage of blood differentiation in the VBI, we further studied whether blood formation was disrupted from the early stage. The basic helix-loophelix factor, SCL, is known to be the earliest blood marker of the VBI. Thus, we examined the pattern of SCL expression at stage 23, when no expression of α-globin or GATA-1 is detected,8,30 and SCL expression shows a V-shaped pattern characteristic of the developing VBI.11 Of the embryos injected with truncated c-met mRNA into 2 dorsal-vegetable blastomeres, 59% showed inhibition of anterior (except most of the anterior region behind the cement gland) SCL expression (Figure 7B), while 26% of embryos showed inhibition throughout the area (except most of the anterior region behind the cement gland) of SCL expression. Some embryos showed a lag of posterior SCL expression (Figure 7C). Of embryos, 15% showed no marked difference in the SCL expression pattern, compared with control (n = 27). Interestingly, the most anterior region of SCL expression, which never showed disturbance of truncated c-met expression, may represent vascular endothelial precursors.11,31
Expression pattern of SCL in experimental embryos at stage 23 shown by in situ hybridization. Embryos were injected with either (A) LacZ mRNA (control) or (B-C) truncated c-met mRNA into 2 dorsal-vegetable blastomeres. Yellow arrowheads indicate the absence of SCL expression. Black arrowheads indicate groups of endothelial precursor cells. Anterior is right for all embryos.
Expression pattern of SCL in experimental embryos at stage 23 shown by in situ hybridization. Embryos were injected with either (A) LacZ mRNA (control) or (B-C) truncated c-met mRNA into 2 dorsal-vegetable blastomeres. Yellow arrowheads indicate the absence of SCL expression. Black arrowheads indicate groups of endothelial precursor cells. Anterior is right for all embryos.
Definitive blood cell formation in DLP
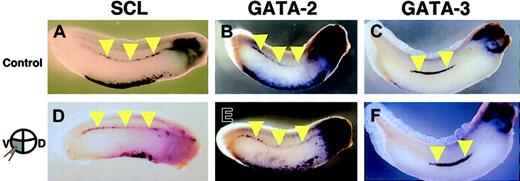
With the striking deficiency of primitive hematopoiesis in the truncated c-met mRNA-injected embryos, we finally examined whether definitive hematopoiesis is also affected by blockade of the HGF signaling pathway. Transplantation studies have demonstrated that definitive hematopoietic progenitors reside in the DLP.2 The expression of hematopoietic transcription factors, SCL, GATA-2, and GATA-3 in the DLP is thought to reflect the initiation of the definitive hematopoietic program in these cells.2,9,11 Thus, we analyzed the expression pattern of these markers in the DLP of embryos injected with truncated c-met mRNA into 2 ventral-vegetable blastomeres, which give rise to the DLP.27,32 Inspection of embryos stained with SCL (n = 49), GATA-2 (n = 33), and GATA-3 (n = 25) failed to reveal obvious defects in the DLP, compared with control embryos (Figure 8).
Expression pattern of SCL, GATA-2, and GATA-3 in AGM shown by in situ hybridization. Embryos were injected with either (A-C) LacZ (control) or (D-F) truncated c-met mRNA into 2 ventral-vegetable blastomeres. (A,D) SCL expression at stage 30/31. (B,E) GATA-2 expression at stage 30/31. (C,F) GATA-3 expression at stage 33. Yellow arrowheads indicate expression of these genes in DLP.
Expression pattern of SCL, GATA-2, and GATA-3 in AGM shown by in situ hybridization. Embryos were injected with either (A-C) LacZ (control) or (D-F) truncated c-met mRNA into 2 ventral-vegetable blastomeres. (A,D) SCL expression at stage 30/31. (B,E) GATA-2 expression at stage 30/31. (C,F) GATA-3 expression at stage 33. Yellow arrowheads indicate expression of these genes in DLP.
Discussion
HGF or c-met null mice showed defects in liver and placental development and migration of myogenic precursor cells into the limb bud.33-35 It was also reported that the erythrocyte count in embryonal blood was reduced in HGF null mice, although fetal hematopoiesis occurred in the impaired liver, suggesting that the definitive hematopoietic program appears normal. Schmidt et al suspected that this reflects a reduction of the size of the liver and extensive cell death.33 However, in the case of lethal knock-out mice, analysis of blood formation has been quite limited. Thus, using Xenopus, we studied the role of HGF and c-met in primitive hematopoiesis. The present study revealed that c-met was detectable during nurula, encompassing a wide domain that includes the VBI field, and it is expressed in a salt-and-pepper fashion, similar to the early expression pattern of SCL.11 HGF was expressed slightly later than c-met in the VBI field. Importantly, HGF and c-met transcripts were particularly abundant in the ventral mesoderm and were detectable prior to the appearance of transcripts encoding SCL and GATA-1. At the late tailbud stage, HGF and c-met expression had shifted and were observed in many organs.
The results of the present study demonstrate that HGF has a pivotal function in primitive blood development in the VBI, since injection of dominant-negative c-met mRNA severely reduced the number of circulating mature primitive red blood cells. In addition, RNA in situ hybridization analysis showed a reduction of α-globin expression in the VBI only in the cells injected with dominant-negative c-met mRNA and morpholino antisense HGF oligonucleotides. Moreover, injection of truncated c-met mRNA reduced the expression of the important transcriptional factors, SCL and GATA-1, but not GATA-2. These findings suggest that the HGF signaling pathway acts upstream of SCL and GATA-1, but not GATA-2. In the present study, a reduction of SCL expression in the VBI was observed before the appearance of GATA-1 expression.8,30 In the yolk sac of SCL-deficient mice and embryoid bodies derived from in vitro differentiation of ES cells, GATA-2 was detected by reverse transcription-polymerase chain reaction at levels comparable with those in wild type, while globin and GATA-1 were undetectable.6,36 Thus, our results suggest that the HGF signaling pathway might regulate blood synthesis via regulation of SCL expression independent of GATA-2 expression. A recent study showed that an enhanceosome responsible for activating SCL transcription was required for GATA-2, Fli-1, and Elf-1.37 As the expression of Fli-1 was induced via the STAT3 transcription factor,38 phosphorylation of STAT3 by the binding of HGF to c-met39 might activate Fli-1 or Elf-1.
In contrast, as early SCL expression in the region immediately posterior to the cement gland was not disturbed by injection of truncated c-met mRNA, gene targeting experiments have indicated an essential role for SCL in hematopoiesis and angiogenesis.40 SCL is expressed in both the hematopoietic and endothelial lineage at an early stage,11 whereas the signal of Flk-1 expression, a marker of vascular endothelial precursors, was detectable in this region.31 Thus, these cells may differentiate into endothelial cells, but not blood cells. In this region, no signal of c-met expression was detectable throughout Xenopus development by in situ hybridization analysis. Thus, the effect of the dominant-negative version of c-met mRNA would be restricted to blood formation, but not angiogenesis. In addition, the present study did not answer whether the blockade of the HGF signaling pathway interferes with the emergence of mature circulating definitive blood cells, since ectopic mRNA has already disappeared by that time.20 However, the early step of the definitive hematopoietic program appears to be undisturbed by blockade of HGF signaling, as no marked decrease in expression of SCL, GATA-2, or GATA-3 was observed in the early DLP of embryos injected with truncated c-met mRNA. Overall, the present study demonstrated that HGF is a specific regulator of primitive hematopoiesis rather than definitive hematopoiesis. Interestingly, these data were clearly different from mouse mutants. In HGF knock-out mice, homozygous mutant embryos have severely impaired placentas with markedly reduced numbers of labyrinthine trophoblast cells and die before birth.34 In addition, one other report documented that the mutation affects the embryonic liver, which is reduced in size and shows extensive loss of parenchymal cells.33 To our knowledge, there is no report that the inhibition of HGF signaling impaired primitive hematopoiesis in the mouse model. Further studies in the role of HGF in the mammalian development must be necessary.
Prepublished online as Blood First Edition Paper, December 30, 2003; DOI 10.1182/blood-2003-02-0352.
Supported in part by a Grant-in-Aid from the Organization for Pharmaceutical Safety and Research, a Grant-in-Aid from the Ministry of Public Health and Welfare, a Grant-in-Aid from Japan Promotion of Science, a Grant-in-Aid from Mitsubishi Pharma Research Foundation, and through Special Coordination Funds of the Ministry of Education, Culture, Sports, Science and Technology of the Japanese government.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Tomoyuki Takahashi and Masahiro Yamaguchi for their technical advice. We are grateful to Paul A. Krieg, Mitsugu Maeno, Koichiro Shiokawa, Kosuke Tashiro, Peter D. Vize, and Leonard I. Zon for providing plasmids.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal