Abstract
We have developed a mouse system by which to track the migration and homing of cells in a setting of bone marrow transplantation (BMT)-induced graft-versus-host disease (GVHD) after systemic infusion using enhanced green fluorescence protein (eGFP) transgenic (Tg) cells and a simple application of a fluorescence stereomicroscope outfitted with a color charge-coupled device (CCD) camera. Whole body images of anesthetized mice taken at various time points after cell infusion revealed the early migration of allogeneic cells to peripheral lymphoid organs, with later infiltration of GVHD target organs. Localization of eGFP Tg cells could be seen through the skin of shaved mice, and internal organs were easily discernible. After allogeneic or syngeneic eGFP Tg cell infusion, representative mice were dissected to better visualize deeper internal organs and tissues. Infusion of different cell populations revealed distinct homing patterns, and this method also provided a simple way to identify the critical time points for expansion of the transplanted cells in various organs. This simple application of the fluorescence stereomicroscope will be valuable for GVHD and graft-versus-tumor studies in which visualization of cellular migration, expansion, and cell-cell interactions will be more informative when analyzed by such an intravital method. (Blood. 2004;103:3590-3598)
Introduction
Hematopoietic or bone marrow stem cell transplantation is used for a variety of conditions, including hematologic disorders, metabolic storage diseases, immune deficiencies, and rescue after treatment for cancer. Although great advances have been made, the problem of graft-versus-host disease (GVHD) remains a major complication after transplantation. In animal models of bone marrow transplantation (BMT), analyses of tissue-specific events usually require the dissociation of cells from the affected organs or sectioning of the harvested tissues, resulting in the loss of spatial and geographic information and limiting the areas being analyzed.
Recently, nonfluorescence techniques, including bioluminescence1 and positron-emission tomography (PET),2 have been developed to track the migration of cells in vivo. The major advantage of these techniques is that the same experimental subjects (usually mice) can be examined sequentially over time, thus providing real-time imaging without the requirement for killing.3 Many features of these systems, however, make them limiting, irrespective of the cost of the hardware that prohibits their purchase by most laboratories and the required use of radiolabeled substrates as in PET. The predominant drawbacks to these imaging systems are their low resolution and low sensitivity. Poor resolution, when using bioluminescence or PET, stems from the system's inability to accurately identify the tissue that is emitting the signal. Identification is inferred from the location of the signal (that generally takes on a roundish shape). Sensitivity is such that at least 1000 cells have to be present in a single location for a signal to be detected by bioluminescence.4 Sensitivity is even lower in PET.5 Concerns have arisen recently about the extent to which the substrate (luciferin, in the case of luciferase) that must be injected before imaging efficiently reaches all tissues consistently, and the stability of the signal has been questioned.6 These issues are important because given that one does not directly see the cells emitting the bioluminescent signal, the quantitative information obtained is dependent on the integrity of the signal. Nevertheless, these imaging techniques have provided invaluable information about the migration patterns of injected cells but will be difficult to apply to situations in which imaging the colocalization and interactions of different cell populations (eg, tumor compared with immune cells) is desired.
We have developed a mouse system by which to track the migration and homing of cells after systemic infusion using enhanced green fluorescence protein (eGFP) transgenic (Tg) cells and a simple application of a Leica MZFLIII (Leica Microsystems, Bannockburn, IL) fluorescence stereomicroscope outfitted with a Magnafire color charge-coupled device (CCD) camera (Optronics, Goleta, CA). We adopted the basis for this application from the reports of the Hoffman group in their investigations of tumor metastasis (for examples, see Hoffman7 and Yang et al8 ). In our BMT model, whole body images of anesthetized mice were taken at various time points after cell infusion. Localization of eGFP Tg cells could be seen through the skin of shaved mice, and internal organs were easily discernible. After allogeneic or syngeneic eGFP Tg cell infusion, representative mice were dissected to better visualize deeper internal organs and tissues. Sensitive to a single-cell resolution level, this real-time spatial-temporal imaging of BMT and the GVHD process provided surprising findings with regard to the extent of donor cell infiltration that had not been appreciated before now.
Materials and methods
Mice
C57BL/6 (H2b) mice were purchased from the National Institutes of Health (Bethesda, MD). B10.BR (H2k) mice were purchased from Jackson Laboratories (Bar Harbor, ME). eGFP Tg mice, on C57BL/6 background, were obtained from the laboratory of Dr Jon Serody (Chapel Hill, NC)9 and subsequently were bred at the University of Minnesota. Mice were housed in microisolator cages in the SPF facility of the University of Minnesota and were cared in accordance with the institution's Research Animal Resources guidelines. For BMT, donors were 8 to 12 weeks of age, and recipients were 8 to 10 weeks of age.
Bone marrow transplantation
B10.BR (for allogeneic studies) or C57BL/6 (for syngeneic studies) mice were lethally irradiated on the day before BMT (7.5 Gy total body irradiation [TBI]) using x-rays at a dose rate of 0.41 Gy/min. Donor C57BL/6 BM (eGFP Tg or non-Tg, as indicated) was T cell depleted (TCD) with anti-Thy 1.2 monoclonal antibody (mAb) (clone 30-H-12, rat IgG2b) plus complement (Nieffenegger, Woodland, CA). Recipient mice underwent transplantation through the caudal vein with 8 × 106 TCD C57BL/6 (H-2b) marrow alone or with 15 × 106 whole spleen cells (BMS), 10 × 106 TCD splenocytes, or 5 × 106 purified T cells from eGFP Tg C57BL/6 mice as a source of GVHD-causing T cells. The cellular composition of the eGFP Tg and non-Tg whole spleen cell inocula did not differ as assessed by flow cytometry analysis (18%-25% CD4+, 14%-17% CD8+, 36%-50% CD19+, 6%-7% CD11b+). TCD splenocytes contained 2% CD4+, 1% CD8+, 80% to 88% CD19+, and 7% to 8% CD11b+ cells. eGFP Tg inocula enriched for T cells were obtained after passage over a goat-antimouse immunoglobulin column (Biotex, Edmonton, AB, Canada) and were 34% CD4+, 37% CD8+, 6% CD19+, and 1% CD11b+. In other experiments, mice received TCD eGFP Tg C57BL/6 BM (containing 1% CD4+, 1% NK1.1+, 23% CD19+, 67% CD11b+ cells) with or without 15 × 106 non-Tg eGFP whole spleen cells that had been depleted of red blood cells.
In vivo imaging
Mice were anesthetized with sodium pentobarbital (Nembutol), and hair was removed with Magic Powder (Carson Products, Savannah, GA) and rinsing with water. Whole body images (0.4- to 1.9-second exposures) were taken over sequential days with a Magnafire color camera (Optronics, Goleta, CA) mounted onto a Leica MZFLIII stereomicroscope using a eGFP2-bandpass filter and a 0.63 × transfer lens (Leica Microsystems, Bannockburn, IL). Zoom factors from 2 × to 10 × were used as indicated in the figure legends. For better resolution of internal organs, a midline incision was made on some of the mice and the rib cage was opened. Imaging was performed on the first day (day 0) at 1 hour and 6 hours after cell infusion and at 24 hours, 48 hours, 72 hours, 5 days, and 7 days after BMT. Mice receiving allogeneic BM only (non-eGFP) or allogeneic BM + spleen (all non-eGFP) served as controls for background autofluorescence in the green channel.
Immunofluorescence costaining
Tissues were embedded in optimal cutting temperature (OCT; Miles, Elkhart, IN) compound, frozen in liquid nitrogen, and stored at -80°C. Cryosections (6 μm) were fixed in acetone and immunofluorescently costained for eGFP and a phenotypic marker. Polyclonal goat-anti-eGFP (Rockland Labs, Gilbertsville, PA) was visualized with Cy3-labeled donkey-antigoat immunoglobulin G (IgG; Jackson Immunoresearch, West Grove, PA). Biotinylated mAbs used for the phenotypic markers were as follows: anti-CD4 (clone GK1.5), anti-CD8 (clone 2.43), anti-CD11b (Mac-1, clone M1/70), anti-CD19 (clone 1D3), anti-Ly6G (Gr-1, clone RB6-8C5), I-Ab (clone KH74), and I-Ak (clone 11-5.2.1). All were purchased from BD PharMingen (San Diego, CA) and were visualized with Cy5-labeled streptavidin (Jackson Immunoresearch). Images were obtained using an Olympus FV500 confocal laser scanning fluorescent microscope with Fluoview software (Olympus America, Melville, NY).
Results
Initial localization to peripheral lymph nodes is not dependent on irradiation-induced injury
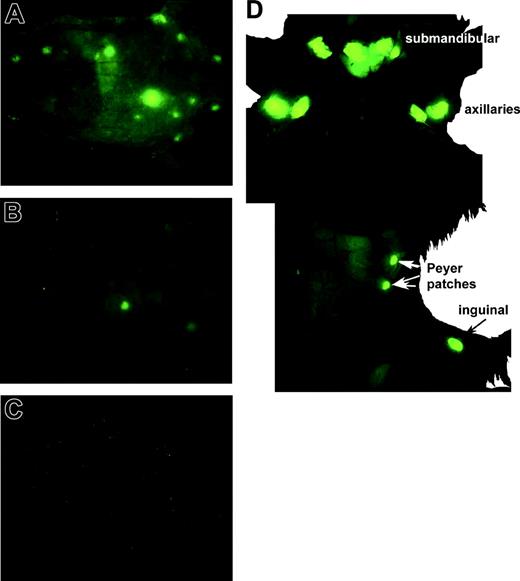
It has generally been presumed that conditioning before BMT creates “space” for the infused cells. Furthermore, there are also presumptions that the damage induced by the conditioning regimens causes the release of acute-phase reactants and inflammatory mediators such as chemokines that direct the homing of the infused cells. Therefore, we wanted first to determine whether pre-BMT conditioning affected initial homing events. Splenocytes from eGFP Tg C57BL/6 mice were injected intravenously into B10.BR mice (for allogeneic studies) or C57BL/6 mice (for syngeneic studies) that were nonirradiated or were lethally irradiated the day before cell transfer. Mice were imaged at 1 hour and 6 hours after infusion. eGFP+ cells immediately localized to lymph nodes and spleen regardless of whether the mice had been irradiated (Figure 1; 6-hour time point shown; irradiated: A, C, E, G; nonirradiated: B, D, F, H). Trafficking to and extravasation into these sites occurred similarly in allogeneic and syngeneic recipient mice (not shown). Green fluorescence was easily distinguished from the yellow autofluorescence of the intestinal contents. eGFP-expressing cells were visualized at a single-cell resolution level using the fluorescence stereomicroscope and were observed to travel through vessels (Figure 1E, inset, white arrows), tether, and extravasate through small venules. Therefore, the early migration of cells to the nodes is dependent neither on the production of conditioning-induced inflammatory mediators or chemokines nor on the recognition of host alloantigens.
Irradiation is not required for early localization of allogeneic donor splenocytes to peripheral lymph nodes. Splenocytes from eGFP Tg C57BL/6 mice were injected intravenously into B10.BR mice that were lethally irradiated (8 Gy; A, C, E, G) or were not irradiated (B, D, F, H) the day before cell infusion. Images captured 6 hours after infusion are shown for the indicated tissues. Tissues from 1 of 3 representative mice per group are shown. Examples of single-cell resolution are indicated by the white arrows (E, inset). Stereomicroscope was set to 10.0 × zoom factor with a 0.63 × transfer lens.
Irradiation is not required for early localization of allogeneic donor splenocytes to peripheral lymph nodes. Splenocytes from eGFP Tg C57BL/6 mice were injected intravenously into B10.BR mice that were lethally irradiated (8 Gy; A, C, E, G) or were not irradiated (B, D, F, H) the day before cell infusion. Images captured 6 hours after infusion are shown for the indicated tissues. Tissues from 1 of 3 representative mice per group are shown. Examples of single-cell resolution are indicated by the white arrows (E, inset). Stereomicroscope was set to 10.0 × zoom factor with a 0.63 × transfer lens.
Homing to lymph nodes is not dependent on allogenicity, but migration to and expansion of donor spleen cells in GVHD target organs occurs only in allogeneic recipients
Although GVHD does not occur very early (ie, first 48 hours) after infusion of GVHD-causing cells in our murine system, we wanted to determine the migration pattern and kinetics after BMT. We also wanted to determine whether host alloantigen recognition influences the homing of cells to various organs, especially those involved in GVHD. B10.BR mice (for allogeneic studies) or C57BL/6 mice (for syngeneic studies) were lethally irradiated and given non-eGFP C57BL/6 BM with or without eGFP Tg C57BL/6 splenocytes. Mice were imaged during the first day at 1 hour and 6 hours after BMT and at 24 hours, 48 hours, 72 hours, 5 days, and 7 days after BMT. Figure 2 shows that by 24 hours after BMT, the eGFP Tg donor splenocytes migrated to lymph nodes in the allogeneic and syngeneic setting. At this 24-hour point, a difference between the allogeneic and syngeneic recipients was only seen for the spleen, where more eGFP+ cells were seen in mice receiving allogeneic splenocytes than in those receiving syngeneic cells. We could not discern any difference in the accumulation of small aggregates of eGFP+ cells in the lungs of allogeneic and syngeneic recipients. eGFP+ cells were not (or were only rarely) seen in the skin and liver at 24 hours after BMT. Figure 3 shows the day-7 post-BMT whole body images (ventral views) of anesthetized, shaved C57BL/6 recipients of either allogeneic BM and spleen (Figure 3A), syngeneic BM and spleen (Figure 3B), and allogeneic BM alone (ie, without eGFP Tg splenocytes) as an autofluorescence control (Figure 3C). Intense eGFP fluorescence was seen in the lymph nodes, Peyer patches, and spleen, with moderate fluorescence in the liver and lungs in the recipients of allogeneic BM and spleen. In contrast, eGFP fluorescence was only seen in Peyer patches and a few peripheral lymph nodes, at lower intensity, in syngeneic recipients. Figure 3B shows a representative syngeneic recipient of an aliquot of the same preparation of cells given to the representative allogeneic mouse shown in Figure 3A. Images of close-up views of several organs are depicted in Figure 4. A cohort of mice receiving an equivalent number of non-eGFP allogeneic splenocytes was also imaged as a control for potential autofluorescence caused by GVHD-induced tissue injury and was shown not to contribute to the fluorescence seen. These photographs demonstrate that although eGFP+ cells are still present in the lymphoid organs at day 7 after BMT in syngeneic mice, these cells are either not present or are present only at low frequencies in GVHD target organs compared with the allogeneic recipients. The imaging results demonstrate that allogeneic splenocytes also migrated to the brain, showing that the brain is a GVHD target organ. We found that several “nonclassical” organs are targeted during GVHD, including the kidneys, connective tissues, gums, tongue, and nasal cavities, where massive infiltration was seen. Therefore, these data were not only consistent with those of the GVHD literature, they also provided surprising findings with regard to the extent of GVHD involvement that had not been appreciated before now.
Localization of donor splenocytes to the lymph nodes at 24 hours after infusion is similar in allogeneic and syngeneic recipients. Splenocytes from eGFP Tg C57BL/6 mice were injected intravenously into lethally irradiated B10.BR mice (A, allogeneic) or C57BL/6 (S, syngeneic) mice. Tissues from 1 of 3 representative mice from each time point per group are shown. Stereomicroscope was set to 10.0 × zoom factor with a 0.63 × transfer lens.
Localization of donor splenocytes to the lymph nodes at 24 hours after infusion is similar in allogeneic and syngeneic recipients. Splenocytes from eGFP Tg C57BL/6 mice were injected intravenously into lethally irradiated B10.BR mice (A, allogeneic) or C57BL/6 (S, syngeneic) mice. Tissues from 1 of 3 representative mice from each time point per group are shown. Stereomicroscope was set to 10.0 × zoom factor with a 0.63 × transfer lens.
Whole body images of mice taken 7 days after infusion of C57BL/6 BM with or without eGFP Tg donor splenocytes demonstrating more expansion of eGFP Tg splenocytes in the allogeneic setting and discernment of internal organs through the skin of anesthetized mice. (A) Allogeneic B10.BR recipient mouse given B6 BM and eGFP Tg splenocytes. (B) Syngeneic C57BL/6 mouse given C57BL/6 BM and eGFP Tg splenocytes. (C) Allogeneic B10.BR recipient given C57BL/6 BM only (to demonstrate low autofluorescence background). (D) Partial dissection (abdominal membrane still intact) of a mouse similar to that in panel A, showing the view of the lymph nodes from the inner side of the skin. Stereomicroscope was set to 1.0 × zoom factor with a 0.63 × transfer lens.
Whole body images of mice taken 7 days after infusion of C57BL/6 BM with or without eGFP Tg donor splenocytes demonstrating more expansion of eGFP Tg splenocytes in the allogeneic setting and discernment of internal organs through the skin of anesthetized mice. (A) Allogeneic B10.BR recipient mouse given B6 BM and eGFP Tg splenocytes. (B) Syngeneic C57BL/6 mouse given C57BL/6 BM and eGFP Tg splenocytes. (C) Allogeneic B10.BR recipient given C57BL/6 BM only (to demonstrate low autofluorescence background). (D) Partial dissection (abdominal membrane still intact) of a mouse similar to that in panel A, showing the view of the lymph nodes from the inner side of the skin. Stereomicroscope was set to 1.0 × zoom factor with a 0.63 × transfer lens.
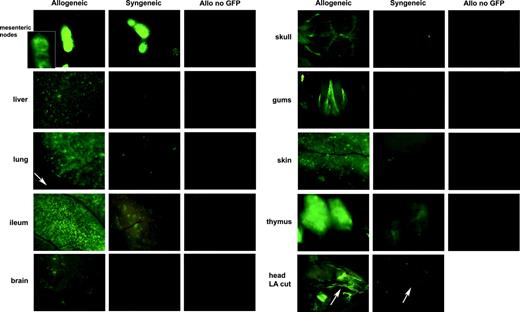
eGFP+ cells are present in the lymphoid organs at day 7 after BMT in syngeneic mice, but these cells are either not present or are present only at low frequencies in GVHD target organs compared with allogeneic recipients. Images of close-up views of several organs captured at day 7 after infusion of C57BL/6 BM and eGFP Tg splenocytes in allogeneic and syngeneic recipients as indicated. A cohort of mice receiving an equivalent number of non-eGFP allogeneic splenocytes was also imaged as a control for potential autofluorescence caused by GVHD-induced tissue injury. All images were captured with 1.09-second exposure times to allow direct comparison. The inset shown in the mesenteric node was taken with a 16-msec exposure time because of the intensity of the fluorescence in the nodes. Stereomicroscope was set to 10.0 × zoom factor with a 0.63 × transfer lens. LA indicates long axis cut of head with stereomicroscope set to 3.0 × zoom factor with a 0.63 transfer lens.
eGFP+ cells are present in the lymphoid organs at day 7 after BMT in syngeneic mice, but these cells are either not present or are present only at low frequencies in GVHD target organs compared with allogeneic recipients. Images of close-up views of several organs captured at day 7 after infusion of C57BL/6 BM and eGFP Tg splenocytes in allogeneic and syngeneic recipients as indicated. A cohort of mice receiving an equivalent number of non-eGFP allogeneic splenocytes was also imaged as a control for potential autofluorescence caused by GVHD-induced tissue injury. All images were captured with 1.09-second exposure times to allow direct comparison. The inset shown in the mesenteric node was taken with a 16-msec exposure time because of the intensity of the fluorescence in the nodes. Stereomicroscope was set to 10.0 × zoom factor with a 0.63 × transfer lens. LA indicates long axis cut of head with stereomicroscope set to 3.0 × zoom factor with a 0.63 transfer lens.
Non-T cells home to the follicular lymphoid compartment (B-cell and myeloid) areas, whereas few T cells initially home to the paracortex but then expand 2 days later
We were intrigued by the patterns of cellular distribution in the lymphoid tissues seen early after transfer of eGFP Tg cells. Because GVHD is ultimately mediated as a consequence of allogeneic effector T cells, we wanted to determine whether there was differential homing of the T-cell and non-T-cell compartments of donor spleen. B10.BR mice (for allogeneic studies) or C57BL/6 mice (for syngeneic studies) were lethally irradiated and given either whole splenocytes, TCD splenocytes, or purified T cells from eGFP Tg C57BL/6 mice. Mice were imaged at 1 hour, 6 hours, 24 hours, 48 hours, 72 hours, 5 days, and 7 days after transplantation. The images depicted in Figure 5 show that TCD splenocytes (non-T cells) immediately home to the follicular areas of the nodes in large numbers (representative Peyer patches and inguinal nodes are shown in Figure 5A-B, respectively). In contrast, when purified T cells are infused, they predominantly home to the deep paracortical areas, but only in small numbers, and appear to remain in close juxtaposition to the venules from which they extravasated. Figure 5 shows that allogeneic T cells begin to expand 48 to 72 hours after transfer in the lymph nodes. They then localize to GVHD target organs 1 day later (ie, 72 hours; see next section). Our data also show that the migration and expansion of the T cells and non-T cells of the spleen migrate to the lymph nodes and expand independently of each other. The addition of the 2 independent migration/expansion patterns is equivalent to the migration/expansion pattern observed when whole splenocytes are infused. In Figure 5C, costaining of lymph node cryosections with an antibody to eGFP and a phenotypic marker, followed by confocal microscopy, demonstrated that the eGFP+ cells seen in the lymphoid follicles at 24 hours after infusion were indeed CD19+ (ie, B cells) of donor origin in recipients of allogeneic whole splenocytes or TCD splenocytes, whereas the eGFP+ T cells (CD4 or CD8) were more apparent at 72 hours after infusion in the paracortical areas in the nodes of mice given whole splenocytes. At 72 hours after infusion, scant eGFP+ T cells were seen in mice receiving TCD splenocytes.
Migration and expansion of donor T cells in the lymph nodes occurs independently of non-T donor splenocytes and vice versa. (A) Peyer patch. (B) Inguinal node. B10.BR mice (for allogeneic studies) or C57BL/6 mice (for syngeneic studies) were lethally irradiated and given C57BL/6 BM with either whole splenocytes, TCD splenocytes, or purified T cells from eGFP Tg C57BL/6 mice. TCD splenocytes homed to the B-cell and myeloid areas (cortical lymphoid nodules), whereas the T cells homed to the T-cell zone (deep paracortex). In the allogeneic setting, donor T cells expanded 48 to 72 hours after infusion. Tissues from 1 of 3 representative mice from each time point per group are shown. Stereomicroscope was set to 10.0 × zoom factor with a 0.63 × transfer lens. (C) Confocal microscopy of lymph node cryosections costained with anti-eGFP antibody (red) and the indicated phenotypic marker (blue). Costained cells are depicted as violet-purple. On the left are nodes taken 24 and 72 hours after BMT of allogeneic BM and eGFP Tg whole splenocytes. On the right are nodes taken 24 and 72 hours after BMT of allogeneic BM and eGFP Tg TCD splenocytes. The images are consistent with the data in Figure 5A and 5B showing that donor cells localized early, at 24 hours after transplantation to the lymphoid follicles, consisted predominantly of B cells (CD19) and fewer myeloid cells (CD11b). eGFP Tg T cells (CD4 and CD8) are predominantly in the paracortex and were more apparent at 72 hours after transplantation. Original magnification × 200.
Migration and expansion of donor T cells in the lymph nodes occurs independently of non-T donor splenocytes and vice versa. (A) Peyer patch. (B) Inguinal node. B10.BR mice (for allogeneic studies) or C57BL/6 mice (for syngeneic studies) were lethally irradiated and given C57BL/6 BM with either whole splenocytes, TCD splenocytes, or purified T cells from eGFP Tg C57BL/6 mice. TCD splenocytes homed to the B-cell and myeloid areas (cortical lymphoid nodules), whereas the T cells homed to the T-cell zone (deep paracortex). In the allogeneic setting, donor T cells expanded 48 to 72 hours after infusion. Tissues from 1 of 3 representative mice from each time point per group are shown. Stereomicroscope was set to 10.0 × zoom factor with a 0.63 × transfer lens. (C) Confocal microscopy of lymph node cryosections costained with anti-eGFP antibody (red) and the indicated phenotypic marker (blue). Costained cells are depicted as violet-purple. On the left are nodes taken 24 and 72 hours after BMT of allogeneic BM and eGFP Tg whole splenocytes. On the right are nodes taken 24 and 72 hours after BMT of allogeneic BM and eGFP Tg TCD splenocytes. The images are consistent with the data in Figure 5A and 5B showing that donor cells localized early, at 24 hours after transplantation to the lymphoid follicles, consisted predominantly of B cells (CD19) and fewer myeloid cells (CD11b). eGFP Tg T cells (CD4 and CD8) are predominantly in the paracortex and were more apparent at 72 hours after transplantation. Original magnification × 200.
The non-T-cell compartment of the donor spleen gets “trapped” in the lungs early (1 hour) after infusion, followed 48 to 72 hours later by donor T cells
After intravenous injection, the cellular inoculum will first traffic through the heart and pulmonary vasculature. Continuing with imaging of the localization of splenic non-T cells and T cells, we found that the allogeneic non-T-cell compartment of the donor spleen comprises the early immigrants into the lungs early after intravenous infusion (Figure 6A). They initially appeared as small nodules that persisted for the duration of the experiment (7 days). In the recipient liver, in contrast to the findings in the lung, non-T cells from donor splenocytes were rarely seen within the first 72 hours of transplantation, whereas T cells appeared as infiltrates starting at 72 hours after BMT (Figure 6B). Therefore, the eGFP+ cells seen 72 hours after whole splenocyte infusion are most likely the T-cell component.
Migration and expansion of donor T cells occurred independently of non-T donor splenocytes and vice versa. (A) Lung, (B) liver, (C) spleen, and (D) femur. B10.BR mice were lethally irradiated and given C57BL/6 BM with either whole splenocytes or TCD splenocytes or purified T cells from eGFP Tg C57BL/6 mice. Tissues from 1 of 3 representative mice from each time point per group are shown. Stereomicroscope was set to 8.0 × (lung, liver, spleen) and 10.0 × (femur) zoom factor with a 0.63 × transfer lens.
Migration and expansion of donor T cells occurred independently of non-T donor splenocytes and vice versa. (A) Lung, (B) liver, (C) spleen, and (D) femur. B10.BR mice were lethally irradiated and given C57BL/6 BM with either whole splenocytes or TCD splenocytes or purified T cells from eGFP Tg C57BL/6 mice. Tissues from 1 of 3 representative mice from each time point per group are shown. Stereomicroscope was set to 8.0 × (lung, liver, spleen) and 10.0 × (femur) zoom factor with a 0.63 × transfer lens.
In the host spleen, the non-T cells appeared early after infusion (Figure 6C, 1 hour and 6 hours) but did not expand. In contrast, donor T cells were detected at 48 to 72 hours after transplantation and continued to increase in number throughout the observation period. Similar findings were seen in the BM (Figure 6D, femur). The apparent independent migration and expansion of the allogeneic T cells compared with the non-T splenocytes (that, when summed, appeared equivalent to the patterns seen after infusion of whole splenocytes) held true for all mouse organs and tissues (eg, non-GVHD target organs, reproductive organs, connective tissue).
Coinfusion of allogeneic splenocytes facilitates engraftment of BM and homing of allogeneic BM-derived cells to GVHD target organs
We next wanted to determine how the coinfusion of splenocytes affected the localization of donor BM cells. B10.BR mice (for allogeneic studies) or C57BL/6 mice (for syngeneic studies) were lethally irradiated and given TCD BM from eGFP Tg C57BL/6 mice with or without whole splenocytes from non-eGFP C57BL/6 mice. Consistent with the data obtained with eGFP Tg non-T cells, we saw early (1-hour) trapping of TCD BM cells to the lungs and, in contrast, also to the liver (Figure 7A). Early migration to the lungs and liver waned in recipients not receiving splenocytes. These findings were similar in allogeneic and syngeneic recipients. In the Peyer patches (Figure 7B), early homing of eGFP+ BM cells was not seen in the absence of coinfused splenocytes. In the presence of coinfused splenocytes, the presence of eGFP+ BM cells rapidly increased by 48 hours after BMT, and this was more apparent in allogeneic recipients (Figure 7B).
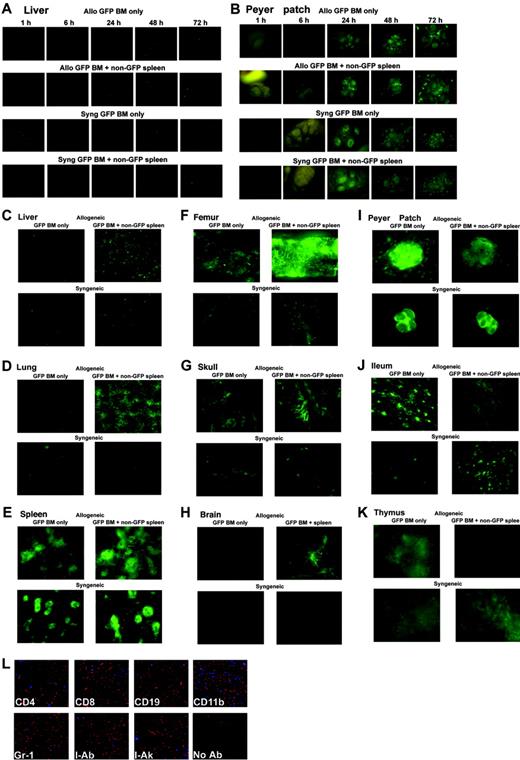
Coinfusion of allogeneic splenocytes facilitates engraftment of BM and homing of allogeneic BM to GVHD target organs. B10.BR mice (for allogeneic studies) or C57BL/6 mice (for syngeneic studies) were lethally irradiated and given BM from eGFP Tg C57BL/6 mice with or without whole splenocytes from non-eGFP C57BL/6 mice. Early time points (1-72 hours after BMT) for the liver (A) and Peyer patches (B) are shown. Day 7 time points are shown for liver (C, L), lung (D), spleen (E), femur (F), skull (G), brain (H), Peyer patch (I), ileum (J), and thymus (K). Tissues from 1 of 3 representative mice from each time point per group are shown. Stereomicroscope was set to 10.0 × zoom factor with a 0.5 × transfer lens. (L) Confocal analysis of immunofluorescence costaining in the liver of a mouse given allogeneic eGFP Tg BM and non-eGFP splenocytes for phenotypic identification of donor eGFP Tg BM-derived cells on day 7 after BMT. Cryosections were costained with anti-eGFP antibody (red) and the indicated phenotypic marker (blue). Costained cells are depicted as violet-purple. Donor eGFP Tg BM-derived cells expressing CD8, CD19, CD11b, Gr-1, and donor MHC class 2(I-Ab) were seen. Original magnification × 200.
Coinfusion of allogeneic splenocytes facilitates engraftment of BM and homing of allogeneic BM to GVHD target organs. B10.BR mice (for allogeneic studies) or C57BL/6 mice (for syngeneic studies) were lethally irradiated and given BM from eGFP Tg C57BL/6 mice with or without whole splenocytes from non-eGFP C57BL/6 mice. Early time points (1-72 hours after BMT) for the liver (A) and Peyer patches (B) are shown. Day 7 time points are shown for liver (C, L), lung (D), spleen (E), femur (F), skull (G), brain (H), Peyer patch (I), ileum (J), and thymus (K). Tissues from 1 of 3 representative mice from each time point per group are shown. Stereomicroscope was set to 10.0 × zoom factor with a 0.5 × transfer lens. (L) Confocal analysis of immunofluorescence costaining in the liver of a mouse given allogeneic eGFP Tg BM and non-eGFP splenocytes for phenotypic identification of donor eGFP Tg BM-derived cells on day 7 after BMT. Cryosections were costained with anti-eGFP antibody (red) and the indicated phenotypic marker (blue). Costained cells are depicted as violet-purple. Donor eGFP Tg BM-derived cells expressing CD8, CD19, CD11b, Gr-1, and donor MHC class 2(I-Ab) were seen. Original magnification × 200.
By day 7 after BMT, the infiltration and expansion of eGFP Tg donor BM cells were seen in GVHD target organs only in recipients receiving spleen cells in an allogeneic setting (Figure 7C, liver; Figure 7D, lung; Figure 7E, spleen). Figure 7L shows an example of confocal analysis of immunofluorescence costaining in the liver of a mouse given allogeneic eGFP Tg BM and non-eGFP splenocytes for phenotypic identification of donor eGFP Tg BM-derived cells on day 7 after BMT. Donor eGFP Tg BM-derived cells expressing CD8, CD19, CD11b, Gr-1, and donor major histocompatibility complex (MHC) class II (I-Ab) were seen. The paucity of donor BM cells in the syngeneic setting supports the concept that the expansion of allogeneic BM is a consequence of alloantigen recognition. More localization of donor BM was seen in the recipient BM cavity if allogeneic splenocytes were coinfused (Figure 7F-G, femur and skull, respectively). Similar findings were seen for the brain in the allogeneic setting (Figure 7H).
Interestingly, some of the secondary lymphoid organs (Peyer patches, ileum, thymus) showed less migration/expansion of eGFP+ BM cells at day 7 after BMT in mice coinfused with splenocytes in the allogeneic setting, when the mice showed signs of clinical GVHD (Figure 7I-K). This might have been because of the conditioning- and GVHD (allo T-cell)-induced destruction of epithelial/stromal cell elements necessary for the maintenance and development of hematopoietic precursors.
The images show pictorially the facilitation of BM engraftment by T cells (as shown in the recipient femur) because mice receiving allogeneic splenocytes had more eGFP+ cells in their BM than mice not receiving splenocytes. We know from BMT experiments, using eGFP Tg spleen cells, that allogeneic T cells migrate to and expand in GVHD target organs, and these data, using eGFP Tg BM, are evidence that allogeneic splenocytes infiltrating GVHD target organs create an environment (or a gradient) conducive to the infiltration of donor BM into these organs (such as the brain).
Discussion
In this report, we present a real-time spatial-temporal analysis of GVHD in our established murine allogeneic BMT model using eGFP Tg donor cells and imaging with a fluorescence stereomicroscope equipped with a color camera. eGFP-expressing cells were visualized at a single-cell resolution level. Although our aim was to track and image the cellular migration by fluorescence in vivo, these data can also be quantitatively expressed as shown by others.10
We also demonstrated the presence of eGFP+ cells in the tissues by immunofluorescence costaining of cryosections. Furthermore, we confirmed the presence of eGFP protein by enzyme-linked immunosorbent assay (ELISA) for eGFP (data not shown) on tissue protein extracts. The results we obtained were consistent with the imaging data. In another experiment, we imaged a cohort of lethally irradiated mice that received irradiated eGFP Tg splenocytes and found only rare radioresistant cells more than 1 day after infusion (data not shown). This provides further evidence that the eGFP+ cells visualized with this technique are not simply phagocytic host cells that have taken up eGFP protein released from dying cells.
We found that initial localization of donor splenocytes to recipient peripheral lymph nodes is not dependent on irradiation-induced injury. We have previously documented the induction of several chemokines following lethal irradiation in mice.11 Such chemokines are presumed to play roles in the directed homing of the infused cells. However, trafficking to, and extravasation into, peripheral lymph nodes occurred similarly in irradiated and nonirradiated mice. Furthermore, this early migration did not require the recognition of host alloantigens. These results are consistent with the recent report of Miller et al12 demonstrating that the initial extravasation of infused T cells occurs in a stochastic manner resembling a random walk. The role of alloantigen becomes apparent in the subsequent expansion of donor spleen cells in GVHD target organs, because this occurred only in allogeneic recipients.
The very early localization of lymphocytes to the lymph nodes after intravenous infusion in allogeneic and syngeneic settings most likely occurs by extravasation through the high endothelial venules. These events are regulated by homing chemokine/chemokine receptor interactions involving CCL19 and CCL21 binding to CCR7 on lymphocytes, but CCR7-independent pathways are also involved.13 We found a 72-hour delay in the infiltration of allogeneic donor T cells into GVHD target organs. This is consistent with the time needed for the lymphocyte response to alloantigen14 and the amount of time it takes for the development of microangiectasia, a process by which focal dilatations of microvessels, in response to inflammation, allow local reductions in vascular wall shear stress such that adhesion and extravasation can occur.15
Our results show that several nonclassical organs are targeted during GVHD, including the brain, kidneys, connective tissues, gums, tongue, and nasal cavities, where massive infiltration was seen. Involvement of the central nervous system and the brain has been previously described in mice16 and humans.17 Infiltration into the nasal cavities has implications for investigations of lung injury after BMT (idiopathic pneumonia syndrome [IPS]) and for pulmonary function studies. Measuring pulmonary resistance parameters by nonintubation methods may not necessarily reflect the lungs but, rather, resistance incurred by the inflamed nasal cavities given that rodents are obligate nose-breathers. Hence, these data are consistent with those of the GVHD literature, and they provide surprising findings with regard to the extent of GVHD involvement that had not been appreciated before now.
Our data also demonstrate that the migration and expansion of T cells into lymph nodes occurs independently of the coinfusion of non-T cells. The addition of the 2 independent migration/expansion patterns is equivalent to the migration/expansion pattern observed when whole splenocytes are infused. These results are consistent with the paradigm of segregation of B- and T-cell homing to the lymph nodes12,18 and the activation of donor T cells by the recipient antigen-presenting cells (APCs).19
We found that the non-T-cell compartment of the donor spleen comprises the early immigrants into the lungs after intravenous infusion. They initially appeared as small nodules that persisted for the duration of the experiments (7 days). Contrary to what has been demonstrated by others as to the early localization of T cells to the lungs (eg, by bioluminescence imaging), we found that the early localization of T cells to “lung areas” is actually localization to the lung-associated lymph nodes and bronchus-associated lymphoid tissue (BALT).20 These findings are consistent with the early reports of our IPS murine system in which we demonstrated by immunohistochemistry of cryosections that donor T cells infused intravenously did not appear as infiltrates into the lungs before day 3 after transplantation.21
Our results also demonstrate that allogeneic, but not syngeneic, splenocytes that infiltrate GVHD target organs create an environment, either directly or indirectly, that is conducive to the infiltration of donor BM into these organs (such as the brain). These findings are potentially relevant for more efficient engraftment of BM and also for the engraftment and subsequent differentiation of multipotential BM-derived cells (such as mesenchymal cells, multipotential adult progenitor cells [MAPCs], or side-population cells) in damaged tissue.22 Microglial engraftment in the brain after BMT with eGFP retrovirus-transduced BM cells in mice is enhanced if there is neuronal injury.23 Therefore, the benefits of having some alloreactivity, or low-grade GVHD that is tolerable, might include not only graft-versus-tumor effects, anti-infectious properties, and facilitation of hematopoietic stem cell engraftment but also facilitation of nonhematopoietic stem cells.
Prepublished online as Blood First Edition Paper, January 8, 2004; DOI 10.1182/blood-2003-08-2827.
Supported by the Children's Cancer Research Fund, National Institutes of Health grants R01 HL-55209 HL-49997 and AI-34495, National Center for Research Resources shared instrumentation grant 1 S10 RR16851, and an anonymous private foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mike Erhardt and Matt Kramer for expert technical assistance and Dr Patricia Taylor for helpful discussions.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal