Abstract
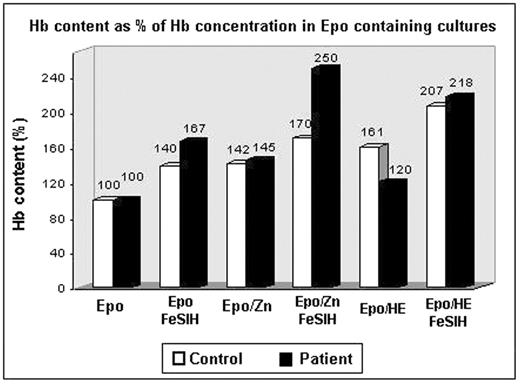
Disorders of iron metabolism and homeostatis belong to the most common diseases in humans; however, neither the regulation of iron metabolism in erythroid cells nor the pathophysiology of these diseases are completely understood. Last year we reported at this meeting a case of severe hypochromic microcytic anemia caused by a homozygous mutation in the divalent metal transporter 1 (DMT1) gene (DMT1 1285G>C). The defective growth of this patient’s erythroid progenitors in vitro was rescued by the addition of 10 μM iron-salicylaldehyde isonicotinoyl hydrazone (Fe-SIH), an iron chelate that has been shown to deliver iron for heme synthesis without involving the transferrin receptor/DMT1 pathway. To further characterize the impact of the DMT1 mutation on the in vitro growth of erythroid progenitors, 10 μM Fe-SIH, 25 μM hemin or 10 μM zinc chloride (ZnCl2) were added to the erythropoietin (Epo) containing cultures. All substances increased the plating efficiency in normal control as well as mutant BFU-Es. Moreover, all substances led to a 1.4 to 1.6 fold increase in hemoglobin (Hb) content in normal BFU-Es. Although hemin did not significantly increase Hb content in the patient’s BFU-E, the addition of Fe-SIH to the Epo/hemin-containing cultures increased Hb content 2.2 fold. Since Hb synthesis in heme-deficient erythroid cells is inhibited by heme-regulated inhibitor (HRI) kinase, which specifically phosphorylates the α subunit of the eukaryotic initiation factor 2 (eIF2α), we have analyzed the level of eIF2α phosphorylation in cytospine-harvested BFU-Es. Immunofluorescence analysis of erythroid cells with phospo-eIF2α (Ser51) antibody revealed increased phosphorylation of eIF2α in mutant BFU-Es compared to control BFU-Es grown under all aforementioned conditions. The most striking differences were observed in Epo and Epo/hemin containing cultures, in which the fractions of phospho-eIF2α-positive cells were significantly higher in mutant BFU-Es. These data suggest that the block of terminal differentiation/hemoglobinization in the DMT1-mutant erythroid cells is only partially caused by translational arrest of globin chain synthesis; the delivery of iron may be required for other cellular functions apart from Hb synthesis. Interestingly, the most prominent positive effect on the proliferation (i.e., BFU-E cellularity) as well as hemoglobinization of mutant BFU-Es had a combination of Epo/Fe-SIH and ZnCl2; this combination led to the highest increase in Hb content that was associated with almost complete lack of eIF2α phosphorylation. This suggests that the addition of Zn2+ to Fe-SIH containing cultures further stimulates processes that are initially rescued in DMT1-mutant erythroid cells by the addition of iron. We conclude, that the DMT1 defect in erythroid cells is complex, probably involving the combination of transcriptional and translational defects that cannot be rescued by hemin alone.
This work was supported by grant #IGA NR/7799-3 and by MSM 151100001.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal