Abstract
Recent studies have demonstrated neutrophil overexpression of the polycythemia rubra vera-1 (PRV-1) gene in polycythemia vera (PV) but not in secondary or spurious polycythemia (SP). To validate as well as expand upon this novel observation, we conducted a prospective study of 88 subjects: 30 with PV, 22 with SP, 14 with essential thrombocythemia (ET), 12 with myelofibrosis with myeloid metaplasia (MMM), and 10 controls. To minimize interstudy methodologic differences, we used a published real-time polymerase chain reaction (PCR)-based assay. The proportion of patients with increased neutrophil PRV-1 expression was 83% in PV, 21% in ET, 42% in MMM, 18% in SP, and 0% in controls. All 5 MMM patients with PRV-1 up-regulation had an antecedent history of PV. We conclude that neutrophil PRV-1 up-regulation is a characteristic feature of PV that may not be affected by fibrotic transformation. However, quantifying neutrophil PRV-1 mRNA, while complementary to other tests, is not in itself sufficient for the diagnosis of PV. (Blood. 2004;103:3547-3548)
Introduction
Although clinical presentation, serum erythropoietin (Epo) level, and bone marrow histology provide adequate information for the diagnosis of polycythemia vera (PV) in most cases, the possibility of either spurious polycythemia (SP) or essential thrombocythemia (ET) is sometimes entertained and needs to be clarified. In this regard, the traditional Polycythemia Vera Study Group “diagnostic criteria”1 lack both accuracy and practicality and are being replaced by a “diagnostic algorithm”2 that utilizes, when necessary, specialized tests including an assay for endogenous erythroid colony (EEC) formation,3 megakaryocyte/platelet Mpl expression,4,5 and the more recently described neutrophil polycythemia rubra vera-1 (PRV-1) expression.6 Although none of these biologic markers are specific to PV, they have certainly provided an additional level of comfort in establishing a working diagnosis.
Quantitative neutrophil PRV-1 measurement has been evaluated in healthy controls as well as patients with PV, SP, ET, and de novo myelofibrosis with myeloid metaplasia (MMM).7-9 In the most recent of such studies, the diagnostic accuracy of a quantitative, neutrophil PRV-1 assay was reported to be 100% in distinguishing PV from SP.7 In this particular study, all 71 patients (100%) with PV but none of 11 patients with SP displayed neutrophil PRV-1 overexpression. In contrast, 2 previous reports that used a similar quantitative assay found neutrophil PRV-1 mRNA not to be elevated in 4 of 13 8 and 2 of 23 9 patients with PV. In regard to ET, while 1 study found PRV-1 up-regulation in only 2 of 12 patients (17%),8 a second study reported a corresponding rate of 67% (10 of 15 patients).9 In the current study, we sought to independently verify the performance of the quantitative PRV-1 assay in distinguishing PV from SP as well as examine neutrophil PRV-1 expression patterns in ET and MMM (both de novo and post-polycythemic).
Study design
This is a prospective, institutional review board (IRB)-approved, single-institutional study involving 88 subjects: 30 with PV, 22 with SP, 14 with ET, 12 with MMM, and 10 controls. All patients with PV, ET, or MMM fulfilled either the updated Polycythemia Vera Study Group (PVSG)10,11 or World Health Organization (WHO)12 diagnostic criteria. Furthermore, the diagnosis was confirmed by characteristic bone marrow histology in all cases of PV, ET, and MMM and in 15 of the 22 patients with SP.13 Among the 22 patients with SP, 13 had secondary erythrocytosis (2 with high-oxygen-affinity hemoglobin variants; 3 with history of exogenous testosterone administration; 4 with a combination of sleep apnea, tobacco use, or pulmonary hypertension; 1 with atrial septal defect; 1 with valvular fibroelastoma; 2 indeterminate causes) and 9 had spurious polycythemia that was defined as an erroneous perception of increased red cell mass that resulted from either volume contraction or a clinician's perception of what constitutes the upper limit of normal values for hematocrit.2 Among the 12 patients with MMM, 7 had de novo MMM and 5 had post-polycythemic myeloid metaplasia (PPMM).
To minimize interstudy methodologic differences, the current study used the original test protocol that was kindly provided by Dr Heike L. Pahl.7 Thirty milliliters of blood, in ethylenediaminetetraacetic acid (EDTA), were obtained from each patient during a clinically indicated phlebotomy. All samples were processed within 6 hours at room temperature. Neutrophils were isolated through double-density gradient centrifugation using Ficoll-Paque (Sigma, St Louis, MO). Total RNA was extracted via Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA). Samples were analyzed for both integrity and quantity using the Agilent 2100 bioanalyzer (Agilent Technologies, Carmel, IN). Reverse transcription and polymerase chain reaction (PCR) were performed in the single-buffer system Taqman One Step reverse transcriptase PCR (RT-PCR) (Applied Biosystems, Foster City, CA [no. 4309169]). PRV-1 transcripts were amplified by using primers created from the human PRV-1 mRNA GenBank sequence (NM_020406): prv1 forward primer (9 μM; 5′-GCTGTCCACCAAAATGAGCAT-3′), prv1 reverse primer (0.5 μM; 5′-TTCTCACGCGCAGAGAAGATC-3′), and prv1 probe (2.5 μM; 5′-FAM-TTCTTGTTGAACCACACCAGACAAATCGG-3′). Cycling conditions were as follows: 30 minutes at 48°C, 10 minutes at 95°C, and 40 cycles of 15 seconds at 95°C and 1 minute at 60°C.
To standardize results, the experiment was run as a relative quantitation assay, incorporating the housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene transcript, which used a JOE reporter dye (Taqman GAPDH Control Reagents, Applied Biosystems [no. 402869]). The amplifications of PRV-1 and GAPDH were performed in separate wells of the same 96-well plate using the Taqman ABI Prism 7700 (Applied Biosystems). Each sample was analyzed in triplicate for both PRV-1 and GAPDH, with RNA diluted to 50 ng in final suspensions of 50 μL. Data were collected for 6-carboxyfluorescein (FAM) (PRV-1) and 2,7-dimethoxy-4,5-dichloro-6-carboxyfluorescein (JOE) (GAPDH) during the 40 cycles. Results were analyzed with the FAM threshold at 0.2 and the JOE threshold at 0.04. The mean cycle threshold (CT) value of the triplicate PRV-1 values was calculated and then divided with the mean CT value for GAPDH, creating a PRV-1/GAPDH ratio. PRV-1 gene expression was considered elevated in the presence of a PRV-1/GAPDH ratio of 1.17 or lower.
Results and discussion
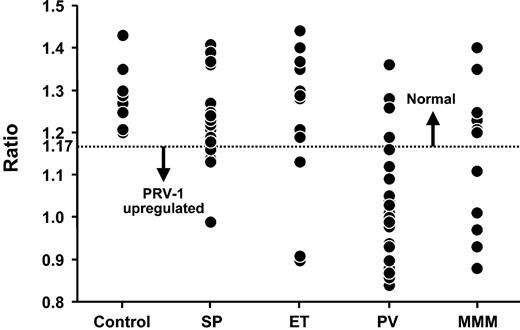
Median ± SD and range of neutrophil PRV-1 expression values, calculated as the ratio of mean cycle of threshold (CT) for each triplicate measurement of PRV-1 to GAPDH, are outlined in Table 1 and graphically presented in Figure 1. The current study confirms previous observations regarding the strong association between PV and neutrophil PRV-1 overexpression. The study also demonstrates, for the first time, neutrophil PRV-1 up-regulation in PPMM but not in de novo MMM, suggesting a distinct biologic difference between PV and MMM. In regard to de novo MMM, our results are different from those of Kralovics et al,9 who used a similar quantitative assay and found PRV-1 overexpression in 4 of 6 patients, but similar to those of Teofili et al,14 who used a qualitative PRV-1 assay and did not detect PRV-1 mRNA in 5 patients. In regard to ET, our results were similar to those of Liu et al,8 who reported a neutrophil PRV-1 overexpression rate of 17%, but different from those of both Kralovics et al9 (67%) and Teofili et al14 (100%).
Comparison of neutrophil polycythemia rubra vera-1 (PRV-1) expression among myeloproliferative disorders and in secondary or spurious causes of polycythemia
. | Control . | SP . | ET . | PV . | MMM . |
|---|---|---|---|---|---|
| No. patients, n = 88 | 10 | 22 | 14 | 30 | 12 |
| Total PRF-1/GAPDH | |||||
| ratio ± SD | 1.28 ± 0.07 | 1.23 ± 0.09 | 1.25 ± 0.16 | 1.01 ± 0.13 | 1.21 ± 0.18 |
| Range of ratio | 1.20-1.43 | 0.99-1.41 | 0.90-1.44 | 0.84-1.36 | 0.88-1.4 |
. | Control . | SP . | ET . | PV . | MMM . |
|---|---|---|---|---|---|
| No. patients, n = 88 | 10 | 22 | 14 | 30 | 12 |
| Total PRF-1/GAPDH | |||||
| ratio ± SD | 1.28 ± 0.07 | 1.23 ± 0.09 | 1.25 ± 0.16 | 1.01 ± 0.13 | 1.21 ± 0.18 |
| Range of ratio | 1.20-1.43 | 0.99-1.41 | 0.90-1.44 | 0.84-1.36 | 0.88-1.4 |
Neutrophil PRV-1 expression in healthy controls, secondary or spurious polycythemia (SP), essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis with myeloid metaplasia (MMM). GAPDH indicates glyceraldehyde-3-phosphate dehydrogenase.
Neutrophil PRV-1 expression in healthy controls, secondary or spurious polycythemia (SP), essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis with myeloid metaplasia (MMM). GAPDH indicates glyceraldehyde-3-phosphate dehydrogenase.
In regard to test sensitivity and specificity, as it pertains to distinguishing PV from SP, our results were different from those of Klippel et al7 (100% accuracy rate) but similar to those of both Liu et al8 and Kralovics et al.9 This clinically relevant discrepancy may not be attributed to methodologic differences because assay performance and interpretation were similar among the 4 studies. In the current study, 20 of the 30 patients with PV were receiving active phlebotomy and 17 myelosuppressive drug therapy at the time of study tests. Among the 5 PV patients with normal PRV-1 expression, 1 was newly diagnosed and manifested all the features of PV (splenomegaly, decreased Epo level, thrombosis). The other 4 patients were diagnosed within 1 month to 10 years of the test date. Three patients were receiving hydroxyurea therapy for 3, 9, and 10 years, respectively, in addition to phlebotomy in 2 cases. None of these 5 patients were receiving interferon-α therapy, a treatment modality that has been associated with correction of PRV-1 up-regulation in PV.15
Four SP patients displayed unexplained (no history of myeloid growth factor use, trauma, or surgery)6 overexpression of neutrophil PRV-1. None of these patients manifested any clinical or laboratory features of PV, and bone marrow histology lacked features of a chronic myeloproliferative process in each instance. One patient was receiving testosterone treatment, and another patient suffered from chronic tobacco-associated obstructive pulmonary disease. The third patient had a stable hemoglobin level of 174 to 185 g/L (174 to 185 g/L) over the preceding 5 years with a normal Epo level. The fourth patient presented with a history of an increased hemoglobin level associated with an increased Epo level. This patient has now been followed for 18 months with a normal hemoglobin level. The current study suggests that PRV-1 up-regulation may not be either an essential or specific component of PV, in the context of its distinction from both SP and ET. Keeping these limitations in mind, however, a carefully standardized quantitative test for measuring neutrophil mRNA should be a useful addition in the diagnostic armamentarium for PV.
Prepublished online as Blood First Edition Paper, December 30, 2003; DOI 10.1182/blood-2003-10-3505.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal