The outcome for adult patients with BCR-ABL–positive acute lymphoblastic leukemia (ALL) remains dismal and long-term survival can hardly be achieved except by allogeneic hematopoietic stem cell transplantation (HSCT). The Japan Adult Leukemia Study Group (JALSG) has recently started a phase 2 trial with intensive chemotherapy and imatinib for newly diagnosed BCR-AB–positive ALL patients, and we present here the interim results for the first 24 patients. All patients except one case of early death (96%) attained complete remission (CR) after a single course of remission induction therapy. Polymerase chain reaction (PCR) negativity was achieved in 28% of the patients on day 28, in 50% on day 63, and in up to 78% during the follow-up period. The toxicity profile was almost similar to that with chemotherapy alone. As a result, 15 patients (63%) could receive an allogeneic HSC transplant during their first CR. Although the number of patients is small and the observation period is too short, the combination therapy is very promising and produces high-quality CR for most newly diagnosed patients with BCR-ABL–positive ALL. This is especially useful because it provides the patients with a better chance to receive an allogeneic HSC transplant.
Introduction
The Philadelphia (Ph) chromosome is the most frequent cytogenetic abnormality, occurring in 20% to 30% of adult acute lymphoblastic leukemia (ALL).1-9 The Ph chromosome is the result of a t(9;22)(q34;q11) reciprocal translocation that forms a BCR-ABL fusion gene.10-14 Two kinds of fusion transcripts, major and minor BCR-ABL, can be distinguished according to the breakpoint of the BCR region. These transcripts, respectively, encode the p210 and p190 oncoprotein, both of which enhance tyrosine kinase activity and play a critical role in leukemic transformation.
The presence of BCR-ABL rearrangement has been recognized as the most adverse prognostic factor for ALL.1-9,15 Although complete remission (CR) is achieved in 50% to 80% of patients after intensive chemotherapy, which is slightly inferior to those without this anomaly, long-term outcome is dismal with overall survival (OS) of approximately 10%. The most common cause of treatment failure is relapse, and most patients suffer a relapse within the first year after achieving CR. Currently, allogeneic hematopoietic stem cell transplantation (HSCT) is thought to be the only curative therapy for the disease in adults.16-22 It is extremely important that the transplant can be delivered during the first CR (CR1) because it has been suggested that disease status at the time of transplantation is a strong indicator for long-term survival.20,22 For this reason, achieving a high-quality CR is preferable for maintaining the remission status until the transplantation is actually performed.
Imatinib is a potent inhibitor of the BCR-ABL protein tyrosine kinase and has been demonstrated to possess substantial activity in chronic myeloid leukemia (CML).23-27 Moreover, its tolerable toxicity and antileukemic activity for patients with Ph chromosome–positive ALL (Ph+ ALL) were confirmed in the phase 1 and phase 2 studies.28,29 Although single-agent imatinib was well tolerated and 60% of the patients with relapsed and refractory Ph+ ALL obtained a hematologic response, the median time to progression was quite short (only 2.2 months).29 In the meantime, Thomas et al30 reported more recent encouraging results for the MD Anderson Cancer Center experience, which used concurrent hyper-CVAD (cyclophosphamide, vincristine, adriamycin, and dexamethasone) and imatinib for patients with Ph+ ALL.
Previously, the Japan Adult Leukemia Study Group (JALSG) conducted 4 trials for adult ALL designated ALL87, ALL90, ALL93, and ALL97.4,8,31 The ALL93 study, published most recently, suggested that an increase in the dose intensity of anthracycline did not improve the outcome of Ph+ ALL with a CR rate of 51%, a 6-year disease-free survival (DFS) of 9.8%, and a 6-year OS of 4.8%, unless patients received an HSC transplant.8 In the current ALL202 study, screening for BCR-ABL is therefore performed at presentation for all patients, and those that were BCR-ABL positive are treated with a combination of intensive chemotherapy and imatinib. This article presents the interim results for 24 newly diagnosed BCR-ABL–positive ALL patients enrolled in the JALSG ALL202 study.
Patients, materials, and methods
Patients
Patients aged 15 to 64 years with newly diagnosed ALL were eligible for the JALSG ALL202 study if they showed adequate functioning of the liver (serum bilirubin level < 34.2 μM [2.0 mg/dL]), kidneys (serum creatinine level < 152.50 μM [2.0 mg/dL]), and heart (no severe abnormalities detected in electrocardiogram and echocardiography) and an Eastern Cooperative Oncology Group (ECOG) performance status between 0 and 3. Patients with other serious underlying medical problems were excluded as were those diagnosed as mature B-lineage ALL (B-ALL). Written informed consent was obtained from every participant before enrollment.
Study design
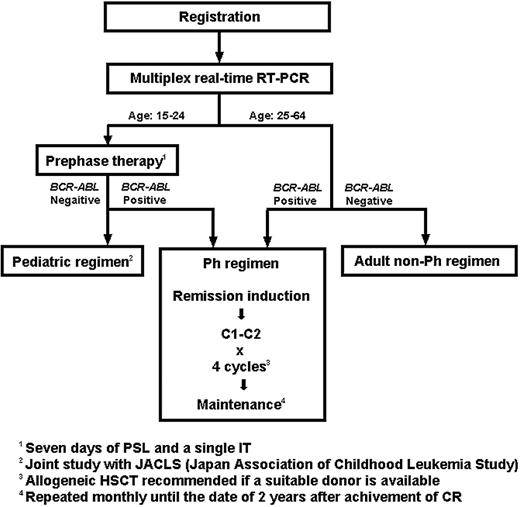
This is a prospective nonrandomized phase 2 trial conducted by the JALSG. The protocol was reviewed and approved by the institutional review board of each of the participating centers and was conducted in accordance with the Declaration of Helsinki. The ALL202 protocol consisted of 3 different regimens, that is, the Ph, the pediatric, and the adult non-Ph regimens (Figure 1). Patients were treated differently according to age and the presence or absence of BCR-ABL fusion transcripts. After registration, pretreatment bone marrow (BM) samples were subjected to a multiplex real-time reverse transcriptase–polymerase chain reaction (RQ-PCR) assay, and the results were obtained within one week. All patients younger than 25 years underwent a 7-day prephase therapy of prednisolone (PSL) and a single intrathecal injection of methotrexate (MTX). Those who were BCR-ABL positive were treated with the Ph regimen and those who were BCR-ABL negative with the pediatric regimen, which was used for the high-risk childhood ALL in the current trial of the Japan Association of Childhood Leukemia Study (JACLS). Patients aged 25 years or older received remission induction therapy immediately after their BM samples for the multiplex RQ-PCR test had been collected. The first 7-day treatment was identical for the Ph and the adult non-Ph regimens, but the patients were treated differently from day 8 on the basis of the BCR-ABL result. The treatment schedule for the Ph regimen is shown in Table 1. Imatinib was administered, in combination with other drugs, at a dose of 600 mg/d from day 8 to day 63. Consolidation therapy consisted of a course with high-dose MTX and high-dose cytarabine (AraC) (C1) and one with 600 mg/d of imatinib alone for 28 days (C2). C1 and C2 were alternatively repeated for 4 cycles. For those patients who did not attain CR with a single course of remission induction therapy, C1 was applied as the second course. If this also failed, the patients were regarded as failure cases. For the initiation of each consolidation course, recovery of peripheral blood (PB) values to neutrophil counts of at least 1 × 109/L (1000/μL), white blood cell (WBC) counts of at least 3000/μL, and platelet counts of at least 80 000/μL were required. After the completion of consolidation therapy, patients received maintenance therapy consisting of vincristine (VCR), PSL, and imatinib until 2 years from the date they had attained CR. Central nervous system (CNS) prophylaxis was performed by intrathecal injection of MTX, AraC, and dexamethasone during the remission induction course and each consolidation course (9 times in total). Patients with cytologic evidence of CNS leukemia received intrathecal injection once a week until the findings disappeared for 2 successive cerebrospinal fluid (CSF) examinations. Whole cranial irradiation was added at a dose of 20 Gy in total after completion of all consolidation courses for those patients having cytologic evidence, with CSF cell counts of 5/μL or more at presentation, which was in accordance with the previous JALSG trials.4,8,31 Symptomatic CNS leukemia was treated by cranial irradiation during induction course if possible. Allogeneic HSCT was recommended if an HLA-identical sibling donor was available. An HSC transplant from an alternative donor was used at the discretion of the institution.
Treatment strategies of the JALSG ALL202 study. Patients were treated differently according to age and the presence or absence of BCR-ABL fusion transcripts. Pretreatment bone marrow samples were analyzed in a multiplex real-time RT-PCR assay, and the results were obtained within one week. Patients younger than 25 years underwent a 7-day prephase therapy. For patients aged 25 years or older, the first 7 days of treatment were identical for the Ph and the adult non-Ph regimen, but patients were treated differently from day 8 on the basis of the BCR-ABL result. IT indicates intrathecal injection.
Treatment strategies of the JALSG ALL202 study. Patients were treated differently according to age and the presence or absence of BCR-ABL fusion transcripts. Pretreatment bone marrow samples were analyzed in a multiplex real-time RT-PCR assay, and the results were obtained within one week. Patients younger than 25 years underwent a 7-day prephase therapy. For patients aged 25 years or older, the first 7 days of treatment were identical for the Ph and the adult non-Ph regimen, but patients were treated differently from day 8 on the basis of the BCR-ABL result. IT indicates intrathecal injection.
Treatment schedule for BCR-ABL-positive ALL in the JALSG ALL202 study
Drug . | Dose . | Route . | Days . |
|---|---|---|---|
| Remission induction | |||
| CPM | 1200 mg/m2* | 3 h IV | 1 |
| DNR | 60 mg/m2† | 1 h IV | 1-3 |
| VCR | 1.3 mg/m2‡ | IV | 1, 8, 15, 22 |
| PSL | 60 mg/m2 | PO | 1-21§ |
| Imatinib | 600 mg/d | PO | 8-63 |
| MTX, AraC, Dex | 15, 40, 4 mg/d | IT | 29 |
| Consolidation 1 (CI) | - | ||
| MTX | 1 g/m2 | 24 h IV | 1 |
| AraC | 2 g/m2∥ | 3 h IV | 2, 3 (12 hourly) |
| MTX, AraC, Dex | 15, 40, 4 mg/d | IT | 1 |
| Consolidation 2 (C2) | |||
| Imatinib | 600 mg/d | PO | 1-28 |
| MTX, AraC, Dex | 15, 40, 4 mg/d | IT | 1 |
| Maintenance | |||
| VCR | 1.3 mg/m2‡ | IV | 1 |
| PSL | 60 mg/m2 | PO | 1-5 |
| Imatinib | 600 mg/d | PO | 1-28 |
Drug . | Dose . | Route . | Days . |
|---|---|---|---|
| Remission induction | |||
| CPM | 1200 mg/m2* | 3 h IV | 1 |
| DNR | 60 mg/m2† | 1 h IV | 1-3 |
| VCR | 1.3 mg/m2‡ | IV | 1, 8, 15, 22 |
| PSL | 60 mg/m2 | PO | 1-21§ |
| Imatinib | 600 mg/d | PO | 8-63 |
| MTX, AraC, Dex | 15, 40, 4 mg/d | IT | 29 |
| Consolidation 1 (CI) | - | ||
| MTX | 1 g/m2 | 24 h IV | 1 |
| AraC | 2 g/m2∥ | 3 h IV | 2, 3 (12 hourly) |
| MTX, AraC, Dex | 15, 40, 4 mg/d | IT | 1 |
| Consolidation 2 (C2) | |||
| Imatinib | 600 mg/d | PO | 1-28 |
| MTX, AraC, Dex | 15, 40, 4 mg/d | IT | 1 |
| Maintenance | |||
| VCR | 1.3 mg/m2‡ | IV | 1 |
| PSL | 60 mg/m2 | PO | 1-5 |
| Imatinib | 600 mg/d | PO | 1-28 |
C1 and C2 are alternatively repeated for 4 cycles. Maintenance is given every 4 weeks for 2 years from the date of CR.
CPM indicates cyclophosphamide; IV, intravenously; DNR, daunorubicin; VCR, vincristine; PSL, prednisolone; PO, per os; MTX, methotrexate; AraC, cytarabine; Dex, dexamethasone; and IT, intrathecally.
CPM 800 mg/m2 in case of patients aged 60 years or older.
DNR 30 mg/m2 in case of patients aged 60 years or older.
Max 2.0 mg in case of patients aged 60 years or older.
PSL for days 1 to 7 in case of patients aged 60 years or older.
AraC 1 g/m2 in case of patients aged 60 years or older.
Dose modification of imatinib
Dose modification of imatinib was generally based on the following conditions. During remission induction courses, dose reduction or interruption of imatinib for hematologic toxicity was not essentially considered, and for grade 3 or 4 nonhematologic toxicity, administration was interrupted until recovery to grade 1 or better and then resumed at 600 mg/d. If grade 3 or 4 toxicity recurred after resuming, dose reduction was implemented. During consolidation or maintenance courses for grade 3 or 4 neutropenia and thrombocytopenia, administration was interrupted until recovery to grade 2 or better and then resumed at 600 mg/d, and for nonhematologic toxicity, the procedure similar to that for remission induction courses was used.
Multiplex real-time RT-PCR
Total RNA was extracted from mononuclear cells in BM and transcribed to cDNA according to the manufacturer's instructions. Multiplex RQ-PCR assay was performed with TaqMan technology as described previously.32 Twelve sets of primers were used for detecting WT1, MDR1, and 9 distinct fusion gene transcripts, namely, major and minor BCR-ABL, TEL-AML1, E2A-PBX1, MLL-AF4, MLL-AF6, MLL-AF9, MLL-ENL, SIL-TAL1, and GAPDH as an internal control. The number of transcript copies was normalized by means of GAPDH and converted into molecules per microgram of RNA. The detection threshold was 50 copies/μg RNA, which responded to a sensitivity of 10–5. The levels under the threshold were distinguished between “not detected” and “slightly detected” and PCR negativity was defined as the former. The negative results were not confirmed by nested PCR. The primers and the detection probe were as follows: Mj-F13 (GATGCTGACCAACTCGTGTGTG), ABL-R21 (TGGCCACAAAATCATACAGTGC), and major-P (CCTTCAGCGGCCAGTAGCATCTGACTTT) for major BCR-ABL; and minor-F1 (ATCGTGGGCGTCCGCAAGAC), ABL-R22 (GCTCAAAGTCAGATGCTACTG), and minor-P1 (CGCCCTCGTCATCGTTGGGCCAGATCT) for minor BCR-ABL. During the follow-up period, only the fusion transcripts detected at diagnosis were evaluated by RQ-PCR.
Evaluation of patients
The primary objective of this study was to assess the CR rate, and the secondary aims were to assess toxicity, response duration, and survival. CR was defined as all of the following: less than 5% of blasts in BM, no leukemic blasts in PB, recovery of PB values to neutrophil counts of at least 1.5 × 109/L (1500/μL) and platelet counts of at least 100 000/μL, and no evidence of extramedullary leukemia. Relapse was defined as the presence of at least one of the following: recurrence of more than 10% leukemic cells in BM, or any leukemic cells in PB or extramedullary sites. BM samples for the RQ-PCR test were to be obtained at diagnosis, on days 28 and 63 of the remission induction course, after the first and third cycles of C1 and C2, after 1 year of treatment, and at the end of the entire therapy course. Toxicity was evaluated on the basis of the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 2.0.
Statistical analysis
Kaplan-Meier survival analysis was performed to estimate event-free survival (EFS) and OS. EFS was defined as the time from the first day of therapy to induction failure, relapse, death, or last visit and OS as the time from the first day of therapy to death or last visit. Patients undergoing HSCT were not censored at the time of transplantation and were evaluated with the inclusion of a posttransplantation period. Stat View 5.0 (SAS Institute, Cary, NC) was used for all statistical analyses.
Results
Patients
A total of 24 patients with newly diagnosed BCR-ABL–positive ALL were enrolled between September 2002 and August 2003. Patient characteristics are listed in Table 2. Only one patient (UPN 3) had CNS leukemia at diagnosis. Major BCR-ABL was present in 9 (39%) of 23 patients and minor BCR-ABL in 14 (61%) of 23 patients. The PCR test could not be performed for one patient (unique patient number [UPN] 23), and the diagnosis of BCR-ABL positivity was based on fluorescent in-situ hybridization (FISH) analysis. This patient was excluded from the subsequent monitoring of minimal residual disease (MRD). Except for this case, MRD was to be evaluated for each patient at the 7 distinct time points. Data at 13 points in total were missing because samples were not subjected to RQ-PCR assay; however, bone marrow examinations were performed during each point, and no evidence of disease recurrence was observed.
Patient characteristics
Age, y | |
| Median | 41.5 |
| Range | 15-59 |
| Sex, male/female | 11/13 |
| ECOG performance status, 0-1/2-3 | 2/22 |
| WBC count, /μL | |
| Median | 19 385 |
| Range | 1700-351 000 |
| PB blast % | |
| Median | 53 |
| Range | 0-95 |
| BM blast % | |
| Median | 89.2 |
| Range | 58.4-98.0 |
| BCR-ABL transcripts*, major/minor | 9/14 |
Age, y | |
| Median | 41.5 |
| Range | 15-59 |
| Sex, male/female | 11/13 |
| ECOG performance status, 0-1/2-3 | 2/22 |
| WBC count, /μL | |
| Median | 19 385 |
| Range | 1700-351 000 |
| PB blast % | |
| Median | 53 |
| Range | 0-95 |
| BM blast % | |
| Median | 89.2 |
| Range | 58.4-98.0 |
| BCR-ABL transcripts*, major/minor | 9/14 |
WBC indicates white blood cell; PB, peripheral blood; and BM, bone marrow.
One patient was excluded because RT-PCR screening at presentation was substituted by the result of FISH test.
Treatment efficacy
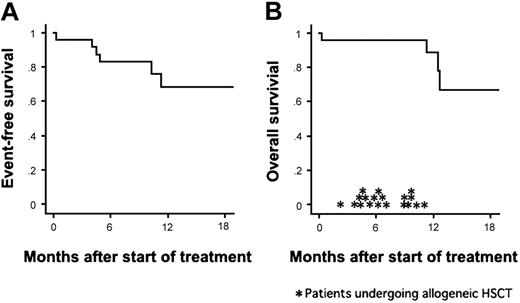
Twenty-three (96%) of the 24 patients achieved CR after a single course of remission induction therapy. The remaining one patient (UPN 9) died of pulmonary bleeding on day 10 (ie, on day 3 of imatinib administration). The median time to CR was 28 days (range, 19 to 62 days). The results of RQ-PCR are shown in Table 3. Negative results, although not confirmed by nested PCR, were demonstrated in 5 (28%) of 18 samples on day 28 and in 10 (50%) of 20 samples on day 63. During the treatment course, 18 patients (78%) achieved PCR negativity. Allogeneic HSCT was performed for 15 patients (6 from a sibling donor, 7 from an unrelated donor, and 2 from unrelated cord blood) during their first CR. Relapse occurred in 4 patients during consolidation therapy after 4.0, 4.5, 4.9, and 10.3 months of CR. One patient had a relapse after chemotherapy course (C1) and 3 after imatinib course (C2). The site of relapse was limited to BM for all of the patients including the patient (UPN 3) with CNS leukemia at presentation. Among these patients, interruption of imatinib was implemented in only one patient (UPN 17) for 14 days due to liver dysfunction, and no unexpected treatment delay was observed. After dropping out of the protocol, the 4 relapsed patients received chemotherapy without imatinib and proceeded to allogeneic HSCT in non-CR status. Only one of them maintained DFS for 7 months after transplantation. The median follow-up period of the whole patients was 12 months. The 1-year EFS and OS rates were estimated at 68% and 89%, respectively (Figure 2).
Kinetics of the number of the BCR-ABL transcripts copies for each patient
Patient . | At diagnosis . | On d 28 . | On d 63 . | After C1-1 . | After C2-1 . | After C1-3 . | After C2-3 . | At 1 y . |
|---|---|---|---|---|---|---|---|---|
| UPN 1 | 84 000 | N | N | < 50 | SIB-HSCT | — | — | — |
| UPN 2 | 2 800 000 | 540 | 260 | N | N | N | N | — |
| UPN 3 | 110 000 | — | < 50 | N | 240 000 | Relapse | — | — |
| UPN 4 | 440 000 | < 50 | N | N | N | N | — | N |
| UPN 5 | 93 000 | N | N | N | N | N | N | N |
| UPN 6 | 140 000 | 99 | N | N | 52 000 | Relapse | — | — |
| UPN 7 | 320 000 | < 50 | SIB-HSCT | — | — | — | — | — |
| UPN 8 | 56 000 | 1 000 | 62 | N | < 50 | < 50 | 1300 | UD-HSCT |
| UPN 9 | 110 000 | ED | — | — | — | — | — | — |
| UPN 10 | 3 500 000 | 73 | < 50 | 110 | 230 | UD-HSCT | — | — |
| UPN 11 | 150 000 | — | — | < 50 | < 50 | N | SIB-HSCT | — |
| UPN 12 | 510 000 | 440 | 8600 | 75 | CB-HSCT | — | — | — |
| UPN 13 | 1 200 000 | 270 | 190 | N | N | N | N | UD-HSCT |
| UPN 14 | 340 000 | 110 | N | — | N | — | 3400 | Relapse |
| UPN 15 | 7 200 000 | — | < 50 | < 50 | < 50 | N | UD-HSCT | — |
| UPN 16 | 290 000 | N | N | N | — | N | 290 | CB-HSCT |
| UPN 17 | 1 500 000 | 260 | < 50 | < 50 | 1 100 | Relapse | — | — |
| UPN 18 | 1 100 000 | — | N | < 50 | N | N | — | N |
| UPN 19 | 420 000 | 38 000 | < 50 | N | SIB-HSCT | — | — | — |
| UPN 20 | 4 600 000 | < 50 | < 50 | N | N | UD-HSCT | — | — |
| UPN 21 | 45 000 | < 50 | N | N | — | SIB-HSCT | — | — |
| UPN 22 | 1 800 000 | N | N | N | SIB-HSCT | — | — | — |
| UPN 23 | —* | — | — | — | — | UD-HSCT | — | — |
| UPN 24 | 2 400 000 | N | N | N | N | N | UD-HSCT | — |
Patient . | At diagnosis . | On d 28 . | On d 63 . | After C1-1 . | After C2-1 . | After C1-3 . | After C2-3 . | At 1 y . |
|---|---|---|---|---|---|---|---|---|
| UPN 1 | 84 000 | N | N | < 50 | SIB-HSCT | — | — | — |
| UPN 2 | 2 800 000 | 540 | 260 | N | N | N | N | — |
| UPN 3 | 110 000 | — | < 50 | N | 240 000 | Relapse | — | — |
| UPN 4 | 440 000 | < 50 | N | N | N | N | — | N |
| UPN 5 | 93 000 | N | N | N | N | N | N | N |
| UPN 6 | 140 000 | 99 | N | N | 52 000 | Relapse | — | — |
| UPN 7 | 320 000 | < 50 | SIB-HSCT | — | — | — | — | — |
| UPN 8 | 56 000 | 1 000 | 62 | N | < 50 | < 50 | 1300 | UD-HSCT |
| UPN 9 | 110 000 | ED | — | — | — | — | — | — |
| UPN 10 | 3 500 000 | 73 | < 50 | 110 | 230 | UD-HSCT | — | — |
| UPN 11 | 150 000 | — | — | < 50 | < 50 | N | SIB-HSCT | — |
| UPN 12 | 510 000 | 440 | 8600 | 75 | CB-HSCT | — | — | — |
| UPN 13 | 1 200 000 | 270 | 190 | N | N | N | N | UD-HSCT |
| UPN 14 | 340 000 | 110 | N | — | N | — | 3400 | Relapse |
| UPN 15 | 7 200 000 | — | < 50 | < 50 | < 50 | N | UD-HSCT | — |
| UPN 16 | 290 000 | N | N | N | — | N | 290 | CB-HSCT |
| UPN 17 | 1 500 000 | 260 | < 50 | < 50 | 1 100 | Relapse | — | — |
| UPN 18 | 1 100 000 | — | N | < 50 | N | N | — | N |
| UPN 19 | 420 000 | 38 000 | < 50 | N | SIB-HSCT | — | — | — |
| UPN 20 | 4 600 000 | < 50 | < 50 | N | N | UD-HSCT | — | — |
| UPN 21 | 45 000 | < 50 | N | N | — | SIB-HSCT | — | — |
| UPN 22 | 1 800 000 | N | N | N | SIB-HSCT | — | — | — |
| UPN 23 | —* | — | — | — | — | UD-HSCT | — | — |
| UPN 24 | 2 400 000 | N | N | N | N | N | UD-HSCT | — |
The detection threshold was 50 copies/μg RNA, and the levels under the threshold were distinguished between “not detected (N)” and “slightly detected (< 50).
N indicates negative for BCR-ABL transcript; SIB, sibling donor; HSCT, hematopoietic stem cell transplantation; —, RQ-PCR test not performed; UD, unrelated donor; ED, early death; and CB, cord blood.
The PCR test could not be performed and the diagnosis of BCR-ABL positivity was based on FISH analysis.
Kaplan-Meier curves of event-free survival and overall survival. The probabilities of event-free survival and overall survival for the 24 patients treated with the combination therapy. Allogeneic HSCT was performed for 19 patients (*), with 15 of them undergoing transplantation during CR1.
Kaplan-Meier curves of event-free survival and overall survival. The probabilities of event-free survival and overall survival for the 24 patients treated with the combination therapy. Allogeneic HSCT was performed for 19 patients (*), with 15 of them undergoing transplantation during CR1.
Toxicity of remission induction course combining dose-intensive chemotherapy with imatinib
Toxicity of the remission induction therapy was almost similar to that observed for remission induction chemotherapy without imatinib. The median time of WBC count recovery to at least 1000/μL was 19 days (range, 14 to 29 days), and the median time of platelet recovery to at least 100 000/μL was 22 days (range, 14 to 30 days). The profile and incidence of grades 2 to 4 nonhematologic toxicity are listed in Table 4. Death occurred in one patient (UPN 9), a 32-year-old man. He complained of sudden chest pain with dyspnea on day 9, and the chest X-ray findings showed infiltration in the S4 section of the left lung. Platelet counts were 54 000/μL when examined just after the symptom appeared. Despite intensive supportive therapy including emergency care and extra platelet transfusion, he died on day 10. Autopsy confirmed the cause of death as pulmonary bleeding. Two patients developed nonneutropenic fever after attaining CR with the presentation of positive cytomegalovirus (CMV) antigenemia test, although no evidence of CMV disease was found. Including the fatal case, interruption of imatinib was required for 7 patients and dose reduction for one patient during the remission induction course because of the following reasons: pulmonary bleeding in one patient, glutamic-pyruvic transaminase (GPT) elevation in 2 patients, and nausea in 4 patients. During consolidation and maintenance courses, interruption of imatinib for its toxicity was implemented for 3 patients, including 2 patients requiring dose reduction. None of the patients dropped out of the protocol due to intolerance of treatment.
Incidence of grades 2 to 4 nonhematologic toxicities during remission induction therapy with intensive chemotherapy and imatinib
Adverse event . | Grade 2, no. (%) . | Grade 3, no. (%) . | Grade 4, no. (%) . |
|---|---|---|---|
| Sepsis | 0 | 3 (13) | 0 |
| Other febrile neutropenia | 1 (4) | 6 (26) | 0 |
| Symptomatic CMV infection | 0 | 2 (8) | 0 |
| Lung hemorrhage | 1 (4) | 0 | 1 (4) |
| Diarrhea | 1 (4) | 1 (4) | 0 |
| Ileus | 1 (4) | 2 (8) | 0 |
| Nausea | 1 (4) | 4 (17) | 0 |
| GPT | 4 (17) | 3 (13) | 0 |
| Jaundice | 1 (4) | 0 | 0 |
| Pancreatitis | 0 | 1 (4) | 0 |
| Hyperglycemia | 2 (8) | 1 (4) | 0 |
| Hypophosphatemia | 1 (4) | 0 | 0 |
| Hypokalemia | 0 | 1 (4) | 0 |
| Fluid retention | 1 (4) | 0 | 0 |
| Depression | 3 (13) | 0 | 0 |
| Muscle weakness | 0 | 1 (4) | 0 |
Adverse event . | Grade 2, no. (%) . | Grade 3, no. (%) . | Grade 4, no. (%) . |
|---|---|---|---|
| Sepsis | 0 | 3 (13) | 0 |
| Other febrile neutropenia | 1 (4) | 6 (26) | 0 |
| Symptomatic CMV infection | 0 | 2 (8) | 0 |
| Lung hemorrhage | 1 (4) | 0 | 1 (4) |
| Diarrhea | 1 (4) | 1 (4) | 0 |
| Ileus | 1 (4) | 2 (8) | 0 |
| Nausea | 1 (4) | 4 (17) | 0 |
| GPT | 4 (17) | 3 (13) | 0 |
| Jaundice | 1 (4) | 0 | 0 |
| Pancreatitis | 0 | 1 (4) | 0 |
| Hyperglycemia | 2 (8) | 1 (4) | 0 |
| Hypophosphatemia | 1 (4) | 0 | 0 |
| Hypokalemia | 0 | 1 (4) | 0 |
| Fluid retention | 1 (4) | 0 | 0 |
| Depression | 3 (13) | 0 | 0 |
| Muscle weakness | 0 | 1 (4) | 0 |
CMV indicates cytomegalovirus.
Discussion
The outcome of Ph+ ALL patients treated with conventional chemotherapy remains extremely poor and the probabilities of DFS and OS are around 10%.1-9,15 Intensification of chemotherapy has had no substantial impact on the unfavorable course. In our previous JALSG ALL93 study, increasing the total dose of doxorubicin up to 180 mg/m2 for the remission induction course did not change the poor outcome for Ph+ ALL, with a CR rate of 51%, a 6-year DFS of 9.8%, and a 6-year OS of 4.8%.8 It has been shown that allogeneic HSCT, if performed during CR1, is associated with a higher DFS of 35% to 65%.16-22 However, results become worse for patients undergoing allogeneic HSCT during the second or subsequent CR and dismal for patients with relapsed or refractory disease.20,22 Thus, maintaining CR status until the transplantation is critical. To provide a better chance of a cure, both CR rate and quality of CR status are important. In this study, the quality of CR was assessed by means of serial quantification of BCR-ABL transcripts.
Of the 24 patients treated in this study, all patients, except for one case of early death, attained CR after a single course of remission induction therapy. The CR rate reached 96% and was significantly higher than the 51% in our previous JALSG ALL93 study.8 Among patients whose sample could be used for MRD analysis, PCR negativity had been achieved in 28% on day 28 and in 50% on day 63. In total, 78% of the patients achieved negative results in the course. As a result of the high-CR rate and long-lasting CR, 15 patients (63%) could be treated with allogeneic HSCT during CR1. EFS and OS appeared superior to those of JALSG ALL93 study, although the follow-up period was too short to reach any definite conclusions.
The toxicity profile was almost similar to that observed with chemotherapy alone. Administration of imatinib had to be interrupted in 7 patients but did not have to be discontinued during a remission induction course for any patient. Addition of imatinib to intensive chemotherapy did not increase toxicity, and this finding is in accordance with the recent publication from the MD Anderson Cancer Center.30 They treated Ph+ ALL patients with concurrent hyper-CVAD regimen and imatinib, and all of the 15 patients with active disease (11 untreated and 4 with primary failure) attained CR. Thus, combination of intensive chemotherapy and imatinib should be considered promising for the treatment of newly diagnosed Ph and/or BCR-ABL–positive ALL. Both the MD Anderson study and our study indicate that the combination therapy is very helpful for providing support for allogeneic HSCT. On the other hand, some differences exist between their regimen and ours. They administer imatinib concurrently with chemotherapy, whereas we alternate the imatinib courses and the chemotherapy courses for consolidation therapy. The dose of imatinib is 400 mg/d in their regimen and 600 mg/d in ours. It should be noted that early relapse was not observed among patients reported in their study. Longer follow-up is needed, however, to draw any conclusion with respect to appropriate dose of imatinib and treatment schedule as well as whether the combination therapy can actually change the prognosis of the disease, which is important, especially for patients not eligible for the transplantation.
In conclusion, our study demonstrated that the combination of intensive chemotherapy and imatinib can produce high-quality CR for a majority of patients with newly diagnosed BCR-ABL–positive ALL without an increase in toxicity. Combination therapy is especially useful in terms of providing patients with a better chance to receive an allogeneic HSC transplant. Long-term efficacy of this combination therapy will be addressed in the final analysis of this study.
Appendix
The following investigators participated in this study: N. Emi (Nagoya University Graduate School of Medicine), Y. Kobayashi (National Cancer Center Hospital), Y. Miyazaki (Nagasaki University School of Medicine), N. Uike (National Kyusyu Cancer Center), M. Tanimoto (Okayama University School of Medicine), M. Takahashi (St Marianna University School of Medicine), N. Kubota (Tokai University School of Medicine), K. Takeshita (Hamamatsu University School of Medicine), T. Ino (Fujita Health University), Y. Kishimoto (Kansai Medical University), K. Shinagawa (Okayama University School of Medicine), T. Kyo (Hiroshima Red Cross Hospital), Y. Sato (Yamaguchi University School of Medicine), K. Miyamura (Tohoku University School of Medicine), M. Matsuda (Kinki University School of Medicine), A. Miyata (Chugoku Central Hospital), Y. Ueda (Kurashiki Central Hospital), T. Matsushima (Gunma University School of Medicine), K. Fujikawa (Saiseikai Narashino Hospital), F. Sano (Yokohama City Seibu Hospital), R. Sakai (Fujisawa Municipal Hospital), Y. Takemoto (Imamura Bun-in Hospital), S. Fujisawa (Yokohama City University School of Medicine), and T. Murayama (Hyogo Medical Center for Adults).
Prepublished online as Blood First Edition Paper, August 17, 2004; DOI 10.1182/blood-2004-04-1389.
A complete list of the members of the Japan Adult Leukemia Study Group appears in the “Appendix.”
Supported in part by a Grant-in-Aid from the Ministry of Health, Labour, and Welfare, Clinical Research for Evidenced Medicine (H15-002).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Imatinib used in this study was kindly provided by Novartis Pharmaceuticals (Basel, Switzerland). We would like to thank Yukie Konishi (Nagoya University Graduate School of Medicine), and Yuko Makino (JALSG) for their secretarial assistance. In addition to the authors, the investigators listed in Appendix are acknowledged for contributing to this trial.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal