Abstract
CLL is considered to be incurable disease by standard therapeutic approaches. We have demonstrated high remission rate including PCR negativity after combined therapeutic modality (rituximab, fludarabine, cyclophosphamide followed by HDT - BEAM - with ASCT) (Blood 2002, 100:804a). We present here longer follow up of larger pts cohort.
Twenty four pts with B-CLL (median age 57 years (32–62)) have been recruited. Pts were eligible for study if they failed previous therapy, progressed without previous tx or if they exhibit at least two adverse prognostic factors at dg (elevated LDH or beta2microglobulin, adverse cytogenetic, diffuse involvement of BM, CD38 pos, low albumin l, short doubling time <1year). The mutational status was examined since 2002. The treatment protocol consists of 4 cycles of R-Flu/Cy (rituximab 375 mg/m2, fludarabine 3x25 mg/m2, cyclophosphamide 3x300 mg/m2), followed by mobilization regimen ESHAP with harvest of progenitor cells and HDT (BEAM) with ASCT. Pts were reassessed after R-Flu/Cy regimen and after HDT therapy. Molecular monitoring was based on CDRIII rearrangement assessment by PCR. Twenty patient were examined before therapy or were informative and they are followed. Fourteen out of 24 pts were treated because of disease progression or previous tx failure.
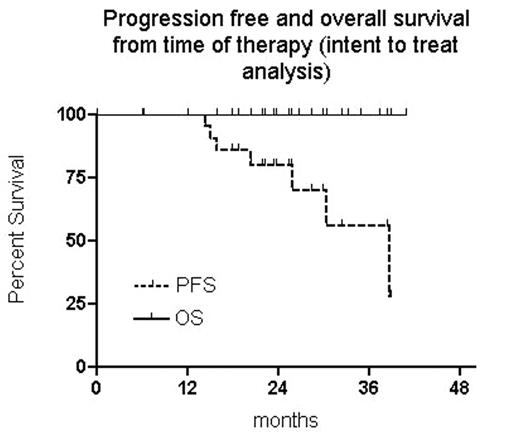
The assessment after R-Flu/Cy therapy revealed 6 CR, 14 nPR and 4 PR. Five out of 20 evaluable pts (25%) achieved PCR neg. Twenty three pts proceed to mobilization and the harvest was succesful in 19 (82,6%). Seventeen pts underwent HDT with ASCT (2 refusals). The assessment after HDT revealed 14 CR and one PR (2 pts with nPR have not been examined yet). Eleven out of 12 informative patients (91,7%) have reached PCR neg. after HDT. The clinical response rate based on intent to treat (ITT) analysis of all pts (24 treated with R-Flu/Cy, 23 mobilized, 17 transplanted, 15 reassessed after HDT) was as follows: 15 CR, 6 nPR and 3 PR. Based on ITT analysis 12 out of 20 informative pts (20 treated with R-Flu/Cy, 19 mobilized, 14 transplanted, 12 reassesed after HDT) have reached PCR negativity (60%). Seven relapses/progressions and no death were observed with median of follow up 26 months from start of tx and 37 from diagnosis. Six out of 7 relapses were found as unmutated CLL, three occurred in patient without HDT and ASCT (including the only one mutated case). Out of 5 progressions in PCR informative cases, 3 occurred in PCR neg group (25%) and 2 in PCR pos. pts (25%). The 2y PFS probability from time of therapy is 80% (CI 95%, 63%–97%) and 4 y PFS probabily from dg is 78% (CI 95%, 59%–98%).
Conclusion: This combined therapeutic approach is very well tollerated and has a high response rate including molecular biology responses. Relapses however have been observed regardless PCR status and they probably occur mainly in unmutated cases.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal