Abstract
There exists controversy regarding the benefit of HSCT in infant ALL. At our institution, the standard treatment for high-risk infant ALL has been intensive chemotherapy per POG 9407 followed by HSCT in CR1. We retrospectively reviewed the outcome of the 15 patients (9 males, 6 females) with infant ALL that underwent allogeneic HSCT since 1992. We defined “very high-risk” as an infant with one or more of the following criteria: age 6 months or less at diagnosis, MLL+ or t(4:11), or WBC>100,000 at diagnosis. Fourteen of fifteen patients are considered “very high-risk.” All except one were under 12 months at diagnosis (range 2–14 months, median 7). The fourteen month old was included as she displayed infant ALL biology, with MLL+ and WBC>500,000. Seven were 6 months or under at diagnosis. Cytogenetics at diagnosis showed MLL+ or t(4:11) in 9, normal in 4, and indeterminate in 2 patients. WBC count at diagnosis was 4,300–1,000,000 (median 360,000). Twelve of the patients were CALLA-negative and four had CNS involvement at diagnosis. Time from diagnosis to transplant was 2.3–13.6 months (median 4.2). All patients were in morphologic CR1 at time of HSCT but one patient had persistent t(4:11).
Cytoreduction: Patients received TBI 150cGy x 8 (d-10 to-7); VP-16 1g/m2 CI (d-5 to-4); cyclophosphamide 60 mg/kg/day x 3(d-4 to-2). GVHD prophylaxis: CSA, short-course MTX, and ATG d +1, +3, +5, +7 (for unrelated cord blood (UCB)). Grafts were not T-cell depleted.
Stem cell sources: 8 UCB, 6 HLA-identical sibling bone marrow, 1 HLA-identical sibling PBSC. Days to neutrophil engraftment was 11–25 days (median 16) and to platelet engraftment was 17–82 days (median 37). CNS prophylaxis consisted of intrathecal methotrexate and Ara-C monthly x 6 post-HSCT. Acute GVHD: 2 grade II and 1 grade III. Chronic GVHD: 3 limited, 1 extensive.
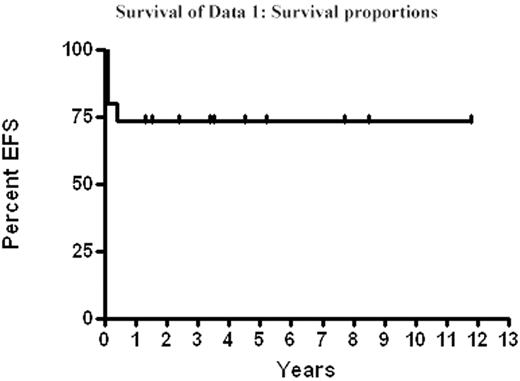
Mortality: 4 patients have died. 2 (13%) of transplant-related mortality (TRM) within 100 days of HSCT and 2 of relapse (15% of evaluable patients), which included the patient with t(4:11) at time of HSCT. Range of follow-up is 0.1–11.8 years with a 73% event-free survival (EFS) and overall-survival at a median follow-up of 3.5 years. We conclude that patients with infant ALL who attain a CR1 (morphologic and cytogenetic) have good EFS with acceptable TRM following HSCT using either UCB or HSC from a matched-sibling. Long-term follow-up, including developmental assessments, are being pursued to document the impact of this intensive therapy in young children.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal