Abstract
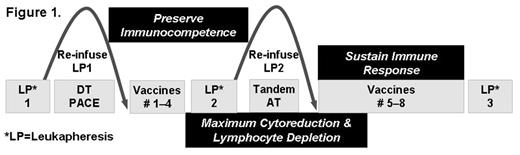
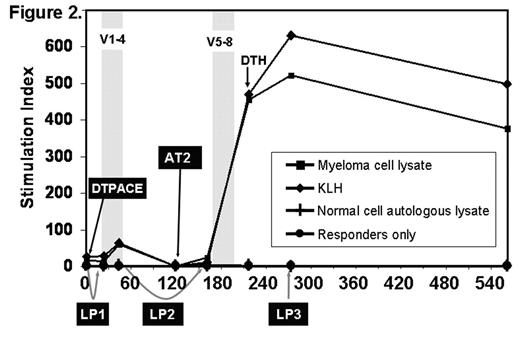
Although high dose chemotherapy and autologous transplantation (AT) can induce complete remission in approximately 50% of multiple myeloma (MM) patients, the majority eventually relapse. We hypothesize that a combination of AT and vaccination may sustain remissions by inducing specific cytotoxic T cells against chemotherapy resistant plasma cells. Vaccinations are typically given after AT when the immune system is very defective. We tested the novel strategy of priming anti-myeloma T-cells before AT and protecting the primed T-cells from high-dose chemotherapy by apheresis and cryopreservation of the primed cells. After high-dose chemotherapy induced cytoreduction, the primed T-cells were returned early after AT and preferentially expanded by a series of booster vaccinations (figure 1). The vaccines are monocyte derived-dendritic cells (DC) loaded with myeloma lysate (ML) and tracer antigen keyhole limpet hemocyanin (KLH), matured with trimeric CD40-ligand, administered intranodally, and followed by 5 days of low dose subcutaneous IL-2. Four patients with poor prognosis MM (characterized by abnormal metaphase cytogenetics) have been vaccinated and three are evaluable (the 4th patient is 60 days post AT2 and only 14 days post vaccine 8). ML specific immune responses were detected by delayed type hypersensitivity reactions and proliferation in 2/3 and by ELISPOT in 3/3 patients. Strong responses to KLH were observed in 3/3 patients. The pattern of response is a dramatic and rapid increase in ML specific T cell proliferation (figure 2) and IFN-g secretion after LP2 infusion plus booster vaccinations post AT, suggesting that the primed T-cells had been protected by collection and storage during AT. A high pre-existing ML specific antibody response was observed in 2/3 patients and a low antibody response pre-vaccination was clearly boosted in a third patient. The patient receiving the lowest DC dose (50 versus 115 and 139 x106) had a response to ML only detected by IFNg secretion assays, but is in near complete remission, 719 days post AT2, while disease progression was observed in the other two patients 496 and 626 days after AT. Our immune monitoring data suggest that the problem of applying vaccination in heavily treated patients may be overcome by early vaccination, collection and subsequent reinfusion of primed cells followed by repeated booster vaccination. Optimization of the vaccine regimen by increasing the frequency of vaccination or the use of highly immunogenic, well characterized tumor-associated antigens may further improve efficacy. We have an ongoing trial for high risk MM patients utilizing NY-ESO-1 and MAGE-A3 peptide vaccines based on a similar strategy to preserve immune competence.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal