Abstract
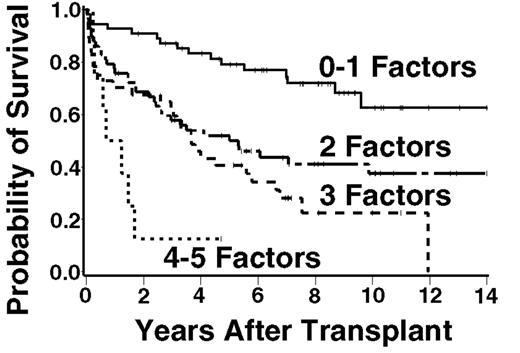
The FLIPI has recently been demonstrated to correlate with survival in patients (pts) with newly diagnosed follicular lymphoma (FL). No such index has been developed or evaluated to predict outcome for FL pts in the setting of myeloablative therapy and ASCT, despite data suggesting that ASCT may improve overall and progression-free survival (PFS). We examined the factors that contribute to the FLIPI as well as other factors assessed at time of transplant for their association with overall survival (OS) in 189 pts undergoing ASCT for FL. Baseline characteristics included: median age = 47 years (range, 24 – 64), stage III–IV = 94%, >4 nodal areas = 7.7%, elevated LDH = 30%, >5 cm maximal bulk of disease = 18%, chemoresistant disease = 13%, median number of prior chemotherapy regimens = 2. The FL histologies included: Grade 1 (49%), Grade 2 (31%), Grade 3 (13%), and transformation to diffuse large B-cell lymphoma (6%). Patients were conditioned with chemotherapy-only (21%), chemo+TBI (45%), or radioimmunotherapy +/− chemo (34%). Among all pts, the five-year estimated OS and PFS are 58% and 39%, respectively, with a median follow-up among surviving pts of 8 years (range, 1 – 18). The five factors that were found to be most significantly associated with OS include two FLIPI factors [age, hazard ratio for death (HR) = 1.37 per ten-year increase in age; elevated LDH, HR = 1.57] and three other clinical factors [>1 maximal extranodal site of disease, HR = 1.67; ≥2 prior chemotherapy regimens, HR = 1.99; chemoresistant disease, HR = 2.8]. Patients with 0 – 1 adverse factors (with age dichotomized as < 45 vs. ≥ 45) had an estimated 5-year OS of 79%, those with 2 factors 50%, 3 factors 41%, 4 or 5 factors 13% (Figure). Although prospective validation of this proposed model is required, this approach may be used to counsel FL pts regarding expected outcome following ASCT, to compare data between trials, and to design future studies.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal