Abstract
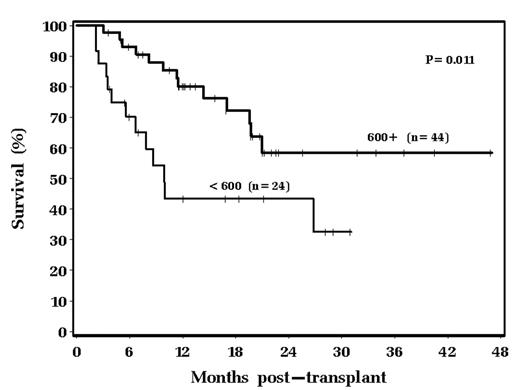
Response to chemotherapy and LDH level at the time of transplant are generally accepted as two variables predictive of clinical outcome in autologous peripheral blood progenitor cell (PBPC) transplantation (ABMT) for diffuse large B cell lymphoma (DLBCL). Several years ago we anecdotally noted that some patients suffered ongoing hypogammaglobulinemia after ABMT. We therefore embarked on a study examining pre and post ABMT IgG levels to determine possible prognostic volume of immunoglobulin levels. This study focuses on 68 patients with relapsed/refractory chemosensitive DLBCL undergoing autologous transplant from 4/2000 to 1/2004. All received PBPCs alone for hematopoietic support, and all received a preparative regimen consisting of Bu/Cy/VP. Median age was 55; patients received a median of 2 courses of prior chemotherapy; 44% received prior radiation therapy; 60% were male; 25% had tumor bulk greater than 10 cm in greatest diameter; 22% had elevated LDH at the time of transplant. Median PBPC dose infused was 7.5 x 106 CD34+ cells/kg. Median pre-transplant IgG level was 689 mg/dL (institutional normal range 717–1411 mg/dL). Six weeks post transplant the median IgG level had fallen to 587 mg/dL. There is a statistically significant difference in survival stratified by baseline IgG: 43% of patients with an IgG level less than 600 mg/dL were alive 18 months post transplant, as compared with 72% with a baseline IgG greater than 600 mg/dL (p=0.011). This is shown graphically below:
18 month relapse-free survival was 44% in the less than 600 mg/dL group versus 63% in the greater than 600 mg/dL group. 13 of 24 in the less than 600 mg/dL group have died (54%) versus 13 of 44 (30%) in the greater than 600 mg/dL group. The majority of these deaths are from disease relapse, however, the relapses occurred faster in the less than 600 mg/dL group. 5 of 7 relapse related deaths occurred by day +200 in the less than 600 mg/dL group, compared to 1 of 10 in the greater than 600 mg/dL group (p=0.009). Additionally, 4 patients in the less than 600 mg/dL group died of diffuse interstitial pulmonary toxicity between day 100 and 165 post chemotherapy. We conclude that pre-transplant hypogammaglobulinemia may be related to a more refractory underlying disease and is associated with the potential for rapid relapse after ABMT. Additionally, those patients entering an autologous transplant with hypogammaglobulinemia are at risk for later non-specific pulmonary toxicity.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal