Abstract
In high risk Essential Thrombocythemia (ET) there is a indication for cytoreductive therapy. However, especially in younger patients long-term use of alkylating agents or hydroxyurea is a matter of concern. Modification of the pharmacokinetic profile of interferon α, a non-leukemogenic agent, through addition of a polyethylenglicol (pegylation) has resulted in slower absorption and lower elimination rate, thus enabling a weekly application with potentially increased compliance compared to conventional interferon α.
In this phase II study we evaluate the safety, tolerability and efficacy of pegylated Interferon α (PegIntron, Essex Pharma) in high risk essential thrombocythemia. PegIntron is administered subcutaneously at a starting dose of 50 μg weekly. In patients not achieving a platelet count < 500 G/l after 8–12 weeks, the dosages can be increased by 25 μg/week up to 150 μg/week. Between 12/01 and 09/03 a total of 36 high risk ET patients (either platelet > 1500 G/l, or age> 60 years, or previous ET related complications) were enrolled by a total of 16 centers (16 male and 20 female patients, median platelet count at diagnosis 900 G/l, median age at diagnosis 54 years [range: 24–72 years]). Treatment was indicated because of at least one of the following reasons: Age > 60 years (38%), platelet count > 1500 G/l (36%) or previous ET related complications (50%). 10 patients (28%) were previously treated (hydroxyurea: 8 patients, anagrelide: 2 patients).
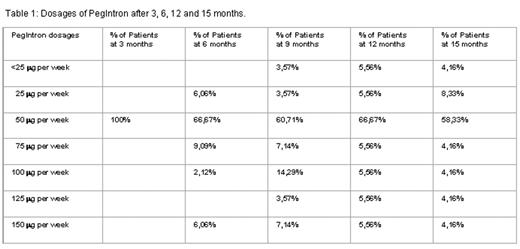
In this analysis there are 35 of 36 patients evaluable, 1 patient was lost to follow-up. Of these 35 patients, 24 (69%) are still on therapy with PegIntron after a median observation time of 18 months. The individual dosages of PegIntron are shown in table 1. A platelet count < 500 G/l was achieved in 57% of the patients after 3 months and in 75% and 89% after 6 and 12 months, respectively.
In 9 patients (26%) therapy was stopped because of drug related side effects (alopecia: 2 patients, arthralgia: 2 patients, flu-like symptoms: 2 patients, psoriasis: 1 patient, depression: 1 patient, diarrhea: 1 patient). In one patient an alternative cytoreductive therapy was initiated because of an insufficient platelet control. One patient suffered from a cerebral stroke and subsequently was taken off PegIntron application. After a median observation time of 18 months, we observed one potentially ET related complication (stroke) during PegIntron therapy so far.
These preliminary data indicate the efficacy of PegIntron in the treatment of high risk ET at relatively low doses and reasonable toxicity and safety compared to conventional interferon α.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal