Since activation-induced cytidine deaminase regulates somatic hypermutation and class-switch recombination, which play key roles in humoral immunity and lymphomagenesis, the elucidation of its expression and activity in B-cell lymphomas is a key issue in current lymphoma research.
Somatic hypermutation and class-switch recombination of immunoglobulin genes strictly depend on the enzyme activation-induced cytidine deaminase (AID). Since these processes play important roles in humoral immune responses, are a hallmark of B-cell differentiation in the germinal center (GC), and are implicated in the pathogenesis of B-cell lymphomas when acting inappropriately, the elucidation of AID expression and activity in human B-cell lymphomas has been the focus of numerous recent investigations. These studies were hampered by the lack of an anti-AID antibody, because detection of AID RNA does not automatically mean that protein also is made. The article by Pasqualucci and colleagues in this issue is therefore a major advance by presenting studies on AID expression with 2 novel anti-AID antibodies. AID protein is found to be expressed in GC B cells and GC-associated B-cell lymphomas, whereas post-GC malignancies lacked detectable amounts of AID. This study also shows that detection of AID transcripts and protein is well correlated, indicating that the previous RNA-based studies yielded valuable information about AID expression. The article by Forconi and colleagues in this issue adds a further facet to this picture by showing that AID transcripts can be regularly detected in hairy cell leukemia. Notably, hairy cell leukemia is considered a malignancy related to memory B cells.
Another important finding in both studies, extending earlier observations, is that the presence of AID is not strictly correlated with somatic hypermutation activity. In the work from Pasqualucci et al, it is shown that some AID-positive lymphomas apparently lack ongoing V gene mutation, and Forconi and colleagues report that AID is transcribed in 2 cases of hairy cell leukemia lacking V gene mutations and intraclonal diversity. Expression of AID does also not correlate with active class-switch recombination, because diffuse large-cell lymphomas of the activated B-cell subtype that show high AID levels mostly retain immunoglobulin M (IgM) expression. Moreover, several hairy cell leukemias analyzed by Forconi et al show an unusual coexpression of various heavy chain isotypes in single cells, likely mediated by differential splicing and not by class-switch recombination, although expressing AID and germ-line heavy chain content region transcripts, which usually initiate class switching.FIG1
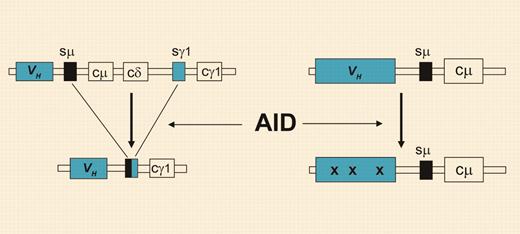
Class-switch recombination and somatic hypermutation. Through class-switch recombination (left), the originally expressed Cμ and Cδ heavy chain constant region genes (coding for IgM and IgD, respectively) are replaced by one of the downstream CH genes. The process of somatic hypermutation (right) introduces mainly point mutations (denoted by “x”) at very high rates, specifically into the Ig variable region genes. Both processes strictly depend on AID activity but can occur independently in B cells.
Class-switch recombination and somatic hypermutation. Through class-switch recombination (left), the originally expressed Cμ and Cδ heavy chain constant region genes (coding for IgM and IgD, respectively) are replaced by one of the downstream CH genes. The process of somatic hypermutation (right) introduces mainly point mutations (denoted by “x”) at very high rates, specifically into the Ig variable region genes. Both processes strictly depend on AID activity but can occur independently in B cells.
That expression of AID is obligatory but not sufficient for hypermutation and class switching in B-cell non-Hodgkin lymphoma is perhaps not so surprising when one considers that in humans, there are many mutated IgM+ (ie, non–class switched) B cells1 and that class switching is known to take place also in T-cell–independent immune responses that are not associated with hypermutation of V genes. There is also indication that class switching takes place in secondary immune responses in activated memory B cells, mainly outside the GC.2 Hence, AID expression is not a strict marker for GC B cells, and AID activity can be stringently and differentially regulated, perhaps by association with specific cofactors for hypermutation and class switching and regulated intracellular localization.3,4
Another seemingly puzzling finding is the detection of intraclonal V gene diversity in cases that lack detectable AID expression. However, it has to be considered that both reverse transcription–polymerase chain reaction and Western blot studies detect AID expression only if a large fraction of cells expresses AID and may therefore miss AID expression if restricted to a small fraction of cells (the antibodies described by Pasqualucci et al unfortunately do not stain on tissue sections). Moreover, it is important to distinguish between the detection of intraclonal V gene diversity and active somatic hypermutation. It may well be that in some lymphomas, somatic hypermutation is still active in an early phase of lymphoma clone expansion, causing intraclonal V gene diversification, but that in later phases of the disease, hypermutation activity is shut down.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal