Abstract
No defects related to deficiency of the Wiskott-Aldrich Syndrome protein (WASp) have been described in osteoclasts. Here we show that there are significant morphologic and functional abnormalities. WASp-null cells spread over a much larger surface area and are highly polykaryotic. In their migratory phase, normal cells assemble clusters of podosomes behind their leading edges, whereas during the bone resorptive phase multiple podosomes are densely aggregated in well-defined actin rings forming the sealing zone. In comparison, WASp-null osteoclasts in either phase are markedly depleted of podosomes. On bone surfaces, this results in a failure to form actin rings at sealing zones. Complementation of WASp-null osteoclasts with an enhanced green fluorescent protein (eGFP)-WASp fusion protein restores normal cytoarchitecture. These structural disturbances translate into abnormal patterns of bone resorption both in vitro on bone slices and in vivo. Although physiologic steady-state levels of bone resorption are maintained, a major impairment is observed when WASp-null animals are exposed to a resorptive challenge. Our results provide clear evidence that WASp is a critical component of podosomes in osteoclasts and indicate a nonredundant role for WASp in the dynamic organization of these actin structures during bone resorption. (Blood. 2004;103:3552-3561)
Introduction
The Wiskott-Aldrich syndrome protein (WASp) is an intracellular protein specifically expressed in hematopoietic cells and belongs to a larger family of more widely expressed proteins that mediate de novo actin polymerization.1,2 At present, the members of the WASp family include WASp, N-WASP (neural WASP), Scar/WAVE (suppressor of G protein-coupled cyclic cAMP receptor cAR/WASp family verprolin-homologous protein) proteins, and Las17p/Bee1p. WASp and WASp-related proteins consist of several functional domains that interact with signaling molecules, actin, and the actin-polymerizing factor, Arp2/3 complex.3 Mutations in the gene coding for WASp leads to the Wiskott-Aldrich syndrome (WAS), a hematologic disorder characterized by eczema, micro-thrombocytopenia, and immunodeficiency. WASp-null leukocytes exhibit defects in the organization and regulation of the actin cytoskeleton,4,5 contributing to the observed defects in cell migration and chemotaxis.6,7
Osteoclasts are highly motile cells derived from the monocytic lineage and are specialized in bone resorption and remodeling, and therefore cycle between migratory and resorptive phases. These processes are regulated by the dynamic organization of the actin cytoskeleton. Osteoclasts assemble actin-based adhesion structures named podosomes, which turn over spatially and temporally with a short half-life of between 2 and 12 minutes.8 Podosomes are present in cells of the monocytic lineage as well as some Src-transformed fibroblasts.9 They consist of an F-actin core surrounded by vinculin, talin, α-actinin, fimbrin, gelsolin, vimentin9,10 adaptor molecules such as Crkl,11 and other molecules participating in cytoskeletal signaling pathways such as pp60c-Src, PI3-K,12 RhoA,13 and PYK-2.14 Uniquely, in nonmigratory or resorptive osteoclasts, clustering and fusion of podosomes in an organized ring around the osteoclast periphery defines a resorbing compartment where protons are released for dissolution of bone mineral.8,15,16 The signaling mechanisms that determine the organization and remodeling of the actin cytoskeleton during the migratory and resorptive phases of osteoclasts are, however, relatively undefined.17,18 It is known that ligation of αvβ3 integrins to bone extracellular matrix proteins such as osteopontin19 induces cSrc-dependent tyrosine phosphorylation, PYK2 activation, and translocation to podosomes, followed by cell polarization and osteoclast migration. Many of these cytoskeletal changes are in part mediated through activation of the Rho-GTPases.13,20 Although WASp plays a prominent role in the formation of many cytoskeletal structures in hematopoietic cells, including podosomes in macrophages and dendritic cells (DCs), no evidence has yet been provided showing that it is necessary for migration or sealing zone formation in osteoclasts. Furthermore, there has been no consistent indication of abnormal bone structure or development in humans or mice deficient in WASp. In the present study we have investigated the participation of WASp in the regulation of the osteoclast cytoskeleton and the consequences of deficiency on resorptive osteoclast function. Our results provide good evidence for an absolute requirement for WASp during the formation of both individual podosomes and the normal osteoclast sealing zone, and, consequently, for the physiologic regulation of bone resorption and remodeling.
Materials and Methods
Media and reagents
Bone marrow-derived osteoclasts were cultured in modified Eagle minimum essential medium (MEM) with Earle's salts plus 10% heat inactivated fetal bovine serum (FBS), 2 mM glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin (Gibco BRL, Grand Island, NY). Recombinant human macrophage colony-stimulating factor (M-CSF) and transforming growth factor beta 1 (TGF-β1) were purchased from R&D Systems (Abington, Oxon, United Kingdom); soluble human recombinant receptor activator of nuclear factor-kappa B (NF-kappaB) ligand (RANKL) was obtained from Insight Biotechnology (Wembley, United Kingdom). Phoenix cells were cultured in Dulbecco modified Eagle medium (DMEM; Gibco BRL) and supplemented with 15% heat inactivated FBS. Antivinculin antibody was purchased from Sigma (Gillingham, Dorset, United Kingdom). Alexa 488-conjugated antimouse IgG antibody and Alexa 568 or Alexa 488 phalloidin were obtained from Molecular Probes (Eugene, OR). Anti-WASp antibody was purchased from Upstate Biotechnology (Milton Keynes, United Kingdom). Anti-green fluorescent protein (GFP) antibody was produced in-house and was a gift from Dr Ng (Randall Centre, KCL, London, United Kingdom). Horseradish peroxidase (HRP)-labeled antimouse and antirabbit antibodies were purchased from Dako (Glostrup, Denmark).
Animals
Pathogen-free SV129 mice purchased from Harlan (Bicester, Oxon, United Kingdom) and WASp-null mice on a SV129 background21 originally obtained from Dr Fred Alt (Howard Hughes Medical Institute) were bred in our own colony in pathogen-free conditions.
Isolation and culture of osteoclast precursors from bone marrow
Preparations of osteoclast precursors from 6- to 8-week-old SV129 or WASp-null mice bone marrow were obtained as previously described.22 Mice were culled by cervical dislocation. Femora and tibiae were aseptically removed and dissected free of adherent soft tissue. The bone ends were cut and the marrow cavity was flushed out into a Petri dish by slowly injecting phosphate buffered saline (PBS) without Ca2+ and Mg2+ at one end of the bone using a sterile 21-gauge needle. The bone marrow cells were carefully agitated with a plastic Pasteur pipette to obtain a single cell suspension. Cells were washed twice in PBS by centrifugation (900 g; 5 minutes), resuspended in MEM supplemented with 10% FBS, and incubated for 24 hours in M-CSF (5 ng/mL) at a density of 3 × 105 cells/mL in 75-cm2 flasks at 37°Cina5%CO2 atmosphere. After 24 hours nonadherent cells were harvested, washed, and seeded in 96-well plates (105 cells/well) containing coverslips or bone slices using as culture medium MEM supplemented with 10% FBS, M-CSF (60 ng/mL), RANKL (30 ng/mL), and TGFβ1 (0.1 ng/mL). Cells were incubated for 7 or 10 days and fed every 3 days. Formation of osteoclasts after incubation was evaluated by quantification of tartrate-resistant acid phosphatase (TRAP)-positive cells. At days 7 and 10, more than 80% of the cells were TRAP-positive multinucleated and mononuclear cells.
Immunocytochemistry
Bone marrow osteoclast precursors were seeded on coverslips or bone slices as described above. At day 7, cells were fixed for 20 minutes in 4% wt/vol paraformaldehyde/3% wt/vol sucrose in PBS warmed to 37°C. Cells were washed 3 times with PBS, permeabilized with 0.5% vol/vol Triton X-100 in PBS for 10 minutes, and blocked with 5% wt/vol bovine serum albumin (BSA) in PBS for 45 minutes at room temperature. Actin filaments were detected by incubation with a solution of 0.1 μg/mL Alexa 568-conjugated phalloidin in PBS for 1 hour at 37°C. For localization of vinculin, cells were incubated for 1 hour at room temperature with a 1/500 dilution of antivinculin antibody in PBS containing 2% wt/vol BSA. Coverslips were washed 3 times with PBS and incubated for 1 hour with a PBS solution of 1/100 anti-MoFc receptor (Research Diagnostics, Flanders, NJ) followed by an incubation with a PBS solution of 1/200 diluted Alexa 488-conjugated anti-mouse IgG. For the simultaneous detection of nuclei and F-actin, cells were incubated with Alexa 488-nm phalloidin as described at the beginning of this section, followed by an incubation with a PBS solution of 5 μg/mL propidium iodide at room temperature for 30 minutes. Coverslips were mounted onto slides using 10% wt/vol Mowiol (Kuraray Specialties Europe, Frankfurt, Germany) in PBS containing 0.1% wt/vol P-phenylenediamine and visualized using a Leica TCS NT confocal laser scanning head attached to a Leica DM RXA optical microscope (Leica, St Gallen, Switzerland). Leica TCS scanning software was used to transpose 4 sequential images from 4 separate optical sections taken at equal distances. The same software was used to obtain merged confocal images.
Quantification of arrangements of actin filaments in osteoclasts
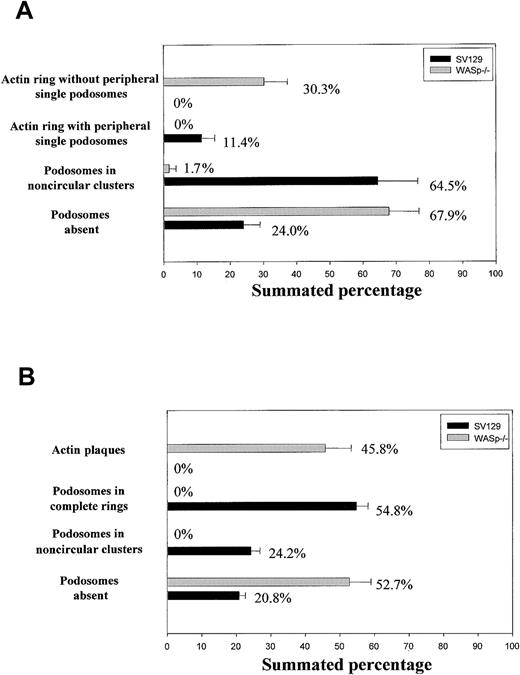
The presence of actin filaments and vinculin was detected by immunofluorescence in osteoclasts plated on glass coverslips, as described in “Immunocytochemistry.” This staining allowed us to detect the presence of podosomes by a characteristic core of actin filaments surrounded by a rim of vinculin. We identified the following possible actin filaments/vinculin arrangements on glass coverslips: (1) actin ring with peripheral single podosomes (dense actin rings with abundant and regularly inserted podosomes surrounded by more dispersed podosomes); (2) actin ring without single podosomes (actin rings depleted of actin and vinculin with few discrete dots of actin filaments within the ring and devoid of surrounding dispersed podosomes); (3) podosomes in noncircular clusters (amorphous aggregations of podosomes); and (4) podosomes absent (absence actin filaments arranged as podosomes). Plating osteoclasts on bone induced the formation of the following arrangements of actin filaments: (1) podosomes in complete rings (dense actin rings colocalizing with vinculin and with inserted podosomes); (2) podosomes in noncircular clusters (organization also identified on glass, see description at the beginning of this section); (3) actin plaques (actin-rich plaques colocalizing with vinculin); (4) podosomes absent (lack of actin filaments arranged into podosomes). We computed 100 cells per experimental condition, and quantification was blinded to the genotypes.
Western blot
Osteoclasts in culture at day 7 were lysed in radioimmunoprecipitation assay (RIPA) lysis buffer containing 1% Triton-X 100, 0.1% sodium dodecyl sulfate (SDS), 150 mM NaCl, 50 mM Tris (trishydroxymethylaminomethane)-HCl, 1 mM EDTA (ethylenediaminetetraacetic acid), 1 mM EGTA (ethyleneglycoltetraacetic acid), 5 mM sodium molybdate, and 20 mM phenylphosphate with protease and phosphatase inhibitors (1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL aprotinin, 20 μg/mL leupeptin, 20 μg/mL pepstatin A, 50 mM NaF, and 1 mM sodium orthovanadate). Twenty micrograms of total cell lysate protein was loaded per lane in a 10% sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel and subjected to electrophoresis. Proteins were blotted onto polyvinylidene-fluoride (PVDF) membranes using a Bio-Rad Mini protein II transfer apparatus (Bio-Rad Laboratories, Hertfordshire, United Kingdom). Blots were blocked with 5% dried milk solution diluted in TBS-T (10 mM Tris, pH 7.5,100 mM NaCl, 0.1% Tween20), incubated with indicated antibody, and signal detected with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (ECL) detection system. Blots were stripped for half an hour with 2% SDS and 0.7% β-mercaptoethanol for 1 hour at 50°C and reprobed for β-actin to check the total amount of protein loaded per lane.
Assessment of bone resorption in vitro
Bone resorption was assessed at day 10 after plating osteoclast precursors on bone slices, as previously described.23 Bone slices were removed from wells, immersed in 10% NaOCl (BDH, Poole, United Kingdom) for 10 minutes to remove cells, washed in distilled water, dried, and sputter-coated with gold. The extent of bone resorption was quantified by counting, using reflected light microscopy, the number of grid intersections in an eyepiece graticule that overlay an area of bone resorption. In addition, the surface of each bone slice was examined in a confocal microscope (Lasertec, London, United Kingdom) to measure the depth and volume of the excavated regions.
Ovariectomy
Ovariectomy (ovx) or sham ovariectomy (sham-ovx) of 22-week-old weight-matched WASp-null and SV129 wild-type mice was performed under general anesthesia (2% halothane [Concord Pharmaceuticals, Essex, United Kingdom], 2l/min O2, 2l/minN2O), using a dorsal approach. During the experiment, animals were housed in solid-bottomed cages, in groups of no more than 6. Food and water were given ad libitum to all animals. Animals were culled 15 days after ovariectomy, and ovariectomy was confirmed by measuring uterine weight. Femurs were removed and freed of soft tissue, fixed in 10% formalin in PBS for 48 hours, demineralized in 10% EDTA in PBS for 7 to 10 days, dehydrated through graded alcohols, and embedded in paraffin wax. Paraffin sections were prepared and mounted onto glass slides. These were then dewaxed, rehydrated through graded alcohols, and stained with toluidine blue or TRAP, using a Leucognost kit (Merck, Darmstadt, Germany). The volume of cancellous bone was measured in a standard zone, situated at least 0.5 mm from the growth plate, excluding the primary spongiosa and trabeculae connected to the cortical bone. All other histomorphometric measurements were carried out in the same zone as that used for assessing bone volume, at an objective magnification of 20 ×. Measurements were made using a digitizer pad, through a camera lucida. The digitizer pad was linked to a computer with dedicated bone software (Osteomeasure, Osteometrics, Atlanta, GA). Osteoclast surface and osteoclast number were measured on the TRAP-stained paraffin sections, in the same way as mentioned for histomorphometric measurement.
Retroviral infection of bone marrow-derived osteoclasts
The retroviral vector backbone used in this study has been described before24 and encodes in our study for enhanced green fluorescent protein (eGFP) or eGFP-WASp fusion proteins under the transcriptional control of a cytomegalovirus promoter. We have previously shown restoration of podosomes in WASp-null dendritic cells using this fusion protein.5 A transient ecotropic packaging cell line (293 HEK, Phoenix) was transfected by standard methods. Cell culture supernatants containing viral particles were collected 48 hours after transfection and filtered through a 0.45-μm filter. Osteoclast precursors were grown on glass coverslips or bone slices for 48 hours after isolation from bone marrow. Transduction was performed by culturing the cells with viral supernatants for 6 hours over 3 cycles, resulting in the expression of eGFP or eGFP-WASp proteins by 70%-80% of osteoclasts at day 7 on glass or day 10 on bone as determined by quantification of the percentage of GFP-positive cells per coverslip or bone slice. Osteoclast cultures were fixed in 4% wt/vol paraformaldehyde/3% wt/vol sucrose in PBS at day 7 and day 10 after isolation on glass and on bone, respectively, and actin organization and cell morphology were examined following the methods given under “Immunocytochemistry.”
Results
Abnormal morphology and actin organization in WASp-null osteoclasts
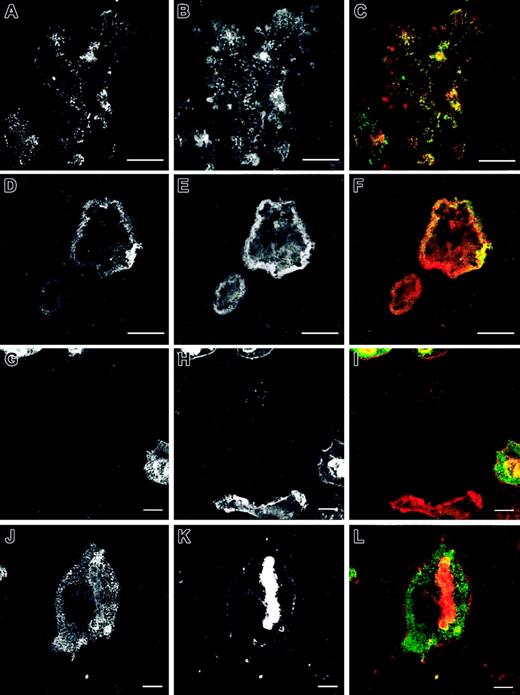
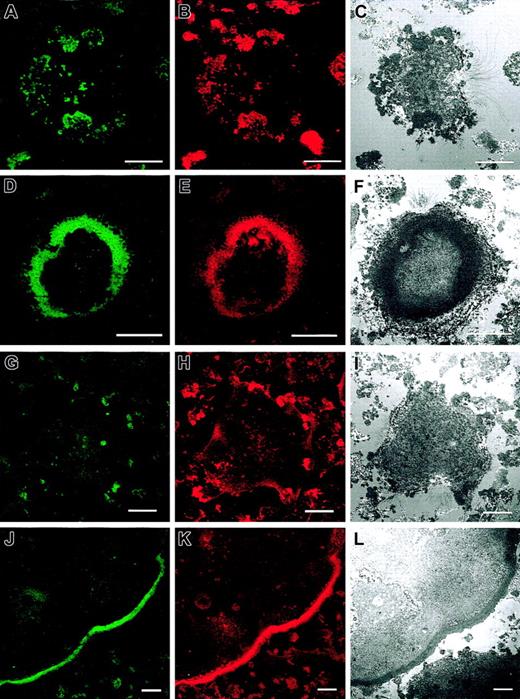
The number and morphology of podosomes and podosome-like structures were first quantified in osteoclast cultures obtained from bone marrow of normal and WASp-null mice and cultured on glass coverslips. The presence of podosomes in osteoclasts was determined by characteristic immunostaining. As shown in Figure 1A, most normal osteoclasts displayed podosomes with an actin core mean size of 1-μm diameter and a well-defined surrounding rim of vinculin. In 64.5% of normal osteoclasts, podosomes were arranged in clusters, very similar to those seen behind the leading edge of migrating macrophages and dendritic cells (Figure 2A-C). In 11.4% of osteoclasts, podosomes were concentrated in dense actin rings (the sealing zone), often surrounded by more dispersed podosome clusters (Figure 2D-F). In cultures derived from WASp-null mice, 67.9% of osteoclasts were completely devoid of podosomes (Figure 2G-I). In contrast, actin and vinculin often colocalized in scattered patches, which were absent in normal cells. Of WASp-null osteoclasts, 30.3% also assembled morphologically abnormal actin rings (Figure 2J-L). These were depleted of actin and vinculin and contained few discrete podosome-like structures within the ring. To determine cytoskeletal organization under more physiologic conditions, cells also were cultured on bone slices. Under these conditions, 24.2% of normal osteoclasts assembled clusters of podosomes, while 54.8% displayed well-organized actin rings, often with several rings per cell (Figure 1B, Figure 3A-F). In summary, a greater percentage of cells formed typical osteoclastic sealing zones on bone slices when compared with cells plated onto glass surfaces, indicating that formation and maturation of actin rings is favored by a physiologic bone substratum. In contrast to normal cells, 52.7% of WASp-null osteoclasts were completely devoid of podosomes (Figure 1B, Figure 3G-I), and 45.8% displayed only dysmorphic actin-rich plaques (Figure 1B, Figure 3J-L). These actin plaques never were observed in normal osteoclasts.
Organization of actin cytoskeleton in normal and WASp-/- osteoclasts. Bone marrow osteoclasts were derived on glass coverslips (A) or bone slices (B), fixed with 3% paraformaldehyde, and permeabilized with 0.05% Triton X-100, and the presence of actin filaments and vinculin was detected by immunofluorescence. We quantified the percentage of cells displaying each of the identified arrangements of actin filaments in 100 cells per experiment. The graphs illustrate the mean percentage of cells ± SD from 3 experiments.
Organization of actin cytoskeleton in normal and WASp-/- osteoclasts. Bone marrow osteoclasts were derived on glass coverslips (A) or bone slices (B), fixed with 3% paraformaldehyde, and permeabilized with 0.05% Triton X-100, and the presence of actin filaments and vinculin was detected by immunofluorescence. We quantified the percentage of cells displaying each of the identified arrangements of actin filaments in 100 cells per experiment. The graphs illustrate the mean percentage of cells ± SD from 3 experiments.
Cytoskeletal organization of normal and WASp-/- osteoclasts on glass coverslips. Bone marrow osteoclasts were derived on glass coverslips. At day 7 after plating, cell cultures were fixed with 3% paraformaldehyde, permeabilized with 0.05% Triton X-100, and stained with a mouse anti-human antibody against vinculin (A, D, G, J) and Alexa 568-conjugated phalloidin, followed by an incubation with an Alexa 488 goat anti-mouse antibody (B, E, H, K). Merged images are shown in panels C, F, I, and L. Normal osteoclasts assemble podosomes in clusters behind leading edges (A-C) or inserted in actin rings (D-F) with the characteristic organization of these adhesion structures: core of actin filaments surrounded by a rim of vinculin (inserts in C, F). WASp-null osteoclasts were defective in the formation of podosomes (G-I), instead assembling actin plaques colocalizing with vinculin (insert in I) or actin rings with few podosome-like structures inserted (J-L), which were depleted of vinculin (insert in L). The micrographs are representative of the cytoskeletal organization of osteoclasts detected in 3 independent experiments. Bar, 20 μm.
Cytoskeletal organization of normal and WASp-/- osteoclasts on glass coverslips. Bone marrow osteoclasts were derived on glass coverslips. At day 7 after plating, cell cultures were fixed with 3% paraformaldehyde, permeabilized with 0.05% Triton X-100, and stained with a mouse anti-human antibody against vinculin (A, D, G, J) and Alexa 568-conjugated phalloidin, followed by an incubation with an Alexa 488 goat anti-mouse antibody (B, E, H, K). Merged images are shown in panels C, F, I, and L. Normal osteoclasts assemble podosomes in clusters behind leading edges (A-C) or inserted in actin rings (D-F) with the characteristic organization of these adhesion structures: core of actin filaments surrounded by a rim of vinculin (inserts in C, F). WASp-null osteoclasts were defective in the formation of podosomes (G-I), instead assembling actin plaques colocalizing with vinculin (insert in I) or actin rings with few podosome-like structures inserted (J-L), which were depleted of vinculin (insert in L). The micrographs are representative of the cytoskeletal organization of osteoclasts detected in 3 independent experiments. Bar, 20 μm.
Cytoskeletal organization of normal and WASp-/- osteoclasts on bone slices. Bone marrow osteoclasts were derived on bone slices. At day 10 after plating, cell cultures were fixed with 3% paraformaldehyde and permeabilized with 0.05% Triton X-100 and vinculin (A, D, G, J), and actin filaments (B, E, H, K) were detected by immunofluorescence as described in “Materials and methods.” Merged images are shown in panels C, F, I, and L. In normal cells, podosomes were assembled either in noncircular clusters across the cell body (A-C) or, more commonly, in actin rings, where they were more diffuse than on glass coverslips (D-F). Vinculin colocalized with podosomes (A-C) and actin rings (D-F). WASp-null cells were devoid of podosomes (G-I) or assembled large plaques of actin (J-L). Vinculin staining was poor in WASp-/- osteoclasts, where actin filaments were almost undetectable (G-I) and distributed throughout the cytoplasm of cells containing actin plaques (J-L). The images are representative of the cytoskeletal organization of osteoclasts detected in 3 independent experiments. Bar, 20 μm.
Cytoskeletal organization of normal and WASp-/- osteoclasts on bone slices. Bone marrow osteoclasts were derived on bone slices. At day 10 after plating, cell cultures were fixed with 3% paraformaldehyde and permeabilized with 0.05% Triton X-100 and vinculin (A, D, G, J), and actin filaments (B, E, H, K) were detected by immunofluorescence as described in “Materials and methods.” Merged images are shown in panels C, F, I, and L. In normal cells, podosomes were assembled either in noncircular clusters across the cell body (A-C) or, more commonly, in actin rings, where they were more diffuse than on glass coverslips (D-F). Vinculin colocalized with podosomes (A-C) and actin rings (D-F). WASp-null cells were devoid of podosomes (G-I) or assembled large plaques of actin (J-L). Vinculin staining was poor in WASp-/- osteoclasts, where actin filaments were almost undetectable (G-I) and distributed throughout the cytoplasm of cells containing actin plaques (J-L). The images are representative of the cytoskeletal organization of osteoclasts detected in 3 independent experiments. Bar, 20 μm.
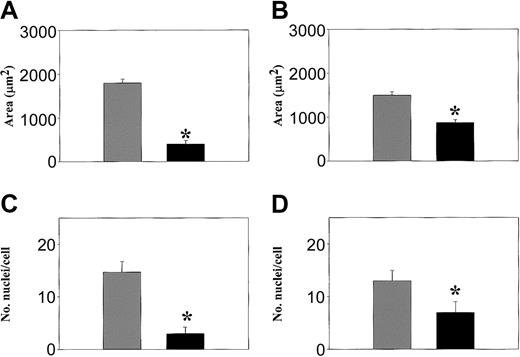
Interestingly, differences in the size of WASp-null osteoclasts cultured in vitro also were noted (Figure 4). When compared to normal cells, the area of spreading of WASp-null osteoclasts on glass and bone was 7.9-fold and 1.8-fold greater, respectively (Figure 4A-B). This increase in the area of spread was associated with a 5.7-fold and a 1.7-fold increase in the number of nuclei per cell on glass and bone, respectively (Figure 4C-D). Therefore, both the size of WASp-null osteoclasts and their individual nuclear number were significantly increased. In addition, in normal cells the area of spread and the number of nuclei within osteoclasts increased by 2.7-fold and 1.6-fold, respectively, when plated on bone compared to glass. This response to the substratum was not observed in WASp-null osteoclasts. Thus, WASp-null osteoclasts were larger than normal and did not undergo any further increase in size when plated on bone.
Increased cell size and number of nuclei per cell in WASp-/- osteoclasts. The area of spreading (A, B) and the number of nuclei per cell (C, D) was quantified on normal (▪) and WASp-null (▦) osteoclasts derived on glass coverslips (A, C) or bone slices (B, D). Cells were fixed and stained with Alexa 468-conjugated phalloidin and propidium iodide. Graphs express the mean ± SEM values from 3 experiments. *P < .05 (Student t test).
Increased cell size and number of nuclei per cell in WASp-/- osteoclasts. The area of spreading (A, B) and the number of nuclei per cell (C, D) was quantified on normal (▪) and WASp-null (▦) osteoclasts derived on glass coverslips (A, C) or bone slices (B, D). Cells were fixed and stained with Alexa 468-conjugated phalloidin and propidium iodide. Graphs express the mean ± SEM values from 3 experiments. *P < .05 (Student t test).
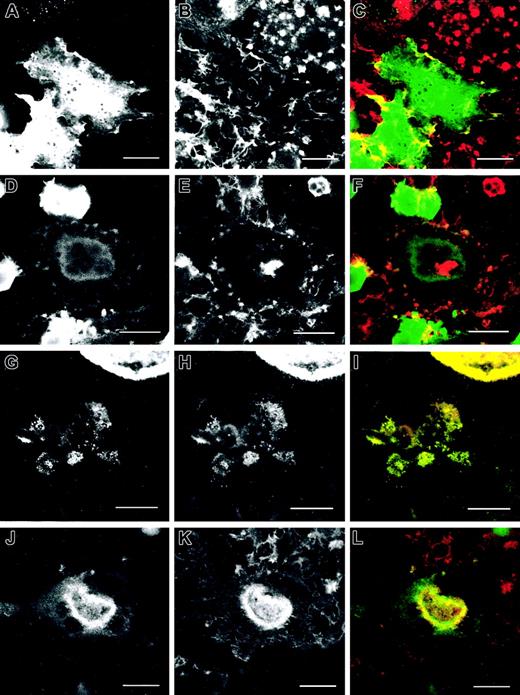
Using interference reflection videomicroscopy (IRM), we observed that the formation and disassembly of the adhesion sites in normal and WASp-null osteoclasts were different. Adhesion points in normal osteoclasts were mediated by podosomes in noncircular clusters (Figure 5A-C) or inserted in actin rings (Figure 5D-F). In WASp-null osteoclasts, attachment of cells to the substratum was mediated by actin patches (Figure 5G-I) or dysmorphic actin rings (Figure 5J-L). As observed by IRM video microscopy of living cells, normal osteoclasts formed highly dynamic well-organized actin rings that assemble and disassembled continuously (to watch Supplemental Video 1, available on the Blood website, see the Supplemental Videos link at the top of the online article). In contrast, WASp-null osteoclasts assembled more static poorly organized actin rings (Supplemental Video 2). At the periphery of these rings very dynamic adhesion points underwent rapid cycles of assembly and disassembly, but they consistently failed to generate a normal podosome morphology.
Organization of actin filaments in normal and WASp-/- osteoclasts at adhesion points on the substratum. Confocal fluorescent and IRM images were taken to detect vinculin (A, D, G, J) and F-actin distribution (B, E, H, K), showing that the adhesion points of the cells with the substratum detected by IRM (C, F, I, L) corresponded to the actin structures we detected by immunofluorescence. Adhesion points in normal osteoclasts were mediated by podosomes in noncircular clusters (A-C) or inserted in actin rings (D-F). In WASp-null osteoclasts, attachment of cells to the substratum was mediated by actin patches (G-I) or dysmorphic actin rings (J-L). Bar, 20 μm.
Organization of actin filaments in normal and WASp-/- osteoclasts at adhesion points on the substratum. Confocal fluorescent and IRM images were taken to detect vinculin (A, D, G, J) and F-actin distribution (B, E, H, K), showing that the adhesion points of the cells with the substratum detected by IRM (C, F, I, L) corresponded to the actin structures we detected by immunofluorescence. Adhesion points in normal osteoclasts were mediated by podosomes in noncircular clusters (A-C) or inserted in actin rings (D-F). In WASp-null osteoclasts, attachment of cells to the substratum was mediated by actin patches (G-I) or dysmorphic actin rings (J-L). Bar, 20 μm.
Reconstitution of podosomes in WASp-null osteoclasts by complementation with eGFP-WASp
Transduction of WASp-null osteoclasts with an eGFP-WASp fusion retroviral vector induced the expression of WASp to levels similar to control cells (Figure 6A-B). Expression of eGFP-WASp resulted in the reconstitution of morphologically normal podosomes. WASp-null osteoclasts transduced with eGFP alone displayed an actin cytoskeletal arrangement similar to nontransduced mutant cells. As expected, eGFP was diffusely distributed in the cytoplasm and did not colocalize specifically with any cellular structures. When plated on glass, 74.8% of eGFP-transduced cells (Figure 6C) were completely devoid of podosomes (Figure 7A-C). In 39.7% of cells, actin rings of poor quality were formed, with few discrete podosomes inserted in the ring (Figure 7D-F). In contrast, 45% of eGFP-WASp-transduced mutant osteoclasts (Figure 6C) assembled clusters of normal podosomes (Figure 7G-I). In addition, 8.8% of the cells displayed actin rings rich in podosomes with some discrete peripheral podosome structures (Figure 7J-L). eGFP-WASp clearly colocalized with the podosome actin core. Similarly, transduction of WASp-null osteoclasts plated on bone slices also resulted in the reconstitution of actin structures, similar to those of wild-type cultures (Figure 6D). Most cells transduced with eGFP alone were devoid of podosomes, and 37.7% assembled abnormal actin plaques (Figure 8A-F). In comparison, 50% of eGFP-WASp-transduced cells assembled morphologically normal actin rings characteristic of the resorptive phase (Figure 8G-I), or in 14.4% of cells, podosome clusters characteristic of the migratory phase (Figure 8J-L). eGFP-WASp again colocalized with the actin rings and the core of individual podosomes. Formation of actin plaques was abrogated in eGFP-WASp-transduced cells. Successful functional reconstitution of WASp-null cells also resulted in a decrease in cell size and nuclear number toward the normal phenotype (Figure 9). The area of spread and the number of nuclei per cell in WASp-null cells transduced with eGFP alone were similar to nontransduced WASp-null cells. However, expression of eGFP WASp in WASp-null osteoclasts induced a 4.5-fold and 1.7-fold decrease in the area of spreading in cells plated on glass and bone, respectively (Figure 9A-B). The number of nuclei in eGFP WASp-expressing osteoclasts also decreased by 4.9-fold and 1.5-fold when cells were plated on glass and bone, respectively (Figure 9C-D).
Expression of eGFP WASp in WASp-null cells results in the reconstitution of the normal organization of the actin cytoskeleton. Bone marrow-derived osteoclasts from normal (SV129), WASp-null (WASp-/-), or WASp-null transduced with eGFP (WASp-/-eGFP) or eGFP-WASp (WASp -/-eGFP-WASp) were lysed in RIPA buffer at day 7 after plating and subjected to a 10% SDS-PAGE gel electrophoresis, probed with anti-WASp (A) or anti-eGFP antibodies (B). Signal was detected with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (ECL) detection system. Visualization of eGFP required a 10-second film exposure, whereas visualization of eGFP-WASp required 90 seconds of exposure. Blots were stripped with 2% SDS and 0.7% β-mercaptoethanol for 1 hour at 50°C and reprobed for β-actin to check the total amount of protein loaded per lane. WASp-null bone marrow osteoclasts derived on glass coverslips (C) or bone slices (D) and transduced with eGFP (▦) or eGFP WASp (▪) were fixed with 3% paraformaldehyde, permeabilized with 0.05% Triton X-100, and the presence of actin filaments and vinculin was detected by immunofluorescence. We quantified the percentage of cells displaying each of the identified arrangements of actin filaments in 100 cells per experiment. The graphs illustrate the mean percentage of cells ± SD from 3 experiments.
Expression of eGFP WASp in WASp-null cells results in the reconstitution of the normal organization of the actin cytoskeleton. Bone marrow-derived osteoclasts from normal (SV129), WASp-null (WASp-/-), or WASp-null transduced with eGFP (WASp-/-eGFP) or eGFP-WASp (WASp -/-eGFP-WASp) were lysed in RIPA buffer at day 7 after plating and subjected to a 10% SDS-PAGE gel electrophoresis, probed with anti-WASp (A) or anti-eGFP antibodies (B). Signal was detected with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (ECL) detection system. Visualization of eGFP required a 10-second film exposure, whereas visualization of eGFP-WASp required 90 seconds of exposure. Blots were stripped with 2% SDS and 0.7% β-mercaptoethanol for 1 hour at 50°C and reprobed for β-actin to check the total amount of protein loaded per lane. WASp-null bone marrow osteoclasts derived on glass coverslips (C) or bone slices (D) and transduced with eGFP (▦) or eGFP WASp (▪) were fixed with 3% paraformaldehyde, permeabilized with 0.05% Triton X-100, and the presence of actin filaments and vinculin was detected by immunofluorescence. We quantified the percentage of cells displaying each of the identified arrangements of actin filaments in 100 cells per experiment. The graphs illustrate the mean percentage of cells ± SD from 3 experiments.
Reconstitution of podosomes in WASp-null osteoclasts plated on glass coverslips by expression of eGFP WASp. WASp-null cells were transduced with eGFP (A-F) or eGFP-WASp (G-L). Distribution of eGFP is shown in panels A and D, and distribution of eGFP-WASp in panels G and J. Actin organization was detected by immunostaining with Alexa 568-conjugated phalloidin (B, E, H, K). Merged images are shown in panels C, F, I, and L. Cells transduced with eGFP were devoid of podosomes (A-C) or assembled abnormal actin rings with few podosome-like structures inserted (D-F). eGFP did not colocalize specifically with any cytoskeletal structure (C, F). Transduction of eGFP WASp resulted in reconstitution of podosomes and formation of clusters similar to normal cells in both migrating cells (G-I) and cells with actin rings (J-L). eGFP WASp colocalized specifically with the actin core of podosomes (insert in I, L). The micrographs are representative of the cytoskeletal organization of osteoclasts detected in 3 independent experiments. Bar, 20 μm.
Reconstitution of podosomes in WASp-null osteoclasts plated on glass coverslips by expression of eGFP WASp. WASp-null cells were transduced with eGFP (A-F) or eGFP-WASp (G-L). Distribution of eGFP is shown in panels A and D, and distribution of eGFP-WASp in panels G and J. Actin organization was detected by immunostaining with Alexa 568-conjugated phalloidin (B, E, H, K). Merged images are shown in panels C, F, I, and L. Cells transduced with eGFP were devoid of podosomes (A-C) or assembled abnormal actin rings with few podosome-like structures inserted (D-F). eGFP did not colocalize specifically with any cytoskeletal structure (C, F). Transduction of eGFP WASp resulted in reconstitution of podosomes and formation of clusters similar to normal cells in both migrating cells (G-I) and cells with actin rings (J-L). eGFP WASp colocalized specifically with the actin core of podosomes (insert in I, L). The micrographs are representative of the cytoskeletal organization of osteoclasts detected in 3 independent experiments. Bar, 20 μm.
Reconstitution of podosomes in WASp-/- osteoclasts plated on bone slices by expression of eGFP WASp. WASp-null cells were transduced with eGFP (A-F) or eGFP-WASp (G-L). Actin organization was detected by immunostaining with Alexa 568-conjugated phalloidin (B, E, H, K). Similarly to nontransduced WASp-null cells, cells transduced with eGFP were devoid of podosomes (A-C) or assembled actin-rich plaques (D-F). eGFP did not colocalize specifically with any cytoskeletal structure (C, F). Expression of eGFP WASp resulted in the formation of actin-based structures similar to wild-type normal cells with podosomes in migratory cells (G-I) and actin rings (J-L). eGFP-WASp colocalized with podosomes (G-I) and actin rings (J-L). The micrographs are representative of the cytoskeletal organization of osteoclasts detected in 3 independent experiments. Bar, 20 μm.
Reconstitution of podosomes in WASp-/- osteoclasts plated on bone slices by expression of eGFP WASp. WASp-null cells were transduced with eGFP (A-F) or eGFP-WASp (G-L). Actin organization was detected by immunostaining with Alexa 568-conjugated phalloidin (B, E, H, K). Similarly to nontransduced WASp-null cells, cells transduced with eGFP were devoid of podosomes (A-C) or assembled actin-rich plaques (D-F). eGFP did not colocalize specifically with any cytoskeletal structure (C, F). Expression of eGFP WASp resulted in the formation of actin-based structures similar to wild-type normal cells with podosomes in migratory cells (G-I) and actin rings (J-L). eGFP-WASp colocalized with podosomes (G-I) and actin rings (J-L). The micrographs are representative of the cytoskeletal organization of osteoclasts detected in 3 independent experiments. Bar, 20 μm.
Reconstitution of normal cell size and number of nuclei of WASp-/- transduced with eGFP-WASp. The area of spreading (A, B) and the number of nuclei per cell (C, D) were quantified in WASp-null osteoclasts expressing eGFP (WASp-/-eGFP, ▦) or eGFP-WASp (WASp-/-eGFP-WASp, ▪) derived on glass coverslips (A, C) or bone slices (B, D). Cells were fixed and stained with Alexa 468-conjugated phalloidin and propidium iodide. Graphs express the mean ± SEM values from 3 experiments. *P < .05 (Student t test).
Reconstitution of normal cell size and number of nuclei of WASp-/- transduced with eGFP-WASp. The area of spreading (A, B) and the number of nuclei per cell (C, D) were quantified in WASp-null osteoclasts expressing eGFP (WASp-/-eGFP, ▦) or eGFP-WASp (WASp-/-eGFP-WASp, ▪) derived on glass coverslips (A, C) or bone slices (B, D). Cells were fixed and stained with Alexa 468-conjugated phalloidin and propidium iodide. Graphs express the mean ± SEM values from 3 experiments. *P < .05 (Student t test).
WASp-null osteoclasts are defective in bone resorption
To determine whether these cytoskeletal disturbances produced abnormalities of cellular function, differences in bone resorption between normal and WASp-null mice were measured, firstly in vitro. Although the number of cells in WASp-null cultures was lower, both normal and WASp-null osteoclasts differentiated on bone routinely covered the same percentage of the total surface (data not shown). This was due to the significantly larger size of WASp-null cells (Figure 4B-D). However, the area of bone surface resorbed by normal osteoclasts was 1.8-fold higher (P < .05) compared to WASp-null cells (Figure 10A). The quality and morphology of pits formed by WASp-null cells also were clearly different (Figure 10B-C). Although both types of osteoclasts were able to form complex pits consisting of multiple individual excavations, the number of individual components per complex pit was higher in WASp-null cultures. However, the total volumes and the maximal depth of the complex pits formed by normal and WASp-null osteoclasts were not notably altered (Figure 10D-E). Expression of eGFP-WASp (but not eGFP alone) induced a 1.4-fold increase in the area of bone surface resorbed by WASp-null cells, reaching values similar to control cells (Figure 10A). As expected, the mean values of the total volume and the maximal depth of the complex pits in WASp-null cells was not altered by infections with either eGFP alone or eGFP-WASp (Figure 10D-E). However, the morphology of the pits excavated by osteoclasts expressing eGFP-WASp (but not eGFP alone) was similar to that of the normal cells (Figure 10F-G).
Bone resorption in vitro of normal, WASp-null osteoclasts, and WASp-null osteoclasts transduced with eGFP or eGFP-WASp. Bone resorption of normal (SV129), WASp-null (WASp-/-) cells, WASp-null cells expressing eGFP (WASp-/-eGFP), or WASp-null cells expressing eGFP-WASp (WASp-/-eGFP WASp) was assessed at day 10 after plating osteoclast precursors on bone slices. Bone slices were removed from wells, immersed in 10% NaOCl for 10 minutes to remove cells, washed in distilled water, dried, and sputter-coated with gold. The extent of bone resorption was quantified according to the following parameters: percent of bone surface resorbed (A) and depth and volume of the excavated regions (D and E, respectively). Both normal (C) and WASp-null osteoclasts (B) formed complex pits consisting of multiple component excavations, however, the number of individual components in complex pits was greater in WASp-null cultures. Expression of eGFP-WASp in WASp-/- cells (G) but not expression of eGFP alone (F) reconstituted the normal morphology of the pits. The graphs illustrate the mean percentage of osteoclasts ± SD from 3 experiments. *P < .05 (Student t test). Bar, 50 μm.
Bone resorption in vitro of normal, WASp-null osteoclasts, and WASp-null osteoclasts transduced with eGFP or eGFP-WASp. Bone resorption of normal (SV129), WASp-null (WASp-/-) cells, WASp-null cells expressing eGFP (WASp-/-eGFP), or WASp-null cells expressing eGFP-WASp (WASp-/-eGFP WASp) was assessed at day 10 after plating osteoclast precursors on bone slices. Bone slices were removed from wells, immersed in 10% NaOCl for 10 minutes to remove cells, washed in distilled water, dried, and sputter-coated with gold. The extent of bone resorption was quantified according to the following parameters: percent of bone surface resorbed (A) and depth and volume of the excavated regions (D and E, respectively). Both normal (C) and WASp-null osteoclasts (B) formed complex pits consisting of multiple component excavations, however, the number of individual components in complex pits was greater in WASp-null cultures. Expression of eGFP-WASp in WASp-/- cells (G) but not expression of eGFP alone (F) reconstituted the normal morphology of the pits. The graphs illustrate the mean percentage of osteoclasts ± SD from 3 experiments. *P < .05 (Student t test). Bar, 50 μm.
Since the abnormalities of bone resorption in vitro were modest, the capacity for bone resorption in vivo was tested following ovariectomy of female mice. This procedure induces a rapid and extensive bone loss of approximately half of the metaphyseal bone within 2 weeks that challenges the capacity of osteoclasts to dissolve the bone matrix. Bone resorption capacity was measured by determining the changes of trabecular bone volume in the metaphyses. Under normal conditions, bone volume did not differ significantly between WASp-null (10.6 ± 7 mm3) and wild-type mice (9.8 ± 1.4 mm3) (Figure 115A). However, following ovariectomy, there were significant differences (Figure 11A). As expected, this procedure induced an extensive bone loss of 38.6% in wild-type control animals compared to a nonsignificant 16.4% of bone loss in ovariectomized mutant mice. Therefore, although WASp-null osteoclasts were capable of physiologic levels of resorption, there was a significant impairment in the function of WASp-null osteoclasts when subjected to a resorptive challenge.
WASp-null osteoclasts are defective in bone resorption in vivo. Weight-matched 22-week-old WASp-null and SV129 wild-type mice were ovariectomized (ovx) or sham ovariectomized (sham). Animals were culled 15 days after ovariectomy, then femurs were removed from each animal, freed of soft tissue, and fixed in 10% formalin in PBS and embedded in paraffin wax. Sections were stained with either toluidine blue for assessment of bone volume (A), eroded surface (B), or TRAP to quantify the percentage of bone area occupied by osteoclast surface (C) and osteoclast number per millimeter (D). The graphs represent the mean ± SEM values. *P < .05 (Student t test) compared to sham-ox.
WASp-null osteoclasts are defective in bone resorption in vivo. Weight-matched 22-week-old WASp-null and SV129 wild-type mice were ovariectomized (ovx) or sham ovariectomized (sham). Animals were culled 15 days after ovariectomy, then femurs were removed from each animal, freed of soft tissue, and fixed in 10% formalin in PBS and embedded in paraffin wax. Sections were stained with either toluidine blue for assessment of bone volume (A), eroded surface (B), or TRAP to quantify the percentage of bone area occupied by osteoclast surface (C) and osteoclast number per millimeter (D). The graphs represent the mean ± SEM values. *P < .05 (Student t test) compared to sham-ox.
We also noted differences in the quality of bone resorption between the 2 cell types. Despite the low levels of bone volume loss in ovariectomized WASp-null mice, we unexpectedly observed a significant 2.75-fold increase in the total eroded surface, similar to that observed in wild-type animals (Figure 11B). Screening TRAP-stained sections to measure osteoclast parameters also revealed that both the percentage of bone surface covered by osteoclasts, and the total number of osteoclasts, were raised by 1.5-fold and 1.7-fold, respectively, as a result of ovariectomy in WASp-null mice. The same indices were not raised in wild-type mice (Figure 11C-D), suggesting that bone loss had been largely completed at the time of analysis. These findings are consistent with the recorded indices of osteoblast activity, which remained high in WASp-null mice, associated with the active remodeling that accompanies bone resorption after ovariectomy (data not shown). Therefore, despite an increase in osteoclast numbers and an increase in eroded surface, bone resorption by WASp-null cells was highly inefficient.
Discussion
WASp is a key regulator of the actin cytoskeleton in hematopoietic cells. In WAS patients, leukocytes display cytoskeletal abnormalities that probably contributes significantly to their abnormal migratory properties.,6,25,26 Although in osteoclasts the reorganization of the actin cytoskeleton is essential for migration and bone remodeling,8,10 no consistent or obvious bone abnormalities have been reported in WAS patients or in WASp-null mice. Three WAS patients have been reported with radiological and clinical features of infantile cortical hyperostosis (Caffey disease).27 This rare and usually self-limited disease is characterized by increased width of bone cortex, resulting in bone expansion and bowing deformities. The frequency with which it appears in WAS is much higher than would be expected, but the etiological link has not been identified.
While normal osteoclasts displayed many podosomes,16 WASp-null osteoclasts cultured on glass failed to assemble podosomes behind their leading edges. As expected, in the presence of bone extracellular matrix, cultures of normal osteoclasts shifted from a predominant migratory phenotype (podosomes in noncircular clusters) on glass to a resorptive phenotype (formation of highly condensed actin rings). In cultures of WASp-null osteoclasts, the formation of actin rings was markedly impaired, and they were replaced by large actin-rich plaques not observed in normal cells. Therefore, in the absence of WASp, osteoclasts retain the capacity to polymerize monomeric actin but fail to arrange these filaments in normal adhesion/resorption complexes. The dynamics of the formation and dissolution of adhesion structures also were disturbed as observed using IRM imaging (see the Supplemental Videos). Although WASp-null osteoclasts were able to rapidly assemble and disassemble adhesion points with the substratum indicating rapid polymerization and depolymerization of actin, these adhesion contacts were not organized in highly dynamic actin rings as in normal cells.
All WASp-family proteins work similarly to promote activation of the Arp2/3 complex.3 The major differences between the members of the WASp-family of proteins are in their binding regions for other interacting adaptor and regulator molecules.3 It is therefore likely that in WASp-null cells, other proteins from the same family such as N-WASp, which is expressed in osteoclasts (data not shown), may partially compensate and enable at least some dysregulated polymerization of actin filaments, as it is known that the WASp-family proteins are unable to compensate for each other completely.28 The restricted occurrence of podosomes in normal hematopoietic cells clearly indicates that WASp itself or specific WASp-interacting factors are absolutely necessary for the successful formation of these structures.6
In terms of bone resorption, the failure to form a normal cytoarchitecture may be compensated under steady-state conditions by the formation of alternative actin-based resorptive structures. Under normal conditions, individual osteoclasts undergo sequential phases of migration and bone matrix resorption.16 Defective adhesion and/or migration of WASp-null osteoclasts may in part be overcome by the very significant increase in the size of the osteoclasts compared to normal cells, thereby allowing a much larger surface area of bone to be covered and potentially exposed to resorptive enzymes. The reasons for the increase in cell size are not clear but may reflect alterations in the normal cell differentiation mechanism of osteoclasts, which may themselves be influenced by signaling through WASp-dependent pathways. For example, formation of osteoclasts results from the fusion of mononuclear hematopoietic precursors in a process mediated by E-cadherin.29 According to recent studies, E-cadherin and the Rho GTPase Cdc42 (of which WASp is a major defined effector) regulate each other's activity, resulting in promotion of cell migration or stability of cell-cell interaction.30 Perturbation of cadherin function in cell-cell junctions induces increased activity of Cdc42 and promotes migration in fibroblasts.31 Activation of Cdc42 also is required for the stabilization of cadherin-based cell contacts in EL cells.32 Therefore, contextual regulation of Cdc42 and activity of effectors such as WASp may determine either cell-cell contact for fusion or formation of adhesion structures for migration. In the absence of WASp, a balance between migration and fusion may be lost and fusion favored, leading to the formation of abnormally large cells. Interestingly, integrin subunit β3-deficient osteoclasts also form highly spread poorly resorbing polykaryotic cells when exposed to high-dose M-CSF.33
Podosome assembly and condensation into typical actin rings is required to tightly seal the compartment between the cell membrane and the bone surface and localize acidic enzymes within the area of resorption.16 WASp-null cells may compensate for their inability to assemble properly sealed rings by forming or maximizing alternative adhesive structures to seal a large portion of the membrane onto the bone surface. It was noticeable that WASp-null cells on bone slices formed large actin plaques, and vinculin distribution was not restricted to these plaques but was diffused across the cell body. Vinculin is normally restricted to the actin rings of the sealing zone and often associated with integrin-linked adhesion structures such as focal complexes, focal adhesions, and podosomes themselves.16,34 The diffuse vinculin staining observed in WASp-null cells may therefore reflect the formation of compensatory adhesive structures, likely associated with the vitronectin receptor, which normally colocalizes with vinculin around the core of osteoclast podosomes19 and interactions with which are required for bone resorption.35
Compensation in bone resorption under steady-state conditions has been observed in mice deficient in the expression of structural components of osteoclasts podosomes such as gelsolin.36 However, we have clearly shown that compensatory mechanisms in WASp-null mice are not sufficient to mediate successful bone matrix degradation in response to the induction of a rapid and extensive bone resorption such as that following ovariectomy. Although ovariectomized WASp-null mice exhibited a highly significant increase in eroded bone surface, they lacked a significant loss of volume. These observations suggest a relatively shallow and inefficient resorption process and are consistent with the tendency of WASp-null osteoclasts to form smaller excavations in vitro than normal cells.
In summary, we have shown that WASp plays a key and nonredundant role in the formation and function of podosomes in osteoclasts. Absence of WASp results in a decrease in the efficiency of bone resorption and is clearly associated with changes in the dynamics of formation/differentiation and function of osteoclasts in vivo. The formation of a spatially and dynamically regulated sealing zone and of optimal bone resorptive capacity is ultimately dependent on the organization of specific actin structures by WASp.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-04-1259.
Supported by grants from the Wellcome Trust (G.E.J., A.T.) and the Arthritis Research Campaign (G.E.J., A.T., Y.C.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal