The Wiskott-Aldrich syndrome protein (WASp) is a key regulator of actin polymerization in hematopoietic cells. The dynamic nature of cytoskeletal changes during a variety of cellular processes demands complex mechanisms for coordinated integration of input signals, precise localization within the cell, and regulated activation of the Arp2/3 complex. Mutations in the Wiskott-Aldrich syndrome gene either inhibit or dysregulate normal WASp function, resulting in clinical diseases with complex and disparate phenotypes. This review highlights recent advances that have enhanced our understanding of the mechanisms by which these molecular defects cause hematologic and immunologic disease.
Introduction
The Wiskott-Aldrich syndrome protein (WASp) is the 502–amino acid product of the gene defective in an X-linked inherited immunodeficiency known as the Wiskott-Aldrich syndrome (WAS).1-3 WASp is expressed only in the cytosol of hematopoietic cells, and is directly responsible for regulating the dynamic actin cytoskeleton during a wide diversity of cellular activities. The majority of WAS gene mutations result in loss of protein function and give rise to a spectrum of clinical disease dictated by the severity or location of the molecular defect and probably by secondary genetic and environmental factors. Classical WAS is characterized by recurrent infections, eczema, and microthrombocytopenia. Within this group of patients, a moderate to severe bleeding disorder is invariable, although other manifestations are heterogeneous in terms of timing and tissue specificity. In general, affected patients demonstrate both cellular and humoral immunodeficiency, are susceptible to bacterial, viral, and fungal infections, and in addition, are prone to autoimmune disease and hematologic malignancy.4-6
At the other end of a phenotypic continuum arising from molecular defects in the same gene, patients with X-linked thrombocytopenia have a less severe bleeding diathesis without the overt immunologic features of classical WAS, 7,8 and in the mildest cases, platelet counts can appear intermittently normal, although the mean platelet volume is invariably low.9 Recently, mutations that give rise to unregulated activation of WASp10 (Ancliff P.J., Blundell M.P., G.O.C., Calle Y., Kempski H., Toscano M., Jones G.E., Ridley A.J., Sinclair J., Kinnon C., Hann I.M., Gale R.E., de Botton S., W.V., Linch D.C., A.J.T., Unregulated activation of the actin cytoskeleton promotes haematopoietic cell death and causes X-linked neutropenia, manuscript submitted, September 1, 2004) have been shown to result in X-linked neutropenia, highlighting the complexity of mechanisms by which WASp can mediate cellular dysfunction.
Regulation of WASp activity
WASp and its more widely expressed homolog, N-WASp, contain several protein domains that regulate their activity and subcellular localization. These enable WASp to mediate actin polymerization in response to a variety of signals at the right time and in the right place (Figure 1). Many investigations into the regulation of WASp/N-WASp have studied one or the other protein, however, the overall similarities between the 2 proteins and comparable results observed suggest that they are regulated in a similar fashion. WASp/N-WASp and the related WAVE (WASp family verprolin-homologous protein, also called Scar) proteins are characterized by a C-terminal tripartite VCA domain (verprolin homology, central, acidic) that is capable of activating the actin related protein 2/3 (Arp2/3) complex (Figure 1) and thereby initiating the formation of new actin filaments.15 Homologous CA domains are found in the ActA16 and RickA17 surface proteins of the intracellular pathogens Listeria monocytogenes and Rickettsia conorii, respectively. These domains activate the host's Arp2/3 complex forming propulsive F-actin tails.18 N-WASp has also been found at the head of actin tails on pathogenic particles and endocytic and other subcellular vesicles, 19-22 suggesting a role for WASp family proteins in membrane trafficking.
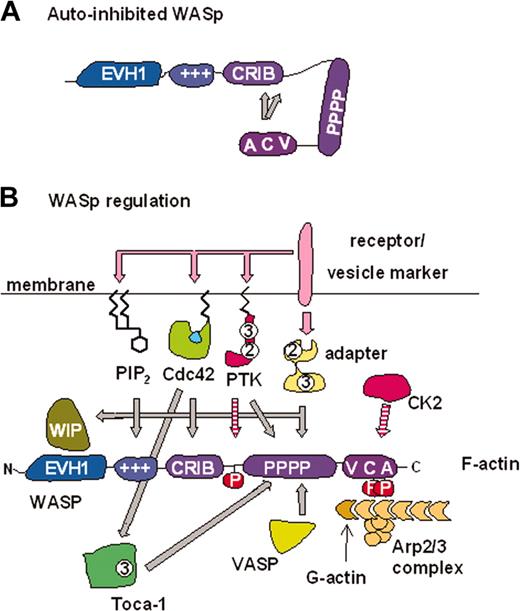
Mechanisms of WASp regulation. (A) Schematic representation of WASp autoinhibition. The VCA domain binds a region from residues 242 to 310, which includes the C-terminal part of the CRIB domain.11 The minimal high-affinity Cdc42 binding site is WASp 230 to 288.12,13 (B) WASp is capable of interpreting signals from many different inputs. These inputs may regulate WASp directly and determine its subcellular localization (see “Regulation of WASp activity”). All regulators/modifications shown activate WASp/N-WASp activity in vitro, with the exception of WIP, which inhibits activation by Cdc42. However, this inhibition may be reversed in the presence of Cdc42–Toca-1 leading to enhanced N-WASp/WASp activity.14 The zigzag lines on PIP2, Cdc42, and protein tyrosine kinases (PTKs) represent lipid modifications responsible for membrane localization. Adaptor proteins such as Nck may also act through WIP to localize WASp. The interplay between these signals remains an important area of study. +++ indicates basic region; 2 indicates SH2 domain; 3 indicates SH3 domain; adaptor indicates, for example, Nck, Grb2, syndapin, intersectin, and PSTPIP1 (proline, serine, threonine-rich phosphatase interacting protein 1); P, phosphate; pink arrows, pathway leading to regulation (or production) of signaling molecule; hatched arrows, phosphorylation events; and solid gray arrows, direct interactions.
Mechanisms of WASp regulation. (A) Schematic representation of WASp autoinhibition. The VCA domain binds a region from residues 242 to 310, which includes the C-terminal part of the CRIB domain.11 The minimal high-affinity Cdc42 binding site is WASp 230 to 288.12,13 (B) WASp is capable of interpreting signals from many different inputs. These inputs may regulate WASp directly and determine its subcellular localization (see “Regulation of WASp activity”). All regulators/modifications shown activate WASp/N-WASp activity in vitro, with the exception of WIP, which inhibits activation by Cdc42. However, this inhibition may be reversed in the presence of Cdc42–Toca-1 leading to enhanced N-WASp/WASp activity.14 The zigzag lines on PIP2, Cdc42, and protein tyrosine kinases (PTKs) represent lipid modifications responsible for membrane localization. Adaptor proteins such as Nck may also act through WIP to localize WASp. The interplay between these signals remains an important area of study. +++ indicates basic region; 2 indicates SH2 domain; 3 indicates SH3 domain; adaptor indicates, for example, Nck, Grb2, syndapin, intersectin, and PSTPIP1 (proline, serine, threonine-rich phosphatase interacting protein 1); P, phosphate; pink arrows, pathway leading to regulation (or production) of signaling molecule; hatched arrows, phosphorylation events; and solid gray arrows, direct interactions.
WASp interacts with several regulators
Poly-proline binding proteins. WASp and WAVE proteins share a proline-rich region, shown to be required for the optimal actin polymerization activity of WASp23,24 and for efficient recruitment to the immune synapse (IS) formed between T cells and antigen-presenting cells (APCs)25,26 (Figure 2). The proline-rich sequence provides binding sites for the vasodilator-stimulated phosphoprotein (VASP), 23 known to promote the formation of filopodia (fingerlike protrusive actin structures believed to important for chemotaxis)30 and also Src homology 3 (SH3) domain–containing proteins such as the adaptor protein Nck, which can also stimulate N-WASp activity in vitro.31 The SH3 domains of several tyrosine kinases bind to WASp, 32-34 which may facilitate localization and regulation by phosphorylation (see “Phosphorylation”). Importantly, several endocytotic proteins including syndapin and intersectin contain SH3 domains that bind WASp/N-WASp, suggesting a role by which WASp/N-WASp may be functionally recruited to sites of membrane remodeling.35-39
Multiple pathways recruit and activate WASp for normal IS assembly. In normal lymphocytes, WASp is recruited to the IS following TCR ligation. (A) Zeta-associated protein 70 (Zap 70) has been shown to localize WASp, after CD3 stimulation, by 2 separate mechanisms.25,57 The first is via EVH1 domain interaction with WIP and formation of a Zap 70-CrkL-WIP-WASp complex27 ; the second is through direct interaction of the proline-rich region (PPR) of WASp with the SH3 domain of Nck.25,28 The adaptor protein SLP76 is phosphorylated by Zap 70 (red hatched arrow) and binds both Nck and Vav-1 (a Cdc42-interacting guanosine diphosphate [GDP]–GTP exchange factor), thereby localizing both WASp and GTP-bound Cdc42 to the IS. (B) A Cdc42-independent route of WASp activation has recently been reported. Fyn phosphorylates WASp at Y291 (red hatched arrow) following TCR stimulation and is critical for multiple TCR-induced WASp effector functions.29 WASp activity can be down-regulated by PST-PEST–mediated dephosphorylation (yellow hatched block), through CD2AP/PSTPIP1, 26,29 indicating that the state of phosphorylation alone can regulate WASp activity.
Multiple pathways recruit and activate WASp for normal IS assembly. In normal lymphocytes, WASp is recruited to the IS following TCR ligation. (A) Zeta-associated protein 70 (Zap 70) has been shown to localize WASp, after CD3 stimulation, by 2 separate mechanisms.25,57 The first is via EVH1 domain interaction with WIP and formation of a Zap 70-CrkL-WIP-WASp complex27 ; the second is through direct interaction of the proline-rich region (PPR) of WASp with the SH3 domain of Nck.25,28 The adaptor protein SLP76 is phosphorylated by Zap 70 (red hatched arrow) and binds both Nck and Vav-1 (a Cdc42-interacting guanosine diphosphate [GDP]–GTP exchange factor), thereby localizing both WASp and GTP-bound Cdc42 to the IS. (B) A Cdc42-independent route of WASp activation has recently been reported. Fyn phosphorylates WASp at Y291 (red hatched arrow) following TCR stimulation and is critical for multiple TCR-induced WASp effector functions.29 WASp activity can be down-regulated by PST-PEST–mediated dephosphorylation (yellow hatched block), through CD2AP/PSTPIP1, 26,29 indicating that the state of phosphorylation alone can regulate WASp activity.
Cdc42. The role of WASp in cytoskeletal regulation is suggested by the presence of a CRIB (Cdc42- and Rac-interactive binding) domain that specifically mediates binding to active guanosine triphosphate (GTP)–bound Cdc42.12,40 Cdc42 is a member of the Rho family of GTPases and regulates the formation of filopodia and cell-substrate adhesions, and controls cell polarity/chemotaxis.41 Initial studies showed that N-WASp cooperated with Cdc42 to induce filopodia42 and that Cdc42 was capable of stimulating WASp/N-WASp activity in actin polymerization assays.43,44 In the absence of Cdc42, the CRIB domain maintains an autoinhibited WASp conformation by binding the VCA domain11 (Figure 1). In the resultant “closed” hairpin structure, the VCA domain is prevented from activating the Arp2/3 complex. Active Cdc42 stimulates WASp by binding its CRIB domain with high affinity, thereby displacing the VCA domain and facilitating a productive interaction with the Arp2/3 complex. The ability of some SH3 domains to activate WASp in place of Cdc42 may reflect alternative mechanisms of destabilizing the autoinhibited conformation (discussed in “Phosphorylation”).
Phosphoinositides. WASp/N-WASp contain a highly basic region N-terminal to the CRIB domain, that in the case of N-WASp binds phosphatidylinositol (4,5) bisphosphate (PIP2).45,46 It remains unclear whether this is the PIP2 binding site in WASp.44 However, PIP2 synergizes with Cdc42 to fully activate WASp/N-WASp in vitro, 44,46 providing a route through which WASp could respond to localized membrane composition. Remarkably, the effects of other phospholipids have been scantly studied.
Phosphorylation. Tyrosine phosphorylation is an important regulatory mechanism for WASp activity. WASp is tyrosine phosphorylated following collagen stimulation of platelets, 47,48 immunoglobulin E (IgE) receptor stimulation of mast cells, 49 and T-cell–receptor (TCR) stimulation.29 A single tyrosine phosphorylation site has been characterized—WASp Y291 (Y256 in N-WASp), which is a target for Src family kinases, Btk, and the protein tyrosine phosphatase (PTP)–PEST.29,50,51 Phosphorylation of this residue disrupts the autoinhibited conformation of WASp52 and activates WASp in vitro and in vivo.51,52 Tyrosine phosphorylation may also pave the way for subsequent activation by SH2 domain binding.52 Phosphorylation at Y291 is critical for multiple WASp effector functions downstream of the T-cell receptor including efficient actin polymerization and IS formation29 (Figure 2). WASp and N-WASp are also phosphorylated on serine residues that lie at the junction of their C and A domains. This is mediated by CK2 and is required for optimal WASp activity.53
WIP. (N-) WASp contains an Ena/VASP homology 1 (EVH1) domain at its N-terminus. This domain binds the verprolin homolog WIP (WASp-interacting protein), 54,55 a widely expressed protein that stabilizes actin filaments and regulates actin polymerization.56 WIP is crucial for localizing WASp/N-WASp activity both in a vaccinia-based actin motility system and to the IS after T-cell–receptor ligation.27,57 In addition, WIP synergizes with N-WASp to induce filopodia when overexpressed in fibroblasts, 58 indicating that WIP/WASp-family interactions can mediate the localized formation of specific actin structures. However, WIP inhibits N-WASp activity in actin polymerization assays by antagonizing the function of Cdc42, 58 perhaps by stabilizing the autoinhibited conformation.
Toca-1. Recently, the Toca-1 protein (transducer of Cdc42-dependent actin assembly) was identified as a crucial intermediate required for Cdc42–N-WASp–Arp2/3 complex–induced actin polymerization in cell extracts, and forms an additional link between Cdc42 and N-WASp.14 Importantly, the ability of Toca-1 to mediate Cdc42-induced activation of purified N-WASp is evident only when N-WASp is complexed with WIP. As the majority of cellular WASp/N-WASp is also complexed with WIP (or the related CR16 protein), 59 this new finding underscores the importance of the WASp/N-WASp–WIP interaction in regulating actin dynamics.
WASp integrates several signals
How the full-length WASp molecule funnels such a diverse array of signals into the coordinated activation of the Arp2/3 complex is still far from fully understood. Cdc42 binding is required for tyrosine phosphorylation of autoinhibited C-terminal WASp fragments in vitro, 52 but not for WASp phosphorylation in vivo.29,51 In addition, the CRIB region of WASp is dispensable for TCR responses in murine thymocytes, whereas phosphorylation of Y291 is required.29 Thus it appears that WASp has the capacity to respond to distinct signals via different molecular interactions. It is worth pointing out that there is likely to be more to the regulation of WASp than merely controlling the accessibility of its VCA domain. Several groups have found that the full-length WASp molecule is a far more potent actin polymerizing molecule than the isolated VCA domain24,44,53 and that N-WASp harbors a second distinct Arp2/3 complex binding site.45,60 Therefore, it remains possible that the nature of the f-actin structures formed by WASp may be influenced by the subset of regulatory interactions involved.
WAS gene defects causing human disease
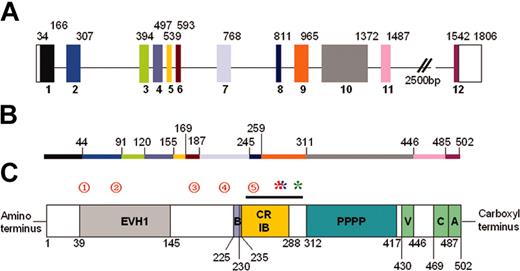
Approximately 300 unique mutations have been reported in the WAS gene (Figure 3), spanning all 12 exons, but clustered in the first 4 (Imai et al6 ; WASpbase: http://homepage.mac.com/kohsukeimai/wasp/WASPbase.html; and Jin et al62 ). In general, missense mutations in the first 3 exons usually are associated with XLT or attenuated WAS, while missense mutations elsewhere and other types of mutations (found predominantly in exons 6-11) are associated with a variable phenotype, indicating that mutation analysis alone is insufficient as a prognostic tool.63-65
WASp genomic organization and protein structure. (A) Schematic diagram of WASp genomic structure. The 12 exons are colored and numbered. Unshaded blocks represent uncoded regions. Numbers above the blocks indicate base pairs. (B) Line diagram representing the WASp protein. Colored regions correspond to encoding exons, and numbers indicate amino acids. (C) Schematic diagram representing the WASp protein. The EVH1, 54 basic (B), minimal high-affinity Cdc42-binding site (CRIB), 12,13 poly-proline (PPPP), and VCA61 domains/regions are shown. Amino acids are numbered below the protein. The black line above the CRIB domain indicates the VCA binding region (amino acids 242-310).11 Red numbered circles denote mutational hotspots62 as follows: 1 indicates T45M; 2, R86S/G/C/H/L; 3, IVS6 (+5g>a); 4, R211X; and 5, IVS8 (+1g>a/c/t). Mutations resulting in constitutive activation are indicated by asterisks: red indicates L270P10 ; blue, L272P (P. Ancliff and A.J.T., unpublished, September 25, 2003); and green, I294T (Ancliff P.J., Blundell M.P., G.O.C., Calle Y., Kempski H., Toscano M., Jones G.E., Ridley A.J., Sinclair J., Kinnon C., Hann I.M., Gale R.E., de Botton S., W.V., Linch D.C., A.J.T., Unregulated activation of the actin cytoskeleton promotes haematopoietic cell death and causes X-linked neutropenia, manuscript submitted, September 1, 2004).
WASp genomic organization and protein structure. (A) Schematic diagram of WASp genomic structure. The 12 exons are colored and numbered. Unshaded blocks represent uncoded regions. Numbers above the blocks indicate base pairs. (B) Line diagram representing the WASp protein. Colored regions correspond to encoding exons, and numbers indicate amino acids. (C) Schematic diagram representing the WASp protein. The EVH1, 54 basic (B), minimal high-affinity Cdc42-binding site (CRIB), 12,13 poly-proline (PPPP), and VCA61 domains/regions are shown. Amino acids are numbered below the protein. The black line above the CRIB domain indicates the VCA binding region (amino acids 242-310).11 Red numbered circles denote mutational hotspots62 as follows: 1 indicates T45M; 2, R86S/G/C/H/L; 3, IVS6 (+5g>a); 4, R211X; and 5, IVS8 (+1g>a/c/t). Mutations resulting in constitutive activation are indicated by asterisks: red indicates L270P10 ; blue, L272P (P. Ancliff and A.J.T., unpublished, September 25, 2003); and green, I294T (Ancliff P.J., Blundell M.P., G.O.C., Calle Y., Kempski H., Toscano M., Jones G.E., Ridley A.J., Sinclair J., Kinnon C., Hann I.M., Gale R.E., de Botton S., W.V., Linch D.C., A.J.T., Unregulated activation of the actin cytoskeleton promotes haematopoietic cell death and causes X-linked neutropenia, manuscript submitted, September 1, 2004).
More usefully, mutations resulting in absence of protein lead to severe clinical disease and, at a molecular level, complete abrogation of WASp-Arp2/3–mediated actin polymerization. As not all actin polymerization is WASp dependent, total cellular f-actin production can appear normal, 66 but spatially and temporally regulated cytoskeletal alterations in response to specific signals are compromised. Expression of a truncated protein similarly correlates with a severe clinical phenotype, but, in contrast, residual full-length protein is predictive of milder disease.6,63-65 In a study of 50 patients, this was associated with significantly fewer infections, less eczema, no malignancy, and longer survival.6 While this information is useful in the clinical setting, certain caution is required as the protein expression phenotype correlation is not absolute and patients with residual full-length protein may still develop severe manifestations.6 This presumably relates to the functionality of the residual protein, and in part to secondary genetic influences and environmental factors.
EVH1 domain mutations interrupt interaction with WIP
A significant proportion of WAS gene defects result in expression of protein with amino acid substitutions within the EVH1 domain69 (Figure 3). This region mediates interaction with WIP, which regulates several important effects on WASp-mediated actin polymerization, in particular spatial localization of WASp activity (see “WIP”). As the entire EVH1 WASp domain is required for WIP binding, 54 many disparate mutations throughout the length of the EVH1 domain are thought to perturb this interaction. Indeed, clinically relevant mutations that disrupt WIP/N-WASp binding have been demonstrated to abolish localization and subsequent actin polymerization, 57 providing a mechanism that could explain many of the cellular defects (see “The effects of WAS gene mutations on cellular function”). What remains unclear, however, is how mutations that appear to have similar biochemical effects result in such variable clinical severity.54,69 While part of the answer may be that specific mutations result in a variable ability to interact with WIP, 70 disruption of alternative EVH1 domain functions, such as transcriptional activity, 71 may also be contributory.
Mutations that result in unregulated activation of WASp
A novel WASp-related disease has been recently described, which is phenotypically quite unlike WAS either in its classical or attenuated form.10 There were 5 male members of a large kindred who presented with recurrent major bacterial infections, severe congenital neutropenia (associated with promyelocyte/myelocyte maturational arrest), and monocytopenia. Lymphocyte function was apparently normal. Thrombocytopenia was not a prominent feature, and platelets were of normal size. The genetic defect was identified as a point mutation in the WAS gene, leading to a single amino acid substitution, L270P (leucine 270 for proline). L270 has a key position in the CRIB domain (Figure 3) and its substitution with proline is predicted to impair VCA-CRIB domain interaction, thereby disrupting autoinhibition. Thus, a constitutively active form of WASp is produced, which mediates unregulated Cdc42-independent Arp2/3-activated actin polymerization.10 A second, unrelated patient with neutropenia, trilineage myelodysplasia, and an I294T point mutation has also been identified (Ancliff P.J., Blundell M.P., G.O.C., Calle Y., Kempski H., Toscano M., Jones G.E., Ridley A.J., Sinclair J., Kinnon C., Hann I.M., Gale R.E., de Botton S., W.V., Linch D.C., A.J.T., Unregulated activation of the actin cytoskeleton promotes haematopoietic cell death and causes X-linked neutropenia, manuscript submitted, September 1, 2004; Figure 3). The mechanism of disease in this case appears to be very similar, as I294 lies adjacent to the important phosphorylation site Y291, on the same face of the α-helix within the hydrophobic core of the CRIB domain, and its substitution with threonine is also predicted to disrupt autoinhibition. In cellular terms, the unregulated expression of WASp appears to enhance apoptosis particularly in myeloid progenitors, and may also interfere with cytokinesis (Ancliff P.J., Blundell M.P., G.O.C., Calle Y., Kempski H., Toscano M., Jones G.E., Ridley A.J., Sinclair J., Kinnon C., Hann I.M., Gale R.E., de Botton S., W.V., Linch D.C., A.J.T., Unregulated activation of the actin cytoskeleton promotes haematopoietic cell death and causes X-linked neutropenia, manuscript submitted, September 1, 2004).
The effects of WAS gene mutations on cellular function
Function of WASp during development and establishment of hematopoiesis
WASp is expressed in CD34+ hematopoietic precursors, as well as in lineage-committed cells, suggesting that it has a role in hematopoiesis and differentiation.72 In fact, WASp is expressed at the site of definitive hematopoiesis in the aorto-gonad-mesonephros (AGM) region of early human embryonic development.73 A recent study has demonstrated that lineage-negative bone marrow cells (a fraction enriched for hematopoietic precursors) isolated from WASp-deficient mice exhibit abnormal stromal cell-derived factor 1a (SDF1a)–stimulated cytoskeletal rearrangement and defective migration, both in vitro and in vivo.74 WASp appears to be important for effective migration of progenitor cells from the fetal liver to bone marrow, as progenitors with a normal copy of the WAS gene preferentially establish hematopoiesis in the bone marrow when in competition. At present it is unclear whether this has a significant bearing on disease pathogenesis, as WAS patients do not demonstrate a global defect in hematopoiesis (apart from predisposition to myelodysplasia). However, it suggests that apparent nonrandom X-inactivation in CD34+ precursors and in all subsequent lineages from WAS carriers75 results from a failure of stem cells with an active mutated WAS allele to transit successfully from the fetal liver to bone marrow, and not from a direct role for WASp in hematopoietic differentiation.
Destruction and abnormal platelet production lead to microthrombocytopenia
Microthrombocytopenia is the most consistent feature of WASp-associated disease, but, as yet, the mechanisms are poorly understood. The persistent occurrence of microthrombocytopenia even in the mildest patient phenotypes is explained by uniform absence of mutant WASp in platelets irrespective of molecular mutation or partial expression in other cell lineages, probably as a result of protein instability.76 Peripheral destruction (which is not usually immune mediated5 ) by the spleen makes a major contribution, as evidenced by substantial correction of the platelet count and size after splenectomy.77,78 It is likely that WASp-deficient platelets are cleared by splenic macrophages, perhaps in response to increased surface exposure of phosphatidylserine, but the escape of microplatelets albeit in small numbers is unexplained.79 It is possible that there is a selective physical restriction to transmigration of larger platelets with a compromised actin cytoskeleton through the splenic vasculature. In favor of this hypothesis, WASp deficiency in mice (which normally have smaller platelets than humans) induces only a marginal thrombocytopenia.67,68 Additional factors may however be contributory, as other studies have demonstrated a defect of platelet production. The mechanism of the latter finding remains unclear as there is no marked defect of megakaryocytopoiesis in vitro, and megakaryocytes (MKs) are usually found in normal numbers in vivo.80 Furthermore, proplatelet formation, which is dependent on dynamic actin polymerization and formation of branching structures, 80,81 and the size of the platelets formed are both normal in liquid culture (albeit in the absence of a normal extracellular and vascular environment). One explanation for these findings may be that MKs normally reside in the extravascular space of bone marrow, and depend on adhesion to the abluminal endothelial surface and transendothelial projection of cytoplasmic processes into the lumen for effective migration and/or proplatelet formation.82 The deficiency of important actin cytoskeletal structures such as filopodia (after adhesion to collagen or to convulxin, a glycoprotein VI ligand) and podosomes, would predict a contribution from defects of this process in vivo (W.V. and S. Sabri, unpublished observations, November 13, 2003). Failure of chemokine-mediated interaction of MK precursors with the endothelium may also participate, much in the same way that WASp-deficient T cells respond abnormally to SDF-1, which itself has been shown recently to contribute significantly to MK maturation and thrombopoiesis.83,84 The functionality of microplatelets in WASp deficiency is also unclear, although signaling downstream of tyrosine kinase–linked and G-protein–coupled receptors, Arp2/3 complex–mediated actin polymerization, and shape change appear normal.48,85
A prominent role for WASp in T-cell signaling
T-cell dysfunction is a major clinical component of WAS-associated immunodeficiency. Early studies on WASp-deficient T cells revealed abnormal morphology and both defective proliferation and actin rearrangement in response to CD3 coreceptor ligation.86-88 The fact that WASp is critical for cytoskeletal remodeling downstream of the TCR is highlighted by its role in formation of the IS—a specialized cell-cell contact site between T-cell and antigen-presenting cell (APC) that mediates the sustained cell-to-cell interaction necessary for optimal T-lymphocyte activation.25,89 In normal cells, TCR ligation leads to IS assembly by clustering of receptor and signaling molecules into membrane compartments called lipid rafts. Subsequent maturation of the IS results in a central concentration of TCRs and costimulatory molecules surrounded by a peripheral ring of the adhesion molecule lymphocyte function–associated antigen 1 (LFA-1).90
In the absence of WASp, IS formation is defective. At the cell surface during activation, WASp-deficient T cells have lower levels of the glycosphingolipid raft marker GM1, which additionally fails to cluster.89 They also are defective in polymerizing actin at the T cell–APC contact site following TCR ligation, either through CD3/CD28 or through CD2.26,89 In addition to, and presumably as a result of, these structural failings, T-cell signaling in WASp-deficient cells is disrupted, evidenced by failure to recruit molecules such as protein kinase C θ to the immune synapse, defective calcium influx after T-cell activation, and ultimately abnormal proliferative responses.26,89
A number of studies have investigated the signaling pathways by which WASp activity is recruited to the IS25-29,89 (Figure 3). WASp activity after TCR ligation appears to be regulated by several diverse upstream signals, including WIP, Cdc42, and SH3 domain–containing proteins. This is consistent with the complex nature of WASp regulation, although further work is required to delineate the relative importance of these various pathways in vivo. Surprisingly, little attention has been paid to the assembly of similar structures on the non–T-cell side of the IS, although clearly this will contribute to the outcome of cell-to-cell interaction. Recently it has been shown that WASp is necessary for APC cytoskeletal remodeling during formation of the dendritic cell (DC)–natural killer (NK) immunostimulatory synapse and for subsequent DC induction of NK-cell interferon gamma production and killing.91
The importance of WASp for T-cell development and function in vivo
Human WASp deficiency results in significant quantitative abnormalities within the peripheral T-cell compartment, which are manifest from infancy.92 This is particularly pronounced for naive CD4+ and CD8+ cells, suggesting abnormal thymopoiesis or peripheral homeostasis. However, the role of WASp during maturation of thymic immunity remains unclear. Variable degrees of thymic hypoplasia and involution have been reported in limited numbers of postmortem studies on human tissue.93,94 There have been 2 independently derived mouse models examined for the effects of WASp deficiency on lymphocyte development. While one study reported normal thymic subsets, 67 the other demonstrated a block in progression from double-negative (CD4–CD8–) to double-positive (CD4+CD8+) compartments and abnormalities in thymocyte proliferation and calcium influx after TCR ligation.68 In support of a role for WASp family proteins, if not WASp itself, during thymopoiesis, mice expressing VCA domain–deficient WASp (which likely has a dominant-negative effect by sequestering molecules that ordinarily bind to endogenous WASp family proteins) also demonstrate defective thymopoiesis and progression through the double-negative to double-positive stages.95
A number of recent studies demonstrating somatic mosaicism (resulting from spontaneous reversion of an inherited disease-causing genetic mutation) have provided important information on the role of WASp in human T cells.96-98 In one study, reversion was detected molecularly in both T and B lymphocytes (presumably due to reversion in a common lymphoid precursor), although only revertant T cells were detected in significant numbers peripherally.98,99 This demonstrates that a normal copy of the WAS allele confers clear survival or growth advantage to T, but not B, cells. Additionally, reversion events restore T-lymphocyte function with regard to CD3-stimulated proliferation and actin rearrangement and may result in significant improvement in clinical phenotype if a sufficient proportion of T cells express the reversion.96,97 These data indirectly indicate a key role for WASp in T-cell proliferation, survival, and function in vivo. In some in vitro studies, WASp-deficient lymphocytes have been shown to exhibit an increased rate of spontaneous apoptosis, 66,100 although another recent study has demonstrated normal survival of naive CD4+ cells.92 Several important issues therefore remain unresolved.
Is WASp important for B-cell function?
The importance of WASp for normal humoral immunity is less well established, partly because of difficulty separating the effects of intrinsic B-cell dysfunction from those of diminished T-cell help and also from architectural changes in secondary lymphoid tissue. However, WAS patients have reduced numbers of circulating B lymphocytes in infancy, poor lymph node follicle formation, and defective immunoglobulin responses to polysaccharide antigens in particular.4,92,101,102 As for other cells, cytoskeletal defects produce abnormalities of morphology, resulting in impaired chemotaxis and B-cell–receptor capping.68,103-105 Despite this, purified murine cells respond relatively normally to antigen receptor stimulation, and antigen presentation in vitro is also not impaired.67,106 Defective migration and/or localization of B cells in vivo may be at least partly responsible for the striking deficiency of splenic marginal zone B cells that has been observed in both human and mouse tissues, and which may therefore provide an explanation for suboptimal T-independent antibody responses.105,107 The modulation of B-cell–receptor signaling pathways by WASp may further contribute to normal human B-cell function, 33,50 although this has not been explored in detail.
Effect of WASp deficiency on cytotoxicity
Susceptibility of patients to viral infections (including herpes viruses) and lymphoreticular malignancy may in part reflect clinically significant cytolytic dysfunction, although this also has not been examined in detail. Recently, impaired actin polymerization and perforin accumulation at the NK-target contact point were shown to result in significantly reduced NK cytolytic activity.108 Functional defects may be in part compensated for by expanded NK cell populations in WAS patients.108
Trafficking and phagocytic defects in myeloid lineage cells
The involvement of the cytoskeleton in cell migration and during phagocytosis has been highlighted by quite profound abnormalities of myeloid cells, suggesting that a significant component of the complex immunodeficiency of WAS patients is attributable to defective cell trafficking, pathogen clearance, and uptake of particulate antigen. Several studies have investigated the functionality of WASp-deficient monocyte/macrophage and dendritic cells (DCs). Phagocytic cup formation and IgG-mediated and apoptotic cell phagocytosis are all impaired in WASp-deficient macrophages.109,110 The latter observation may have implications for initiation of some autoimmune phenomena as noted in other pathologic states in which clearance of apoptotic cells is compromised.111 WASp-deficient murine DCs also process particulate antigen abnormally, although the mechanism for this is not established.106 Migration of macrophages and DCs is defective at several levels. Chemotactic responses (directional motility in chemical gradients) of WASp-deficient monocytes and macrophages are abnormal in response to a variety of stimuli.112-114 In addition, multiple specific defects of migration-related cytoskeletal regulation have been reported, including abnormal actin distribution, protrusive activity, and cell polarization.109,113,115 Perhaps most strikingly, there is a complete failure to assemble specialized substratum contacts, called podosomes, which are sites of integrin localization thought to be important for adhesion and motility.115,116 The absence of podosomes in WASp-deficient murine osteoclasts also results in the failure to form normal sealing zones and translates into defective bone resorption when animals are challenged by oophorectomy.117 Whether these skeletal defects are clinically important in humans has not been investigated. Both macrophage and osteoclast podosome assembly and macrophage chemotaxis are restored in WASp-deficient cells by introduction of a normal copy of the WAS gene, causally linking WASp with these functions.117,118
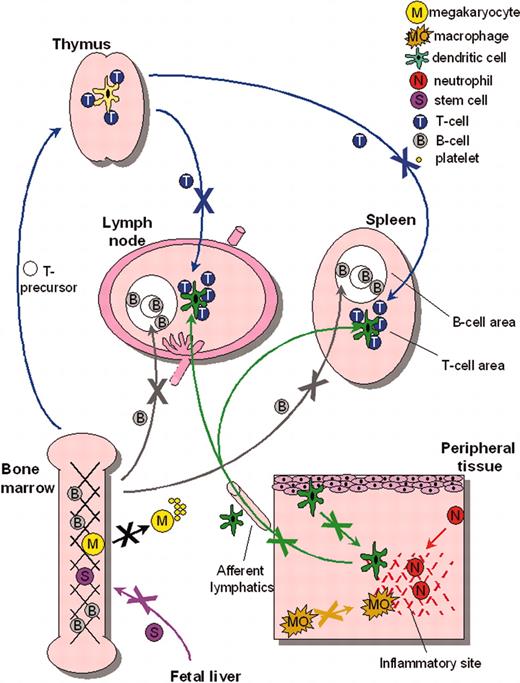
Correctable cytoskeletal defects have also been reported for WASp-deficient DCs, which similarly lack podosomes, and exhibit abnormal polarization and aberrant lamellipodia formation. Consequently, translocation on fibronectin is severely compromised.119,120 WASp-deficient DCs fail to cluster β2 integrins at podosome sites, with a resultant defect in adhesion to the endothelial adhesion molecule, ICAM-1.121 This may have relevance for trans-endothelial migration of DCs, for example during transit from skin to lymph vessels. Abnormalities of DC migration have been identified in vivo using a murine model (de Noronha S., Hardy S., Sinclair J., Blundell M.P., Strid J., Schulz O., Jones G.E., Katz D.R., Kinnon C., A.J.T., Impaired dendritic cell homing in vivo in the absence of Wiskott-Aldrich syndrome protein, manuscript submitted, August 9, 2004), underlining the fact that defective DC trafficking, and therefore impaired initiation of immune responses, is likely to contribute to the immunodeficiency associated with WAS in humans (and possibly to the development of autoimmunity). In fact, the identification of chemotactic or homing defects in WASp-deficient T cells, B cells, DCs, and stem cells74,83 Westerberg et al, 105 is supportive of a hypothesis that a more generalized defect in blood cell traffic is a major contributor to the pathogenesis of WAS122,123 (Figure 4).
Migratory defects in multiple WASp-deficient lineages. Multiple cell lineages, including stem cells, megakaryocytes, lymphocytes, and myeloid cells, have been shown to exhibit migratory deficiency either in vitro or in vivo. Together, these data indicate the importance of WASp for normal cell motility and suggest that dysregulated trafficking may be a major contributor to the pathogenesis of WASp-related human disease. Arrows depict transport through blood vessels unless otherwise indicated.
Migratory defects in multiple WASp-deficient lineages. Multiple cell lineages, including stem cells, megakaryocytes, lymphocytes, and myeloid cells, have been shown to exhibit migratory deficiency either in vitro or in vivo. Together, these data indicate the importance of WASp for normal cell motility and suggest that dysregulated trafficking may be a major contributor to the pathogenesis of WASp-related human disease. Arrows depict transport through blood vessels unless otherwise indicated.
Somewhat surprisingly, the function of WASp in neutrophils has not been clarified in functional studies even though it is expressed at high levels.76,61 Given that a proportion of WAS patients suffer from infections classically attributed to neutrophil dysfunction, it is likely that defects in this lineage are present.4,6 In fact, although defective Boyden chamber neutrophil chemotaxis was identified many years ago, it was correctable using normal serum, implicating an inhibitory factor in serum of WAS patients rather than an intrinsic neutrophil defect.124 Using more stringent tools for measurement of cell chemotaxis, neutrophils have more recently been shown to respond normally (in terms of directional response and speed of motility in Dunn chambers) to both formyl-methionyl-leucyl-phenylalanine and interleukin-8.114 This analysis was not, however, comprehensive, and the response to other chemoattractants has not yet been studied. Phagocytic defects have been reported for murine but not for human WASp-deficient neutrophils.68,101 Interestingly, normal neutrophils do not assemble typical podosomes, and deficiencies of adhesion in WASp-deficient cells have not been studied.
Murine WASp-deficient mast cells have been shown to exhibit abnormal IgE-induced cytoskeletal rearrangement, granule exocytosis, and cytokine production.125 Tyrosine phosphorylation of phospholipase Cγ and Ca2+ mobilization is defective after IgE receptor ligation, indicating a role for WASp in the signaling pathways downstream of Fcϵ receptor I. The reproducibility of these results in human mast cells and their clinical relevance will require further investigation.
Mechanisms for WAS-related autoimmunity and malignancy
The incidence of autoimmune disease associated with classical WAS is high (40% and 72% in 2 independent patient cohorts4,5 ) and is not necessarily associated with overall disease severity or complete absence of WASp expression.6 Surprisingly, therefore, this complication can also develop in patients with otherwise milder disease.69 Despite being an important cause of morbidity and mortality, there is no direct data to explain the mechanisms of WAS-associated autoimmunity. WASp may be required for normal thymic maturation (see “The importance of WASp for T-cell development and function in vivo”) and theoretically self-reactive T cells may escape negative selection because of defective TCR-induced apoptosis, as described for WASp-deficient murine lymphocytes.68 Impaired function of WASp-deficient T cells, B cells, macrophages, or DCs could all destabilize important mechanisms participating in the maintenance of normal tolerance.
There is a similar paucity of data available to explain the propensity for lymphoreticular malignancy (reported incidence: 13%-22%4,6 ). Immunodeficiency is likely to be contributory as a proportion of malignancies are associated with Epstein-Barr virus (EBV) and there may be specific surveillance defects (such as impaired NK or cytotoxic T-cell cytolysis).108 However, this is unlikely to be the full explanation, as the highest lymphoma risk (44%) may be conferred by a single splice site mutation that is otherwise associated with a mild clinical phenotype.126 Interestingly, there also appears to be a significant incidence of myelodysplasia in severe WAS patients. Although the mechanisms of malignancy remain undetermined, there may be a role for WASp in human cytokinesis and maintenance of genomic stability (Ancliff P.J., Blundell M.P., G.O.C., Calle Y., Kempski H., Toscano M., Jones G.E., Ridley A.J., Sinclair J., Kinnon C., Hann I.M., Gale R.E., de Botton S., W.V., Linch D.C., A.J.T., Unregulated activation of the actin cytoskeleton promotes haematopoietic cell death and causes X-linked neutropenia, manuscript submitted, September 1, 2004), as has been reported for the WASp homolog in fission yeast.127
Tailoring therapy for predictable phenotypes?
Management of patients with WAS continues to present significant challenges, particularly in attenuated phenotypes where the natural history of disease progression is unclear. Emerging data indicate that there is a reasonable correlation between expression of WASp and clinical severity, 6,62 which may therefore be a useful prognostic indicator, although this methodology needs to be standardized. However, the occurrence of autoimmune disease and IgA nephropathy in significant numbers of patients with residual protein expression and otherwise mild phenotypes6 suggests that this group is not necessarily immunologically benign and that close long-term monitoring is necessary. Splenectomy is an effective treatment for thrombocytopenia, but carries a significant long-term risk of sepsis and is therefore probably indicated only in severe disease where there is no prospect for curative intervention. Bone marrow transplantation is the only curative therapy at present, with good results for patients with HLA-matched family donors and young patients with HLA-matched unrelated donors but less satisfactory outcomes for other donor types.128 Gene therapy has attracted significant interest and encouraging data have emerged from a number of sources. Multiple individual cellular defects, including macrophage migration, DC cytoskeletal arrangement, and T-cell proliferation, have been corrected in human and murine cells in vitro through gene transfer.98,118,120,129 In addition, gene transfer to hematopoietic stem cells in murine models of WASp deficiency partially restores T-cell function in vivo.129,130 Whether reconstitution of a normal T-cell compartment alone will be sufficient for correction of immunologic disease remains unclear and is an important issue to be resolved. Certainly, correction of platelet defects will require efficient gene transfer to stem cell populations.
Concluding comments
A remarkably wide and variable clinical spectrum of human disease is associated with mutations in the WAS gene. By far, the most scientific progress has been made in defining cytoskeletal mechanisms underlying the immunodeficiency caused by loss of function, and illustrating the importance of WASp during effective signal transduction, phagocytosis, and cell migration. The elucidation of these mechanisms continues to provide a fascinating insight into the functionality of the actin cytoskeleton in the hematopoietic system, and will hopefully provide the platform for the development of safe and effective novel therapies for both WAS and other immunologic disorders.
Prepublished online as Blood First Edition Paper, August 12, 2004; DOI 10.1182/blood-2004-04-1678.
Supported by the Wellcome Trust (A.J.T., G.O.C.), European Commission (contract no. QLG1CT 1999-01090, WASPNEST program; A.J.T., W.V.), La Ligue Nationale contre la cancer (équipe labellisée 2004/2008; W.V.), the French Ministry of Research ACI (W.V.), and the Institute of Child Health, University College London (S.B.).

![Figure 2. Multiple pathways recruit and activate WASp for normal IS assembly. In normal lymphocytes, WASp is recruited to the IS following TCR ligation. (A) Zeta-associated protein 70 (Zap 70) has been shown to localize WASp, after CD3 stimulation, by 2 separate mechanisms.25,57 The first is via EVH1 domain interaction with WIP and formation of a Zap 70-CrkL-WIP-WASp complex27; the second is through direct interaction of the proline-rich region (PPR) of WASp with the SH3 domain of Nck.25,28 The adaptor protein SLP76 is phosphorylated by Zap 70 (red hatched arrow) and binds both Nck and Vav-1 (a Cdc42-interacting guanosine diphosphate [GDP]–GTP exchange factor), thereby localizing both WASp and GTP-bound Cdc42 to the IS. (B) A Cdc42-independent route of WASp activation has recently been reported. Fyn phosphorylates WASp at Y291 (red hatched arrow) following TCR stimulation and is critical for multiple TCR-induced WASp effector functions.29 WASp activity can be down-regulated by PST-PEST–mediated dephosphorylation (yellow hatched block), through CD2AP/PSTPIP1, 26,29 indicating that the state of phosphorylation alone can regulate WASp activity.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/12/10.1182_blood-2004-04-1678/5/m_zh80230470470002.jpeg?Expires=1769083896&Signature=3GYI--bROCuskFKKkytb-SuC9F8~FHHJLDonSTiVwwji19oBJP7j9tmsXhvA6VruroEsV5gFFH3i9HDrCjoG6j86eGNw6dKYIBNo1RUK~AtWqHSrQTusPACm9mH1Ky322mzfLuH0eBAZnnLK8b9BDq7z3EfXusn87qIxzLtSp3gOKoaoKlEuLnM-EXGyv0AH9ROmNhRNUxAGTEX9SB~9Y7~yhOQIfJhUjuiles9q0w3JvjUXu8vT5NTx~GrOFxvqZID~VxHMFuOkUwAcKa9LxtDf~-Iq1O~eYSCBvSnSQwv70mh6q3guonRMW9WONt9MWiRnJ-xWa5kGk~mt5gqwzA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal