Abstract
Stimulation of the erythropoietin (EPO) receptor triggers a cascade of signaling events. We reported that EPO upregulates c-myc expression through 2 pathways in BaF3-EpoR cells—a phosphatidylinositol 3-kinase (PI3K) pathway operating on transcriptional initiation and a Raf-1–mitogen-activated protein kinase (MAPK) pathway affecting elongation. We now show that EPO induces phosphorylation of Raf-1 at serine 338 and within the carboxy-terminal domain, resulting in an electrophoretic mobility change (hyperphosphorylation). Importantly, MEK 1 inhibitor PD98059 blocked only the hyperphosphorylation of Raf-1 but not the phosphorylation at serine 338. This inhibition of Raf-1 hyperphosphorylation resulted in increased kinase activity of Raf-1 and increased phosphorylation of MEK, suggesting that the hyperphosphorylation of Raf-1 inhibits its MEK kinase activity. Deletion of the first 184 amino acids of Raf-1, which are involved in its interaction with Ras, had no effect on EPO-induced phosphorylation. Introducing the dominant-negative N17Ras or GAP had no effect on EPO-induced kinase activity of Raf-1 and ELK activation. N17Ras failed to inhibit ELK activation in another cell line—Rauscher murine erythroleukemia— which expresses the EPO receptor endogenously and differentiates in response to the hormone. These results indicate the presence of a Ras-independent mechanism for Raf-1 and MEK activation in these cells.
Introduction
The Ras-Raf-MEK-ERK pathway is at the center of signaling networks that control cell proliferation, survival, and differentiation. Although the basic regulatory components and steps have been known for almost a decade, work from many laboratories has revealed that this pathway is, in reality, a multistep process that requires the assembly of several protein complexes1 that are subject to a variety of positive and negative regulatory factors.
Raf-1 is 74-kDa kinase and is composed of an amino-terminal regulatory domain and a carboxy-terminal catalytic domain. Two Ras-binding domains are located at residues 51 to 131 and residues 139 to 184 within amino-terminal domain of Raf-1.2-5 It has been proposed that the amino-terminal regulatory domain acts as an inhibitor of the carboxy-terminal catalytic domain6 or that it may serve as the receptor for the upstream signal. In quiescent cells, the amino-terminal domain inhibits the activity of the carboxyterminal catalytic domain through an intramolecular interaction. In response to activating signals, the amino-terminal domain mediates the interaction of Raf-1 with Ras. Interaction with guanosine triphosphate (GTP)–bound Ras localizes Raf-1 to the plasma membrane and is the first step in Raf-1 activation.7,8 The translocation of Raf-1 to the plasma membrane facilitates further interaction with kinases and other proteins.9 These interactions lead to the phosphorylation of Raf-1 at multiple tyrosine, serine, and threonine residues, resulting in its activation10 and in an electrophoretic mobility decrease/shift (hyperphosphorylation).11,12 The kinases and other proteins that interact with Raf-1 include protein kinase Pak3 phosphorylating Raf-1 at serine 338,13 the Src family of kinases phosphorylating Raf-1 at tyrosines 340 and 341,3,12,14 protein kinase C (PKC),15-17 Janus kinase 2 (JAK2),18 kinase suppressor of Ras (KSR),19,20 MEKK1,21 abl tyrosine kinase,22 the 14-3-3 protein family,23,24 RKIP,25 heat-shock protein 90 (Hsp90),26 p50cdc37,27 bcr,28 and cdc25.29 Although there are different models of Raf-1 activation.24,30-32 the interaction of Raf-1 with GTP-bound Ras is the first step in each. However, emerging data are challenging this view. For example, the phosphorylation of Raf-1 can occur without interacting with Ras.14,33 Therefore, Raf-1 can be activated in a Ras-dependent and a Ras-independent manner. Activated Raf-1 then phosphorylates its downstream targets,34,35 including MEK, which appears to be involved in the feedback regulation of Raf-1.36
Erythropoietin (EPO) has been shown to activate all the components of the Ras-Raf-MEK-ERK pathway.37-41 One study showed that EPO activation of Raf-1 is Ras dependent.18 In studies of the signal transduction pathways from the EPO receptor to c-myc, we showed previously that EPO up-regulates c-myc by 2 distinct pathways, one of which is PI3K dependent and operates on transcriptional initiation, whereas the other is Raf-1–mitogenactivated protein kinase (MAPK) dependent and operates on elongation.38 We have now further investigated EPO regulation of the Raf-MEK-ERK pathway and have found that EPO triggers the phosphorylation of Raf-1 at serine 338 and independently within the carboxy-terminal domain (hyperphosphorylation). This hyperphosphorylation of Raf-1 is dependent on MEK and does not appear to require the interaction of Raf-1 with Ras. Importantly, in the course of these studies, we found that inhibiting MEK kinase activity results in the inhibition of Raf-1 hyperphosphorylation as expected, but that it also results in a simultaneous increase in Raf-1 kinase activity and MEK phosphorylation, showing that the hyperphosphorylation of Raf-1 inhibits its kinase activity toward MEK, thus defining the role of carboxy-terminal hyperphosphorylation in EPO regulation of Raf-1 signaling.
Materials and methods
Cell culture and EPO treatment
Three types of EPO-responsive cells were used in these studies. First, BaF3 cells stably transfected with the human EPO receptor (EpoR) cDNA (BaF3-EpoR cells; generous gift of M. Showers) were maintained in a humidified atmosphere of 95% air/5% CO2 at 36.5° C in RPMI 1640 medium (Life Technologies, Bethesda, MD) supplemented with 10% fetal bovine serum (FBS) (Hyclone Laboratories, Logan, UT) and 1 U EPO/mL (Elanex Pharmaceuticals, Bothell, WA). Cells were incubated overnight in the absence of FBS and EPO and then were treated with 5 U EPO/mL for specified times. In some experiments, after overnight serum-free incubation, cells were incubated in the presence of specified concentrations of the PI3K inhibitor LY294002 or of the MEK inhibitors PD98059 and U0126 (Sigma-Aldrich, St Louis, MO) for 10 minutes. Then 5 U EPO/mL was added, and the incubation was continued for an additional 5 minutes. Cells were harvested by centrifugation for nuclear and cytoplasmic extraction. Second, normal erythroid cells were obtained from the spleens of phenylhydrazine-treated mice, as described previously.38 Cells were washed and resuspended in α-minimum essential medium (α-MEM) (108 cells/mL). An equal volume of cold ammonium chloride solution (0.83% in 0.01 M Tris-HCl, pH 7.5) was added to lyse erythrocytes. After 10 minutes on ice, the cells were washed again and were plated at 1 × 107/mL in 37° C α-MEM with 10% FBS for 4 hours. Then the cells (BaF3-EpoR or splenic erythroid) were incubated in the absence or presence of 12 U EPO/mL for 10 minutes. In several experiments, BaF3-EpoR cells were pretreated with specified concentrations of PD98059 for 10 minutes followed by the addition of 12 U EPO/mL. The cells were harvested by centrifugation for nuclear and cytoplasmic extraction and Western blotting. Third, Rauscher murine erythroleukemia cells42-48 were maintained in a humidified atmosphere of 95% air/5% CO2 at 36.5° C in DMEM supplemented with 10% FBS for transfection experiments.
Expression of flag-tagged, wild-type, and amino-terminal truncated Raf-1
Raf-1-Flag, p(Δ1-184)Raf-1-Flag, and dominant-negative Raf-1(S338339A and K375M) were constructed by inserting the full-length human Raf-1 (Upstate Biotechnology, Lake Placid, NY) or the amino-terminal 184–amino acid truncated human Raf-1 or mutant Raf-1 cDNA into the flag-tagged mammalian expression vector pCMV-Tag2A (Stratagene, La Jolla, CA) at EcoRI and XhoI sites. Gal4-Elk fusion protein expression plasmid pFA2-ELK and luciferase reporter plasmid pFR-Luc were purchased from Stratagene. Ha-Ras expression plasmid pH06T1, control plasmid Homer 6, and GTPase-activated protein (GAP) expression plasmid were generous gifts from Dr M. C. Ostrowski (Ohio State University, Columbus). N17Ras was inserted into mammalian expression vector pCMV. After confirmation by sequencing, the expression constructs were purified using Qiagen (Valencia, CA) kits. Transfection was carried out by electroporation. Cells (BaF3-EpoR or Rauscher murine erythroleukemia) growing in log phase were counted and resuspended in RPMI 1640 containing 10% FBS to 3 × 107/mL. Cells (1.0 × 107) (330 μL) were transferred to each electroporation cuvette (4 mm; Bio-Rad, Hercules, CA) and were mixed with 10 μg plasmid DNAs. Cells were then transfected by electroporation at 260 V/cm and 960 μF capacitance and were returned immediately to prewarmed, serum-free RPMI 1640 to be cultured at 37° C, 5% CO2, and 99% humidity overnight. Cells were incubated in the presence of the MEK inhibitors PD98059 and U0126 for 10 minutes. Then 5 U EPO/mL was added, and, for nuclear and cytoplasmic extraction, the incubation was continued for an additional 5 minutes. Cells were harvested by centrifugation for nuclear and cytoplasmic extraction. For luciferase assays, the incubation was continued for another 5 hours.
Tryptic digestion
One microgram tosylphenylalanyl chloromethyl ketone-trypsin (Worthington Biochemicals, Freehold, NJ) was added to 155 μL cytoplasmic extract. After incubation, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer was added to tryptic digestion reaction; this was followed by heating at 100° C for 3 minutes.
Dephosphorylation
Eighteen microliters cytoplasmic extract was incubated with 250 ng protein tyrosine phosphatase 1B (PTP-1B) and 250 ng protein phosphatase 2A (PP2A) (Upstate Biotechnology) at 30° C for 20 minutes. After incubation, SDS-PAGE sample buffer was added to the reaction and was heated at 100° C for 3 minutes.
Western blot analysis
Western blot analyses were performed on cytoplasmic or on nuclear extracts. Cytoplasmic and nuclear extracts were prepared using NE-PER Extraction Reagents (Pierce, Rockford, IL) according to the manufacturer's instructions. Briefly, 5 × 106 cells were suspended in 200 μL CER I buffer. After incubation on ice for 10 minutes, 11 μL CER II buffer was added for 1 minute to lyse cells. After centrifugation for 5 minutes, the supernatant (cytoplasmic extract) fraction was transferred to new tubes, and 100 μL NER buffer was added to the pellet (nuclei) fraction to lyse nuclei for 40 minutes. Then samples were centrifuged at 10 000g for 5 minutes in a microfuge. Twenty microliters of each sample was subjected to 8% SDS-PAGE and then transferred electrophoretically to polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA). The membrane was washed with 10 mL 1 × Tris-buffered saline (TBS; 20 mM Tris-HCl, 140 mM NaCl, pH 7.6) and then was incubated in 10 mL blocking buffer (1 × TBS, 0.1% Tween-20, 5% wt/vol nonfat dry milk) for 1 hour at room temperature. The membrane was incubated with a 1:1000 dilution of the specified primary antibody (New England Biolabs, Beverly, MA) in 10 mL blocking buffer with gentle shaking overnight at 4° C. After three 5-minute washes in blocking buffer, the membrane was incubated with 1:1000 alkaline phosphatase-conjugated secondary antibody (New England Biolabs) in 10 mL blocking buffer with gentle shaking for 1 hour at room temperature. Proteins were detected using CDP-Star Western Blotting Kit (New England Biolabs, Beverly, MA). For reprobing of membrane with a second primary antibody, the membrane was incubated in strip buffer (100 mM β-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.7) for 30 minutes at 50° C, then washed and blocked. Protein amounts in the bands were quantified by densitometric analysis of scanned images using Gel-Pro Analyzer, version 3.1 (Media Cybernetics).
Immunoprecipitation and kinase assay
Cells (5 × 106) were lysed in 1 mL RIPA buffer. After incubation at 4° C with rocking for 15 minutes and centrifugation for 15 minutes, the supernatant fractions were transferred to new tubes, and 100 μL protein A Sepharose beads was added. After incubation at 4° C with rocking for 30 minutes, the samples were centrifuged at 10 000g for 1 minute in a microfuge; then 500 μL supernatant was mixed with 10 μL agaroseconjugated anti–Raf-1 antibody. After incubation at 4° C with rocking for 2 hours, the Raf-1 and antibody complexes were pelleted and washed 4 times with 1 mL cold RIPA buffer. Raf-1 and antibody complexes were resuspended in 40 μL RIPA buffer and were designated immunoprecipitated Raf-1 (IP Raf-1). The Raf-1 kinase assay was carried out according to the manufacturer's instructions (Upstate Biotechnology). Briefly, 20 μL ADBI buffer, 10 μL magnesium/adenosine triphosphate (ATP) cocktail, 0.3 μg inactive MEK1, and 0.75 μg inactive ERK2 were mixed (total volume, 34 μL). Then 8 μL IP Raf-1 or water as control was added. The reaction was incubated at 30° C for 30 minutes in a shaking incubator to activate MEK1 and ERK2 (first-stage reaction). The second-stage reaction—that is, the phosphorylation of myelin basic protein (MBP) by ERK2—was carried out by adding 4 μL first-stage reaction to 30 μL second-stage component mixture that consisted of 10 μL ADBI buffer, 10 μL MBP, and 10 μL diluted γ-[32P] ATP. The second-stage reaction was incubated for 10 minutes at 30° C. After that, 25 μL of the second-stage reaction was pipetted onto the center of a 2 cm × 2 cm Whatman P81 paper (Clifton, NJ). After washing 3 times with 0.75% phosphoric acid, the radioactivity bound to the P81 paper was determined by liquid scintillation spectrometry. The remainder of the first-stage reaction was used for Western blotting.
Results
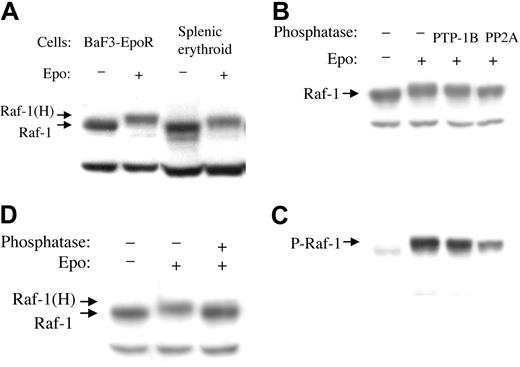
EPO treatment of BaF3-EpoR cells and of normal mouse splenic erythroid cells causes a decrease in the SDS-PAGE electrophoretic mobility of Raf-1. Cells were exposed to 5 U EPO/mL for 5 or 10 minutes, and cytoplasmic extracts were subjected to SDS-PAGE and Western blotting with anti–Raf-1 antibody (Figure 1A). In the absence of EPO, the antibody detected a 74- to 76-kDa Raf-1 band. In addition, a cross-reacting band of 56 kDa was seen. EPO treatment resulted in a reduced mobility (hypershift) of Raf-1, but not of the cross-reacting band. An equivalent EPO-induced mobility shift of Raf-1 was seen in both cell types. The mobility shift was partially reversed by pretreatment of the BaF3-EpoR extract with protein tyrosine phosphatase 1B (PTP-1B) or with protein phosphatase 2A (PP2A), respectively (Figure 1B-C). PTP-1B reduced the hyperphosphorylation (mobility shift) of Raf-1 but not the serine 338 phosphorylation. PP2A reduced both the hyperphosphorylation and the serine 338 phosphorylation. Pretreatment of the extract with combination PTP-1B and PP2A reduced the mobility shift completely (Figure 1D), strongly suggesting that the EPO-induced mobility shift was caused by tyrosine and serine/threonine phosphorylation. BaF3-EpoR cells were used for all further experiments.
Raf-1 undergoes a mobility shift in response to EPO, which can be reversed by phosphatase treatment. (A) BaF3-EpoR cells or normal murine splenic erythroid cells were treated with EPO for 5 minutes, and cytoplasmic extracts were subjected to Western blotting with anti–Raf-1 antibody. Note mobility shift (hypershift) Raf-1(H). (B) BaF3-EpoR cell cytoplasmic extract was incubated with phosphatase PTP-1B or PP2A for 20 minutes at 30° C, followed by Western blotting with anti–Raf-1 antibody. (C) The membrane shown in panel B was stripped and reprobed with anti–Raf-1 phosphoserine 338 antibody. (D) Cytoplasmic extract was incubated with a combination of phosphatases PTP-1B and PP2A for 20 minutes at 30° C, followed by Western blotting with anti–Raf-1 antibody.
Raf-1 undergoes a mobility shift in response to EPO, which can be reversed by phosphatase treatment. (A) BaF3-EpoR cells or normal murine splenic erythroid cells were treated with EPO for 5 minutes, and cytoplasmic extracts were subjected to Western blotting with anti–Raf-1 antibody. Note mobility shift (hypershift) Raf-1(H). (B) BaF3-EpoR cell cytoplasmic extract was incubated with phosphatase PTP-1B or PP2A for 20 minutes at 30° C, followed by Western blotting with anti–Raf-1 antibody. (C) The membrane shown in panel B was stripped and reprobed with anti–Raf-1 phosphoserine 338 antibody. (D) Cytoplasmic extract was incubated with a combination of phosphatases PTP-1B and PP2A for 20 minutes at 30° C, followed by Western blotting with anti–Raf-1 antibody.
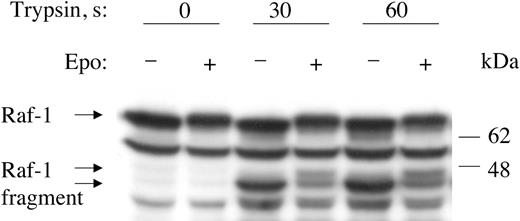
The EPO-induced hyperphosphorylation of endogenous Raf-1 responsible for the mobility shift occurred within its carboxy-terminal domain. We incubated cytoplasmic extracts from EPO-untreated and EPO-treated BaF3-EpoR cells in the absence or presence of trypsin and then subjected the extracts to SDS-PAGE and Western blotting with an antibody specific for the carboxy-terminal region of Raf-1. Tryptic digestion of untreated cell lysates resulted in the appearance of an approximately 44-kDa fragment recognized by the antibody. In lysates of EPO-treated cells, this carboxy-terminal fragment exhibited an electrophoretic mobility shift similar to that of intact endogenous Raf-1 (Figure 2).
Tryptic digestion of Raf-1 localizes hyperphosphorylation to the carboxy-terminal region. Cytoplasmic extracts of EPO-untreated or EPO-treated BaF3-EpoR cells were incubated with 1 μg TPCK-trypsin for specified times, followed by Western blotting with anti–Raf-1 carboxy-terminal domain antibody. Note mobility shift of approximately 44 kDa carboxy-terminal Raf-1 fragment on EPO treatment.
Tryptic digestion of Raf-1 localizes hyperphosphorylation to the carboxy-terminal region. Cytoplasmic extracts of EPO-untreated or EPO-treated BaF3-EpoR cells were incubated with 1 μg TPCK-trypsin for specified times, followed by Western blotting with anti–Raf-1 carboxy-terminal domain antibody. Note mobility shift of approximately 44 kDa carboxy-terminal Raf-1 fragment on EPO treatment.
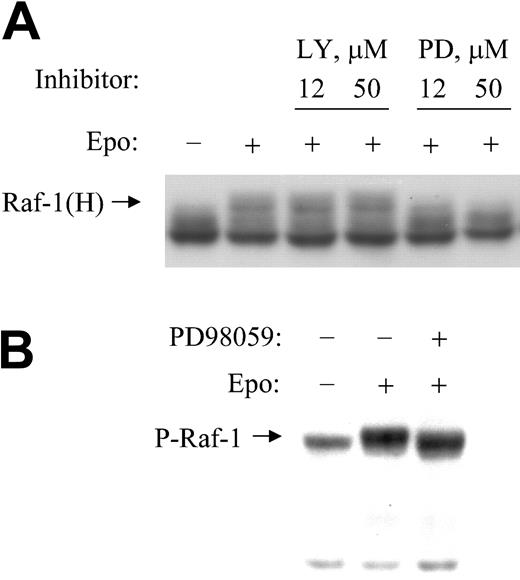
The EPO-induced hyperphosphorylation of Raf-1 required active MEK. To determine which kinase(s) is(are) involved in the hyperphosphorylation of Raf-1, we incubated cells in the absence or presence of specified concentrations of MEK1 inhibitor PD98059 or PI3K inhibitor LY294002 before treatment with EPO and then subjected cytoplasmic extracts to SDS-PAGE and Western blotting with anti–Raf-1 antibody (Figure 3A). We found that only the MEK1 inhibitor PD98059 blocked the hyperphosphorylation of Raf-1. In contrast, LY294002 had no effect on Raf-1 hyperphosphorylation. LY294002 did block EPO-induced Akt phosphorylation, confirming its inhibition of PI3K (not shown). PD98059 inhibition of Raf-1 hyperphosphorylation did not result from a generalized toxic effect because it did not inhibit the phosphorylation of Akt and did not induce apoptosis. Virtually identical results were obtained with another MEK inhibitor, U0126 (not shown). When we probed the membrane with antibody specific for the phosphoserine 338 region of Raf-1, we found that the MEK inhibitor PD98059 selectively blocked the hyperphosphorylation (mobility shift) of Raf-1 but had no effect on the EPO-induced phosphorylation of serine 338 (Figure 3B). This is consistent with evidence that serine 338 phosphorylation is dependent on other kinases.13
EPO-induced hyperphosphorylation of Raf-1 requires active MEK. (A) BaF3-EpoR cells were treated with specified concentrations of PI3K inhibitor LY294002 or MEK inhibitor PD98059 for 10 minutes, then treated with EPO for 5 minutes. Cytoplasmic extracts were subjected to Western blotting with anti–Raf-1 antibody. (B) Cytoplasmic extracts were subjected to Western blotting with anti–Raf-1 phosphoserine 338 antibody.
EPO-induced hyperphosphorylation of Raf-1 requires active MEK. (A) BaF3-EpoR cells were treated with specified concentrations of PI3K inhibitor LY294002 or MEK inhibitor PD98059 for 10 minutes, then treated with EPO for 5 minutes. Cytoplasmic extracts were subjected to Western blotting with anti–Raf-1 antibody. (B) Cytoplasmic extracts were subjected to Western blotting with anti–Raf-1 phosphoserine 338 antibody.
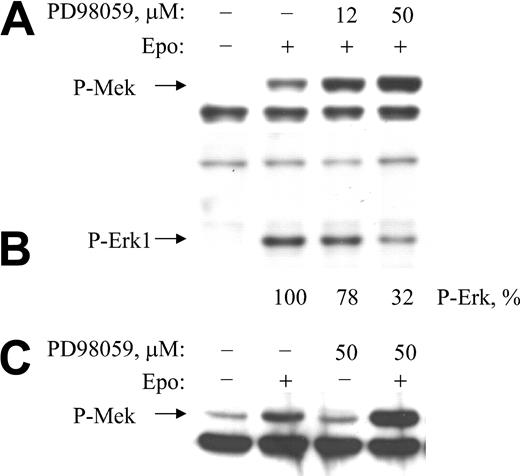
The effect of carboxy-terminal hyperphosphorylation of Raf-1 on Raf-1 kinase activity has been controversial. Both activation and inhibition have been proposed.36,49 To investigate this effect, we incubated cells in the absence or the presence of PD98059 for 10 minutes and then by EPO for 5 minutes. Cytoplasmic extracts were subjected to SDS-PAGE and Western blot analysis with anti–phospho-MEK1 antibody (Figure 4). EPO induced MEK phosphorylation as expected. Importantly, MEK inhibitor PD98059 enhanced the EPO-induced phosphorylation of MEK markedly, indicating an increase in Raf-1 kinase activity toward MEK in the presence of the MEK inhibitor, concomitant with the inhibition of Raf-1 hyperphosphorylation (Figure 4A). This result provides strong evidence that MEK-dependent hyperphosphorylation of Raf-1 inhibits Raf-1 kinase activity toward MEK. To confirm that PD98059 inhibited MEK in this experiment, we stripped the membrane and probed it with anti–phospho-ERK1/2 antibody (Figure 4B). EPO induction of ERK phosphorylation was clearly inhibited by PD98059. Further, we determined that PD98059 alone, in the absence of EPO treatment, did not result in enhanced MEK phosphorylation (Figure 4C).
MEK inhibitor PD98059 enhances EPO-induced phosphorylation of MEK. (A) BaF3-EpoR cells were treated with specified concentrations of PD98059 for 10 minutes, then treated with EPO for 5 minutes. Cytoplasmic extracts were subjected to Western blotting with anti–phospho-MEK antibody. (B) The same membrane was stripped and reprobed with anti–phospho-ERK1/2 antibody. (C) Cells were treated with EPO alone, inhibitor alone, or inhibitor then with EPO for 5 minutes. Cytoplasmic extracts were subjected to Western blotting with anti–phospho-MEK antibody.
MEK inhibitor PD98059 enhances EPO-induced phosphorylation of MEK. (A) BaF3-EpoR cells were treated with specified concentrations of PD98059 for 10 minutes, then treated with EPO for 5 minutes. Cytoplasmic extracts were subjected to Western blotting with anti–phospho-MEK antibody. (B) The same membrane was stripped and reprobed with anti–phospho-ERK1/2 antibody. (C) Cells were treated with EPO alone, inhibitor alone, or inhibitor then with EPO for 5 minutes. Cytoplasmic extracts were subjected to Western blotting with anti–phospho-MEK antibody.
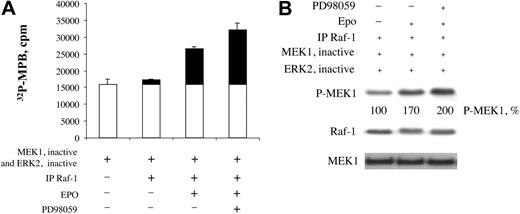
Inhibition of Raf-1 hyperphosphorylation directly results in its increased kinase activity. To demonstrate this, we carried out Raf-1 kinase assays (see “Materials and methods”). This assay is based upon the kinase cascade initiated by Raf-1 leading to the phosphorylation of myelin basic protein (MBP) in vitro (Raf-1 → MEK1 → ERK2 → MBP). As seen in Figure 5A, there was an intrinsic background level of MBP phosphorylation in the system, even in the absence of added IP Raf-1. Adding immunoprecipitated Raf-1 (IP Raf-1) from EPO nontreated (EPO–) cells did not increase background MBP phosphorylation significantly. In contrast, adding IP Raf-1 from EPO-treated (EPO+) cells increased MBP phosphorylation 72% above background. Importantly, MBP phosphorylation was increased even further, to 106% above background, when IP Raf-1 from EPO+ and PD98059-treated cells was added (P < .02). This was confirmed by Western blot analysis results (Figure 5B) showing the phosphorylation of recombinant MEK1 by IP Raf-1. There was a background level of phosphorylated MEK1 (P-MEK1) when IP Raf-1 from EPO–cells was used. Adding IP Raf-1 from EPO+ cells increased the amount of P-MEK1 to 170% of that observed with IP Raf-1 from EPO–cells. Notably, adding IP Raf-1 from EPO+ cells that were also treated with PD98059 increased P-MEK1 further, to 200%. The membrane was stripped and reprobed with anti–Raf-1 antibody (Figure 5B, middle panel). IP Raf-1 exhibited an EPO-induced mobility shift, and this mobility shift was inhibited by PD98059. The membrane was again stripped and reprobed, this time with anti-MEK antibody as control (Figure 5B, bottom panel). These results indicated that the inhibition of EPO-induced Raf-1 hyperphosphorylation in the cell by the MEK inhibitor PD98059 increased Raf-1 kinase activity.
Inhibition of Raf-1 hyperphosphorylation by PD98059 increases Raf-1 kinase activity. (A) Raf-1 kinase assay. BaF3-EpoR cells were serum-starved overnight and were incubated in the absence or presence of inhibitor PD98059 for 10 minutes, then in the absence or presence of EPO for 5 minutes. Cell lysates were immunoprecipitated with anti–Raf-1 antibody, and the immunoprecipitate was used for Raf-1 kinase assay. Four microliters of the first-stage reaction of kinase assay was used to phosphorylate MBP. Black bars show increase above background. Error bars represent means ± SD. (B) Western blot analysis of the first-stage reaction from kinase assay.
Inhibition of Raf-1 hyperphosphorylation by PD98059 increases Raf-1 kinase activity. (A) Raf-1 kinase assay. BaF3-EpoR cells were serum-starved overnight and were incubated in the absence or presence of inhibitor PD98059 for 10 minutes, then in the absence or presence of EPO for 5 minutes. Cell lysates were immunoprecipitated with anti–Raf-1 antibody, and the immunoprecipitate was used for Raf-1 kinase assay. Four microliters of the first-stage reaction of kinase assay was used to phosphorylate MBP. Black bars show increase above background. Error bars represent means ± SD. (B) Western blot analysis of the first-stage reaction from kinase assay.
We also obtained data using immunoprecipitated B-Raf in kinase assays, suggesting that B-Raf may also be involved in MEK activation in a manner similar to that of Raf-1 (data not shown). However, Western blot analyses were inconclusive.
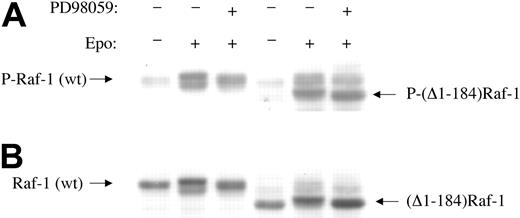
It has been reported that EPO activation of Raf-1 is Ras dependent in Sf21 insect cells.18 To examine the role of Ras in EPO-induced Raf-1 hyperphosphorylation in BaF3-EpoR cells, we expressed wild-type Raf-1 and an amino-terminal deletion mutant of Raf-1, (Δ1-184)Raf-1, which is unable to interact with Ras,3,50 as flag-tagged fusion proteins in BaF3-EpoR cells. EPO treatment induced carboxy-terminal domain hyperphosphorylation and serine 338 phosphorylation in the wild-type and truncated proteins (Figure 6A-B). MEK1 inhibitor PD98059 blocked EPO-induced hyperphosphorylation, but not serine 338 phosphorylation, in wild-type and truncated Raf-1. These data indicate that EPO-induced serine 338 phosphorylation and MEK-mediated hyperphosphorylation of Raf-1 occur in the absence of interactions with Ras and, presumably, without associations with the plasma membrane.
Amino-terminal Ras-binding domain of Raf-1 is not required for EPO-induced serine 338 phosphorylation or for carboxy-terminal domain phosphorylation. (A) Wild-type Raf-1 and amino-terminal truncated (Δ1-184)Raf-1 were transfected into BaF3-EpoR cells as flag-tagged fusion proteins. Cytoplasmic extracts were subjected to Western blotting with anti–Raf-1 phosphoserine 338 antibody. The left 3 lanes are mixtures of endogenous Raf-1 and transfected wild-type flag-Raf-1. The upper bands in the right 3 lanes are endogenous Raf-1, and the lower bands in the right 3 lanes are transfected truncated flag-(Δ1-184)Raf-1. Note that EPO induces a hypershift and serine 338 phosphorylation (increased band intensity) in (Δ1-184)Raf-1 (B). The same membrane was stripped and reprobed with antiflag antibody.
Amino-terminal Ras-binding domain of Raf-1 is not required for EPO-induced serine 338 phosphorylation or for carboxy-terminal domain phosphorylation. (A) Wild-type Raf-1 and amino-terminal truncated (Δ1-184)Raf-1 were transfected into BaF3-EpoR cells as flag-tagged fusion proteins. Cytoplasmic extracts were subjected to Western blotting with anti–Raf-1 phosphoserine 338 antibody. The left 3 lanes are mixtures of endogenous Raf-1 and transfected wild-type flag-Raf-1. The upper bands in the right 3 lanes are endogenous Raf-1, and the lower bands in the right 3 lanes are transfected truncated flag-(Δ1-184)Raf-1. Note that EPO induces a hypershift and serine 338 phosphorylation (increased band intensity) in (Δ1-184)Raf-1 (B). The same membrane was stripped and reprobed with antiflag antibody.
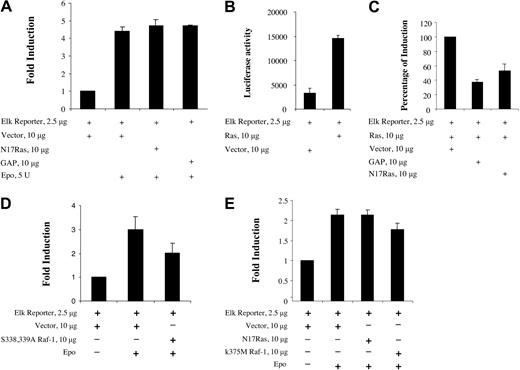
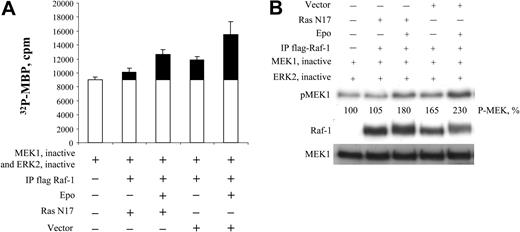
EPO-induced Raf-1-MEK1-ERK-ELK signaling appears to be Ras independent in BaF3-EpoR cells. We transfected BaF3-EpoR cells with a Gal4-ELK reporter and dominant-negative Ras, N17Ras, or GAP. As shown in Figure 7A, neither dominant-negative Ras N17Ras nor GAP had an effect on EPO-induced Raf-1-MEK1-ERK-ELK signaling. In Figure 7B-C, we show that the Gal4-ELK reporter is Ras responsive in BaF3-EpoR cells and that dominant-negative Ras, N17Ras, and GAP inhibit this Ras response. To confirm that this Gal4-ELK reporter was activated by the Raf-1-MEK1-ERK pathway, we cotransfected a MEK-binding deficient Raf-1 mutant (S338A, S339A) (dominant negative)51 along with the Gal4-ELK reporter into BaF3-EpoR cells (Figure 7D). This dominant-negative mutant partially inhibited EPO-dependent ELK activation. Experiments with reporter plasmids (eg, β-gal) and initial preliminary work demonstrated an average transfection efficiency of 40% to 50%. Correcting for the transfection efficiency, this dominant-negative Raf-1 mutant inhibited EPO-dependent Elk activation by 50% to 67%, consistent with a major Ras-independent role for Raf-1 in EPO-dependent MEK activation in BaF3-EpoR cells. The remainder of ELK activation not inhibited by the mutant might have been mediated by B-Raf (see above in this section). We also confirmed this by transfecting another dominant-negative Raf-1 mutant (K375M)33 or N17Ras along with the Gal4-ELK reporter into a different cell line expressing the EpoR endogenously, Rauscher murine erythroleukemia. This dominant-negative mutant also partially inhibited EPO-dependent ELK activation (Figure 7E). Correcting for transfection efficiency, this dominant-negative Raf-1 mutant inhibited EPO-dependent Elk activation by 40% to 50%. The remaining ELK activation not inhibited by the mutants might have been mediated by B-Raf (see above in this section). Furthermore, we cotransfected N17Ras with full-length Raf-1 fused to flag tag and then immunoprecipitated transfected Raf-1 with antiflag antibody. The immunoprecipitation was used for Raf-1 kinase assays and for Western blotting. As shown in Figure 8A-B, dominant-negative Ras N17Ras reduced the endogenous, noninduced Raf-1 kinase activity detected in the absence of EPO. However, there was no inhibitory effect on the EPO-dependent fold induction of Raf-1 activity. In the presence of N17Ras, EPO induced Raf-1 activity by 3.2-fold, whereas in the vector control cells, EPO induction of Raf-1 was 2.3-fold. Taken together, the data support the hypothesis that the major portion of this EPO-induced activation of Raf-1 is Ras independent.
Dominant-negative Ras N17Ras and GAP have no effect on the EPO-induced activation of ELK. (A) Ten micrograms N17Ras or GAP or empty vector along with 2.5 μg Gal4-Elk fusion protein expression plasmid and luciferase reporter plasmid containing Gal4 binding site were cotransfected into BaF3-EpoR cells. Cells were incubated in the absence of EPO for 14 hours. Then 5 U EPO/mL was added, and the incubation was continued for an additional 5 hours. Cells were harvested for luciferase assay. (B) Ten micrograms activated H-Ras or vector along with 2.5 μg Gal4-Elk fusion protein expression plasmid and luciferase reporter plasmid were cotransfected into BaF3-EpoR cells. Cells were incubated in the presence of EPO for 14 hours and then harvested for luciferase assay. (C) Ten micrograms activated H-Ras along with 10 μg N17Ras or GAP or empty vector and 2.5 μg Gal4-Elk fusion protein expression plasmid and luciferase reporter plasmid were cotransfected into BaF3-EpoR cells. Cells were incubated in the presence of EPO for 14 hours and then harvested for luciferase assay. (D) Ten micrograms dominant-negative Raf-1 S338A, S339A, or empty pCMV-tag2A vector along with 2.5 μg Gal4-Elk fusion protein expression plasmid and luciferase reporter plasmid containing Gal4 binding site were cotransfected into BaF3-EpoR cells. Cells were incubated in serum-free medium for 14 hours. After 25 U EPO/mL was added, the incubation was continued for another 5 hours. Cells were harvested for luciferase assay. (E) Ten micrograms N17Ras or dominant-negative Raf-1 K375M or empty pCMV-tag2A vector along with 2.5 μg Gal4-Elk fusion protein expression plasmid and luciferase reporter plasmid containing Gal4 binding site were cotransfected into Rauscher murine erythroleukemia cells. Cells were incubated in serum-free medium for 14 hours. After 25 U EPO/mL was added, the incubation was continued for another 5 hours. Cells were harvested for luciferase assay. Error bars represent means ± SD.
Dominant-negative Ras N17Ras and GAP have no effect on the EPO-induced activation of ELK. (A) Ten micrograms N17Ras or GAP or empty vector along with 2.5 μg Gal4-Elk fusion protein expression plasmid and luciferase reporter plasmid containing Gal4 binding site were cotransfected into BaF3-EpoR cells. Cells were incubated in the absence of EPO for 14 hours. Then 5 U EPO/mL was added, and the incubation was continued for an additional 5 hours. Cells were harvested for luciferase assay. (B) Ten micrograms activated H-Ras or vector along with 2.5 μg Gal4-Elk fusion protein expression plasmid and luciferase reporter plasmid were cotransfected into BaF3-EpoR cells. Cells were incubated in the presence of EPO for 14 hours and then harvested for luciferase assay. (C) Ten micrograms activated H-Ras along with 10 μg N17Ras or GAP or empty vector and 2.5 μg Gal4-Elk fusion protein expression plasmid and luciferase reporter plasmid were cotransfected into BaF3-EpoR cells. Cells were incubated in the presence of EPO for 14 hours and then harvested for luciferase assay. (D) Ten micrograms dominant-negative Raf-1 S338A, S339A, or empty pCMV-tag2A vector along with 2.5 μg Gal4-Elk fusion protein expression plasmid and luciferase reporter plasmid containing Gal4 binding site were cotransfected into BaF3-EpoR cells. Cells were incubated in serum-free medium for 14 hours. After 25 U EPO/mL was added, the incubation was continued for another 5 hours. Cells were harvested for luciferase assay. (E) Ten micrograms N17Ras or dominant-negative Raf-1 K375M or empty pCMV-tag2A vector along with 2.5 μg Gal4-Elk fusion protein expression plasmid and luciferase reporter plasmid containing Gal4 binding site were cotransfected into Rauscher murine erythroleukemia cells. Cells were incubated in serum-free medium for 14 hours. After 25 U EPO/mL was added, the incubation was continued for another 5 hours. Cells were harvested for luciferase assay. Error bars represent means ± SD.
Dominant-negative Ras N17Ras does not inhibit the EPO-dependent induction of Raf-1 activity. (A) Raf-1 kinase assay. BaF3-EpoR cells were cotransfected with 10 μg Raf-1 and 30 μg dominant-negative Ras (N17 Ras) expression plasmid DNA. Cells were incubated in the absence or presence of EPO for 5 minutes. Cells were lysed, and Raf-1 was immunoprecipitated with antiflag antibody. The immunoprecipitate was used for the Raf-1 kinase assay. Four microliters of the first-stage reaction of the kinase assay was used to phosphorylate MBP. Error bars represent means ± SD. (B) Western blot analysis of the first-stage reaction from kinase assay.
Dominant-negative Ras N17Ras does not inhibit the EPO-dependent induction of Raf-1 activity. (A) Raf-1 kinase assay. BaF3-EpoR cells were cotransfected with 10 μg Raf-1 and 30 μg dominant-negative Ras (N17 Ras) expression plasmid DNA. Cells were incubated in the absence or presence of EPO for 5 minutes. Cells were lysed, and Raf-1 was immunoprecipitated with antiflag antibody. The immunoprecipitate was used for the Raf-1 kinase assay. Four microliters of the first-stage reaction of the kinase assay was used to phosphorylate MBP. Error bars represent means ± SD. (B) Western blot analysis of the first-stage reaction from kinase assay.
Discussion
Although the basic regulatory components and steps in the “classic” pathway, Receptor → Shc or Grb2 → Sos1 → Ras → Raf-1 → MEK → ERK → ELK, have been known for almost a decade, work from several laboratories is challenging this model. The regulation of Raf-1 phosphorylation is subject to a multiplicity of influences because of the numerous kinases and other proteins with which Raf-1 can interact.
Wartmann49 showed that phorbol 12-myristate 13-acetate (PMA) treatment of Chinese hamster ovary cells resulted in a rapid increase in Raf-1, MEK, and MAPK activities along with a Raf-1 mobility shift. A similar hypershift was observed in a 33-kDa carboxy-terminal Raf-1 fragment transfected into NIH 3T3 cells on serum or phorbol ester treatment.52 Using carboxy-terminal Raf-1 fragments transfected into Sf9 insect cells, Ferrier et al11 demonstrated that the hypershift was caused by the phosphorylation of serine 621 and serine 624 and that cotransfection of an activated MEK1 mutant induced the shift independently of external stimuli. This is especially interesting in view of the observation that protein kinase A (PKA)–dependent phosphorylation of serine 621 reduced Raf-1 kinase activity.53 Zimmermann et al36 later reported that MEK1 mediated a positive feedback on Raf-1 activity and mediated the hyperphosphorylation of the amino-terminal and the carboxy-terminal domains. The exact role of the hyperphosphorylation in Raf-MEK regulation was unclear.
The participation of Raf-1 in EPO signaling was first demonstrated by Carroll et al,39 who observed that EPO treatment of HCD-57 and FDC-P1/ER hematopoietic cell lines resulted in the phosphorylation of Raf-1 on serine and tyrosine residues and a concomitant increase in Raf-1 kinase activity. Another study showed that Raf-1 activation by EPO was Ras dependent.18 Studies of EPO-induced Raf-1 activation have also revealed that Raf-1 interacts with numerous proteins that appear to be relevant to the activation process and to Raf's downstream effectors, including JAK2,18 Shc,54 CrkL-C3G,55 Lyn,56 and Rac.57
In the EPO signaling experiments we have presented here in BaF3-EpoR cells, we have emphasized as much as possible the study of endogenous proteins. We have shown that EPO signaling results in a hyperphosphorylation of Raf-1 because of the phosphorylation of tyrosine, serine, and threonine in the carboxy-terminal domain. This EPO-induced hyperphosphorylation is MEK dependent because the MEK inhibitor PD98059, but not the PI3K inhibitor LY294002, specifically blocked the mobility shift. Simultaneously, PD98059 inhibition of Raf-1 hyperphosphorylation resulted in an increase in Raf-1 kinase activity and MEK phosphorylation, demonstrating that reversing the hyperphosphorylation of Raf-1 increases its kinase activity toward MEK in vivo. We obtained these same results using MEK kinase inhibitor U0126 (data not shown).
How MEK regulates Raf-1 is not fully understood. We performed preliminary in vitro kinase assays in which active MEK or ERK was used as a kinase and immunoprecipitated inactive Raf-1 was used as substrate. We did not find that MEK or ERK phosphorylated Raf-1 directly in these assays. Therefore, one or more unknown kinases may be involved in the MEK-dependent carboxy-terminal phosphorylation of Raf-1.
EPO activates all the components in the Ras-Raf-1-MEK-ERK pathway, and the activation of Raf-1 by EPO has been reported to be strictly Ras dependent in several cell backgrounds.37-41 Here, we showed that dominant-negative Ras N17Ras (and GAP) had no inhibitory activity on EPO-induced Raf-1 kinase activity or Raf-1-MEK-ERK-ELK signaling in BaF3-EpoR cells and that N17Ras also failed to block EPO-induced ELK activation in Rauscher murine erythroleukemia cells, a distinctly erythroid line that expresses EpoR endogenously. Moreover, an amino-terminal 184-amino acid truncated Raf-1 unable to interact with Ras3 responded to EPO with serine 338 phosphorylation and hyperphosphorylation. The phosphorylation of serine 338 by Pak3 is related to the activation of Raf-1.58 Therefore, our data suggest that the interaction of Raf-1 with GTP-bound Ras may not be the necessary first step in all Raf-1 activation by EPO in the hematopoietic cells described here and that Raf-1 is sequentially phosphorylated by different kinases, in response to EPO. One of the kinases is MEK independent, phosphorylating serine 338 (possibly Pak3) to activate Raf-1, and the other is MEK dependent, phosphorylating the carboxy-terminal region of Raf-1 to reduce the MEK kinase activity of Raf-1.
The significance of MEK-dependent carboxy-terminal phosphorylation has been controversial. Mishak et al53 reported that the mutation of serine 621 to alanine in full-length Raf-1 prevented its mobility shift and its Src- or Ras-dependent activation in Sf9 cells, leading the authors to conclude that serine 621 has a dual function. It appeared to be required for Raf-1 kinase activity and yet served as a negative regulator when phosphorylated. In view of the numerous and sometimes conflicting studies on Raf-1 phosphorylation, most of which used different cell types and different stimuli for the activation of Raf-1, we speculate that the effect of the numerous phosphorylation events on Raf-1 activity may depend on the amounts of the various interacting proteins available in the cellular milieu.
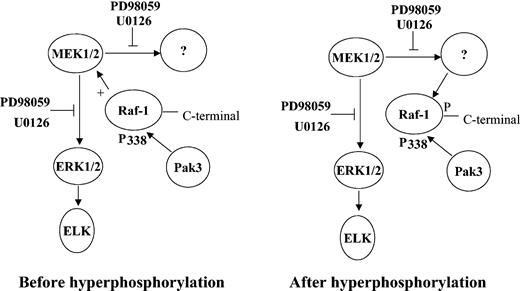
Based on our data, we propose a model for the Ras-independent Raf-1–MEK regulation in response to EPO reported here (Figure 9). Raf-1 is initially phosphorylated on some residues, such as serine 338, by Pak313 and on tyrosine 340 and 341, possibly by Src.14 This active Raf-1 then phosphorylates MEK, which, in turn, phosphorylates MAPK, ERK1/2, and unknown, kinase(s). The phosphorylated unknown kinase(s) then hyperphosphorylates Raf-1 at its carboxy-terminal domain to reduce its MEK kinase activity, which is to say the hyperphosphorylation of Raf-1 acts as negative feedback to prevent further phosphorylation of MEK1. The hyperphosphorylated Raf-1 dissociates from MEK and translocates to the membrane to interact with other proteins, such as Ras. In the presence of MEK inhibitor, MEK cannot phosphorylate the unknown kinase(s), which in turn cannot hyperphosphorylate Raf-1 at its carboxy-terminal domain to inactivate Raf-1 or to promote Raf-1 disassociation from MEK. Therefore, active Raf-1 prolongs the phosphorylation of MEK1. Although this model is speculative, it has sufficient experimental support to warrant its further investigation.
Model for Ras-independent regulation of Raf-1 and MEK by EPO. Kinase(s) such as Pak3 phosphorylates Raf-1 at serine 338 to activate it. The activated Raf-1 phosphorylates MEK, which, in turn, phosphorylates ERK and unknown kinase(s). The unknown kinases then hyperphosphorylate Raf-1 in the carboxy-terminal domain to inactivate Raf-1 MEK kinase activity or to promote Raf-1 disassociation from MEK and translocation to Ras. In the presence of inhibitors, MEK cannot phosphorylate unknown kinase, and unknown kinase cannot phosphorylate Raf-1 in the carboxy-terminal domain to inactivate its MEK kinase activity or to promote its dissociation from MEK. Active Raf-1 prolongs the phosphorylation of MEK.
Model for Ras-independent regulation of Raf-1 and MEK by EPO. Kinase(s) such as Pak3 phosphorylates Raf-1 at serine 338 to activate it. The activated Raf-1 phosphorylates MEK, which, in turn, phosphorylates ERK and unknown kinase(s). The unknown kinases then hyperphosphorylate Raf-1 in the carboxy-terminal domain to inactivate Raf-1 MEK kinase activity or to promote Raf-1 disassociation from MEK and translocation to Ras. In the presence of inhibitors, MEK cannot phosphorylate unknown kinase, and unknown kinase cannot phosphorylate Raf-1 in the carboxy-terminal domain to inactivate its MEK kinase activity or to promote its dissociation from MEK. Active Raf-1 prolongs the phosphorylation of MEK.
Prepublished online as Blood First Edition Paper, March 16, 2004; DOI 10.1182/blood-2003-04-1340.
Supported in part by National Institutes of Health grants KO1 DK62113 (C.C.) and R01 CA89204, National Aeronautics and Space Administration grants NAG9-1368 and NAG2-1592, and US Army Medical Research and Materiel Command grant DAMD 17-03-1-0233 (A.J.S).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal