Unrelated cord blood transplantation (CBT) has now become more common, but as yet there have been only a few reports on its outcome compared with bone marrow transplantation (BMT), especially for adults. We studied the clinical outcomes of 113 adult patients with hematologic malignancies who received unrelated BM transplants (n = 45) or unrelated CB transplants (n = 68). We analyzed the hematopoietic recovery, rates of graft-versus-host disease (GVHD), risks of transplantation-related mortality (TRM) and relapse, and disease-free survival (DFS) using Cox proportional hazards models. The time from donor search to transplantation was significantly shorter among CB transplant recipients (median, 2 months) than BM transplant recipients (median, 11 months; P < .01). Multivariate analysis demonstrated slow neutrophil (P < .01) and platelet (P < .01) recoveries in CBT patients compared with BMT patients. Despite rapid tapering of immunosuppressants after transplantation and infrequent use of steroids to treat severe acute GVHD, there were no GVHD-related deaths among CB transplant recipients compared with 10 deaths of 24 among BM transplant recipients. Unrelated CBT showed better TRM and DFS results compared with BMT (P = .02 and P < .01, respectively), despite the higher human leukocyte antigen mismatching rate and lower number of infused cells. These data strongly suggest that CBT could be safely and effectively used for adult patients with hematologic malignancies.
Introduction
Although hematopoietic stem cell transplantation from human leukocyte antigen (HLA)–identical siblings offers the best results for selected hematologic and nonhematologic diseases, many patients do not have a suitable related donor. Bone marrow from HLA-matched unrelated donors has been an alternative graft source for these patients.1-3 While cord blood cells have been increasingly used as a source of hematopoietic stem cells for allogeneic transplantation, most recipients were pediatric4-8 because of the relatively lower cell doses in cord blood grafts.4-8 Recently, multicenter and single-center studies of unrelated cord blood transplantation (CBT) in adults after myeloablative9,10 or nonmyeloablative11 conditioning regimens showed cord blood could effectively restore hematopoiesis and was associated with acceptable levels of graft-versus-host disease (GVHD). There have also been 2 clinical comparisons of CBT and bone marrow transplantation (BMT) from unrelated donors: one a multicenter study of acute leukemia12 and the other a single-institution matched-pair analysis.13 In both, CBT produced comparable results to BMT from unrelated donors among pediatric patients. With regard to adult patients, Laughlin and colleagues (Hamza et al14 ) compared the outcomes of unrelated CBT and BMT. They observed slow neutrophil recovery after CBT with a high incidence of early Gram-positive bacterial infections during the first 50 days after transplantation. The event-free survival at 3-year follow-up was 25% in CB transplant and 35% in BM transplant recipients.14
We recently reported promising results of a pilot study in which adults with advanced myelodysplastic syndrome (MDS)15 and de novo acute myelogenous leukemia (AML)16 received CB transplants. The present clinical analysis aimed to confirm the safety of cord blood as a stem cell source for adults with hematologic malignancies and to compare the outcomes of unrelated CBT and BMT. In this study, we evaluated a total of 113 adult patients with hematologic malignancies who received BM transplants (n = 45) and CB transplants (n = 68) from unrelated donors after myeloablative regimens, including cyclophosphamide (CY) and total body irradiation (TBI), between 1996 and 2003 in a single center. We also analyzed factors related to the probability of engraftment, GVHD, transplant-related mortality (TRM), relapse, and disease-free survival (DFS).
Patients, materials, and methods
Patients and controls
The study includes data from 113 patients, 16 years or older, who received unrelated BM transplants (n = 45) or unrelated CB transplants (n = 68) for AML, acute lymphoblastic leukemia (ALL), chronic myelogenous leukemia (CML), MDS, or non-Hodgkin lymphoma. All consecutive patients receiving unrelated donor transplants between 1996 and 2003 at the Institute of Medical Science, University of Tokyo were included. Patients qualified as standard risk if they were in first or second complete remission (CR), had chronic phase CML or refractory anemia MDS, or had no high-risk cytogenetics [eg, ALL with t(4;11) or t(9;22); AML with complex karyotype -5, del(5q), -7; or abnormalities of 3q]. Patients in third CR, relapse, CML beyond chronic phase, or with high-risk cytogenetics were classified as high risk. Patients receiving CB transplants or BM transplants as a second transplant following relapse after a first allogeneic transplantation were excluded. Median follow-up was 14 months (range, 1-100 months) for BMT and 25 months (range, 0-68 months) for CBT (P = .77), as shown in Table 1. The clinical protocol was approved by the institutional review board of the Institute of Medical Science, University of Tokyo, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Characteristics of patients and grafts
Characteristics . | Bone marrow transplantation, n = 45 . | Cord blood transplantation, n = 68 . | P* . |
|---|---|---|---|
| Age, y | < .01 | ||
| Median | 26 | 36 | |
| Range | 16-50 | 16-53 | |
| Weight, kg | .01 | ||
| Median | 59.6 | 55.1 | |
| Range | 37.2-84.6 | 36.2-76.2 | |
| Sex, no. (%) | .65 | ||
| Male | 30 (67) | 42 (62) | |
| Female | 15 (33) | 26 (38) | |
| CMV serologic status, no. (%) | .07 | ||
| Negative | 12 (27) | 9 (13) | |
| Positive | 33 (73) | 59 (87) | |
| Diagnosis, no. (%) | < .01 | ||
| AML | |||
| CR1, CR2 | 5 (11) | 15 (22) | |
| Advanced | 10 (22) | 24 (35) | |
| ALL | |||
| CR1, CR2 | 4 (9) | 6 (9) | |
| Advanced | 4 (9) | 9 (13) | |
| CML | |||
| CP | 7 (16) | 1 (1)† | |
| Advanced | 11 (24) | 4 (6) | |
| MDS | |||
| RA | 3 (7) | 3 (4) | |
| Advanced | 1 (2) | 4 (6) | |
| NHL | |||
| CR1, CR2 | 0 | 3 (4)† | |
| Advanced | 0 | 0 | |
| Duration from diagnosis to transplantation, mo | .06 | ||
| Median | 20 | 17 | |
| Range | 4-146 | 3-148 | |
| Duration of donor search, mo | < .01 | ||
| Median | 11 | 3 | |
| Range | 4-52 | 1-36 | |
| Conditioning, no. (%)‡ | .60 | ||
| TBI + Ara-C/G-CSF + CY | 36 (80) | 49 (72) | |
| TBI + CY | 5 (11) | 12 (18) | |
| TBI + α | 4 (9) | 7 (10) | |
| GVHD prophylaxis, no. (%) | < .01 | ||
| CsA | 0 | 3 (4) | |
| CsA + sMTX | 23 (51) | 65 (96) | |
| FK506 + sMTX | 21 (47) | 0 | |
| CsA + mPSL | 1 (2) | 0 | |
| G-CSF administration on first 7days, no. (%) | < .01 | ||
| Yes | 39 (87) | 68 (100) | |
| No | 6 (13) | 0 | |
| Year of transplantation, no. (%) | < .01 | ||
| 1997-1999 | 33 (73) | 9 (13) | |
| 2000-2003 | 12 (27) | 59 (87) | |
| Follow-up time, mo | .77 | ||
| Median | 14 | 25.2 | |
| Range | 1-100 | 1-68 | |
| Follow-up time in survivors, mo | < .01 | ||
| No. of evaluable patients | 21 | 52 | |
| Median | 59 | 26 | |
| Range | 13-100 | 4-68 | |
| No. of leukocytes for transplantation, × 107/kg | < .01 | ||
| Median | 33.0 | 2.47 | |
| Range | 6.6-50 | 1.1-5.29 | |
| No. of HLA-A, -B, and -DRB1 mismatches, no. (%) | < .01 | ||
| 0 | 39 (87) | 0 | |
| 1 | 6 (13) | 14 (21) | |
| 2 | 0 | 37 (54) | |
| 3 | 0 | 15 (22) | |
| 4 | 0 | 2 (3) | |
| Sex of donor/recipient, no. (%) | .22 | ||
| Male/male | 22 (49) | 21 (31) | |
| Female/female | 5 (11) | 10 (15) | |
| Female/male | 8 (18) | 21 (31) | |
| Male/female | 10 (22) | 16 (24) | |
| Extent of ABO match, no. (%) | .75 | ||
| Match | 16 (36) | 20 (29) | |
| Minor mismatch | 11 (24) | 20 (29) | |
| Major mismatch | 18 (40) | 28 (41) |
Characteristics . | Bone marrow transplantation, n = 45 . | Cord blood transplantation, n = 68 . | P* . |
|---|---|---|---|
| Age, y | < .01 | ||
| Median | 26 | 36 | |
| Range | 16-50 | 16-53 | |
| Weight, kg | .01 | ||
| Median | 59.6 | 55.1 | |
| Range | 37.2-84.6 | 36.2-76.2 | |
| Sex, no. (%) | .65 | ||
| Male | 30 (67) | 42 (62) | |
| Female | 15 (33) | 26 (38) | |
| CMV serologic status, no. (%) | .07 | ||
| Negative | 12 (27) | 9 (13) | |
| Positive | 33 (73) | 59 (87) | |
| Diagnosis, no. (%) | < .01 | ||
| AML | |||
| CR1, CR2 | 5 (11) | 15 (22) | |
| Advanced | 10 (22) | 24 (35) | |
| ALL | |||
| CR1, CR2 | 4 (9) | 6 (9) | |
| Advanced | 4 (9) | 9 (13) | |
| CML | |||
| CP | 7 (16) | 1 (1)† | |
| Advanced | 11 (24) | 4 (6) | |
| MDS | |||
| RA | 3 (7) | 3 (4) | |
| Advanced | 1 (2) | 4 (6) | |
| NHL | |||
| CR1, CR2 | 0 | 3 (4)† | |
| Advanced | 0 | 0 | |
| Duration from diagnosis to transplantation, mo | .06 | ||
| Median | 20 | 17 | |
| Range | 4-146 | 3-148 | |
| Duration of donor search, mo | < .01 | ||
| Median | 11 | 3 | |
| Range | 4-52 | 1-36 | |
| Conditioning, no. (%)‡ | .60 | ||
| TBI + Ara-C/G-CSF + CY | 36 (80) | 49 (72) | |
| TBI + CY | 5 (11) | 12 (18) | |
| TBI + α | 4 (9) | 7 (10) | |
| GVHD prophylaxis, no. (%) | < .01 | ||
| CsA | 0 | 3 (4) | |
| CsA + sMTX | 23 (51) | 65 (96) | |
| FK506 + sMTX | 21 (47) | 0 | |
| CsA + mPSL | 1 (2) | 0 | |
| G-CSF administration on first 7days, no. (%) | < .01 | ||
| Yes | 39 (87) | 68 (100) | |
| No | 6 (13) | 0 | |
| Year of transplantation, no. (%) | < .01 | ||
| 1997-1999 | 33 (73) | 9 (13) | |
| 2000-2003 | 12 (27) | 59 (87) | |
| Follow-up time, mo | .77 | ||
| Median | 14 | 25.2 | |
| Range | 1-100 | 1-68 | |
| Follow-up time in survivors, mo | < .01 | ||
| No. of evaluable patients | 21 | 52 | |
| Median | 59 | 26 | |
| Range | 13-100 | 4-68 | |
| No. of leukocytes for transplantation, × 107/kg | < .01 | ||
| Median | 33.0 | 2.47 | |
| Range | 6.6-50 | 1.1-5.29 | |
| No. of HLA-A, -B, and -DRB1 mismatches, no. (%) | < .01 | ||
| 0 | 39 (87) | 0 | |
| 1 | 6 (13) | 14 (21) | |
| 2 | 0 | 37 (54) | |
| 3 | 0 | 15 (22) | |
| 4 | 0 | 2 (3) | |
| Sex of donor/recipient, no. (%) | .22 | ||
| Male/male | 22 (49) | 21 (31) | |
| Female/female | 5 (11) | 10 (15) | |
| Female/male | 8 (18) | 21 (31) | |
| Male/female | 10 (22) | 16 (24) | |
| Extent of ABO match, no. (%) | .75 | ||
| Match | 16 (36) | 20 (29) | |
| Minor mismatch | 11 (24) | 20 (29) | |
| Major mismatch | 18 (40) | 28 (41) |
CMV indicates cytomegalovirus; AML, acute myelogenous leukemia; CR1, CR2, first and second complete remission; Advanced, patients in third complete remission, relapse, CML beyond chronic phase, or who had high-risk cytogenetics were classified as high risk; ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; CP, chronic phase; MDS, myelodysplastic syndrome; RA, refractory anemia; NHL, non-Hodgkin lymphoma; TBI, total body irradiation; Ara-C, cytosine arabinoside; G-CSF, granulocyte colony-stimulating factor; CY, cyclophosphamide; CsA, cyclosporine; sMTX, short-term methotrexate; FK506, tacrolimus; and mPSL, methylprednisolone.
The chi-square test was used for categoric variables; the Mann-Whitney U test was used for continuous variables
One patient with CML in chronic phase and NHL in first complete remission received cord blood for the treatment of both diseases and was categorized as standard risk
All conditioning regimens included 12 Gy of TBI. In BMT group, 2 patients received TBI + CY + etoposide, 1 patient received TBI + Ara-C + antithymocyte globulin, and 1 patient received TBI + CY + antithymocyte globulin. In CBT group, 2 patients received TBI + G-CSF-combined Ara-C + one drug (fludarabine 30 mg/m2 × 3 days or antithymocyte globulin) and 5 patients received TBI + CY + one drug (etoposide in 3 patients, thiotepa in 1 patient, and antithymocyte globulin in 1 patient)
HLA typing and donor selection
HLA-A and HLA-B antigens were identified by serologic typing. HLA-DRB1 alleles were determined by high-resolution molecular typing using polymerase chain reaction method with sequence-specific primers (PCR-SSP). Patients without a suitable closely HLA-matched family donor, namely, with 5 of 6 or 6 of 6 matching HLA loci, were eligible for CBT if a matched unrelated bone marrow donor was unavailable as a first treatment option. If there was insufficient time for an unrelated bone marrow donor search due to disease status or if the preliminary search indicated a low likelihood of obtaining a matched unrelated bone marrow donor, we attempted to locate cord blood grafts. All cord blood grafts were evaluated by HLA-A, HLA-B, and HLA-DRB1 typing and nucleated cell counts. Preferred cord blood units matched 4 of 6 to 6 of 6 HLA loci and contained a minimal cell count of 1.5 × 107 nucleated cells/kg body weight before freezing. T-lymphocyte depletion was not performed on cord blood or bone marrow grafts. All unrelated bone marrow donor searches were processed through the Japan Marrow Donor Program. Sixty-seven of 68 cord blood units came from cord blood banks (CBBs) in the Japan Cord Blood Bank Network and one from another CBB.
Conditioning regimen, GVHD prophylaxis, and supportive care
All patients received a TBI-containing myeloablative pretransplantation conditioning regimen of 12 Gy fractionated in 4 or 6 doses on days -9, -8, and -7, or -8 and -7. Eighty-five of 113 patients also received cytosine arabinoside (Ara-C) intravenously over 2 hours at 3 g/m2 of body surface every 12 hours on days -6 and -5 or -5 and -4 (total dose 12 g/m2), as well as continuous infusion of recombinant human granulocyte colony-stimulating factor (G-CSF) at 5 μg/kg/d starting 12 hours before the first Ara-C dose and stopping after the last dose.17 CY was administered intravenously over 2 hours at 60 mg/kg once daily on days -4 and -3 or -3 and -2 (total dose 120 mg/kg). Seventeen additional patients received 12 Gy of TBI and CY (60 mg/kg intravenously on days -4 and -3). Two patients received TBI + G-CSF–combined Ara-C + 120 mg/m2 fludarabine and 5 received TBI + CY + one immunosuppressant (60 mg/kg etoposide in 3 patients, 60 mg/kg thiotepa in 1 patient, and 60 mg/kg antithymocyte globulin in 1 patient). Three patients received TBI + Ara-C + one immunosuppressant (120 mg/m2 fludarabine in 2 patients or 60 mg/kg antithymocyte globulin in 1 patient). One patient received TBI + 120 mg/m2 fludarabine + 140 mg/m2 melphalan (Table 1). Essentially, the TBI + CY + Ara-C combined with G-CSF regimen has been chosen in patients with myeloid leukemias17-19 and CY was avoided and changed to other drugs in the case of recipients who had risk of organ dysfunction, especially in the heart.
Eighty-eight of 113 patients received a standard cyclosporine (CsA) and methotrexate (MTX) combination as GVHD prophylaxis. CsA was administered daily from day -1 at 3 mg/kg/d intravenously and MTX at 15 mg/m2 intravenously on day 1, followed by 10 mg/m2 on days 3, 6, and 11. MTX on day 11 was omitted in all patients receiving cord blood. Twenty-one patients received tacrolimus (FK-506, 0.03 mg/kg/d continuous intravenously from day -1; Fujisawa Pharmaceuticals, Osaka, Japan) combined with MTX (15 mg/m2 intravenously on day 1 and 10 mg/m2 intravenously on days 3, 6, and 11). Three patients received only CsA (3 mg/kg/d from day -1) and one received CsA + methylprednisolone (2 mg/kg/d from day 1). Once oral intake could be tolerated, patients were administered oral CsA or FK506 at a dose ratio of 1:2.5, in 2 divided doses/d based on the last intravenous dose. In the absence of GVHD, CsA or FK506 was tapered beginning between weeks 6 and 9 until it could be discontinued in the absence of chronic GVHD between 6 and 12 months after transplantation. CsA or FK506 was reduced when serum creatinine levels rose above 1.5 times baseline or other serious agent-associated toxicities occurred. Physicians could freely modify the CsA dose for patients experiencing severe acute GVHD or risk of disease relapse. Corticosteroid-based treatment was considered when grade II or higher severe acute GVHD occurred (1 to 2 mg/kg).
Gut decontamination using ofloxacin and fluconazole and isolation in laminar airflow were begun on day -14 and day -7, respectively. These were continued until the absolute neutrophil count reached more than 0.5 × 109/L (500/mm3). Patients also received a daily oral administration of trimethoprim-sulfamethoxazole from day -21 to day -2, intravenous high-titer anticytomegalovirus immunoglobulin from day -3 to day 120 if the immunoglobulin level in the serum was lower than 5 g/L (500 mg/dL), and daily oral acyclovir from day -5 to day 35. All patients who received CB transplants and 39 of 45 patients who received BM transplants received G-CSF (5 μg/kg/d intravenous infusion) starting on day 1 until durable granulocyte recovery was achieved. Both groups of unrelated recipients received the same supportive care except for the G-CSF administration.
End points, definitions, and assessments of hematopoietic recovery, GVHD, TRM, disease relapse, and DFS
We focused on hematologic recovery, acute and chronic GVHD, TRM, disease relapse, and DFS after unrelated BMT compared with unrelated CBT. The primary measure of hematopoietic recovery was the time required for myeloid and platelet recovery. The myeloid cell recovery time was defined as the first of 3 consecutive days during which the absolute neutrophil count in the blood was at least 0.5 × 109/L (500/mm3). Platelet recovery time was achieved on the first of 3 days when the platelet count was above 20 × 109/L (20 000/mm3) (or 50 × 109/L [50 000/mm3]) without transfusion support. Primary engraftment failure was defined as the absence of donor-derived myeloid cells at day 60 in patients surviving beyond day 28 after transplantation or when patients were given a second allogeneic transplant or reconstituted with autologous cells. Chimerism was evaluated by fluorescence in situ hybridization for the Y chromosome or quantitative PCR analysis for microsatellite DNA markers. Acute GVHD was graded 0 to IV according to the criteria of Glucksberg et al,20 and chronic GVHD was defined as none, limited, or extensive.21 The incidence and time to acute GVHD development were evaluated in patients surviving 21 days or longer with evidence of engraftment. Time to occurrence of any chronic GVHD disease was evaluated in patients surviving 100 days or longer after transplantation with allogeneic engraftment. TRM was defined as death from any cause except relapse. Relapse was defined by morphologic evidence of disease in peripheral blood, marrow, or extramedullary sites or the recurrence and sustained presence of pretransplantation chromosomal abnormalities on cytogenetic analysis of bone marrow cells. Patients showing minimal residual disease (eg, the presence of bcr/abl RNA transcripts by PCR) were not classified as having relapsed. DFS was defined as survival in continuous CR.
Statistical analysis
The probability of DFS was estimated from the time of transplantation according to the Kaplan-Meier product limit method. Cumulative incidences were estimated for hematopoietic recovery, GVHD, TRM, and relapse to take account of competing risks. Associations between graft type and outcome were evaluated using Cox proportional hazard regression models. In addition to the hematopoietic stem cell source, the following variables were considered as covariates: recipient age at transplantation; weight; status regarding cytomegalovirus (CMV; determined by serologic testing); recipient and donor sex; degree of ABO matching; degree of HLA-matching; type (ALL, AML, CML, MDS, or non-Hodgkin lymphoma) and pretransplantation duration of the underlying disease; disease status at transplantation (standard or high risk); conditioning regimen; GVHD prophylaxis used; use or nonuse of G-CSF during the first 7 days after transplantation; dose of nucleated cells infused (above or below 0.25 × 108/kg or 2.5 × 108/kg for CBT or BMT, respectively); and time of transplantation (between 1996 and 1999 or 2000 and 2003). We used a stepwise procedure at a significance level of 5% to construct prognostic models in which we tried to maintain the graft source (bone marrow or cord blood) as a variable until the last step. The proportional hazards assumption of the Cox model was assessed mainly by a graphic approach. When groups were compared according to continuous covariates, we calculated the mean or median of each group and t or Mann-Whitney U tests were used. A chi-square test was used to compare categoric covariates; SAS version 8.2 (SAS Institute, Cary, NC) and S Plus 2000 (Mathsoft, Seattle, WA) were used for all analyses. End points were calculated at the last contact, the date of the latest follow-up being April 1, 2004.
Results
Characteristics of patients and donors
Although patient characteristics were relatively balanced between the BM transplant and CB transplant recipients, significant differences occurred in the following variables (Table 1). Patients who received CB transplants were older, had lower bodyweight, and received transplants in a later year. The rate of patients with CML among the BM transplant recipients was significantly higher than that among the CB transplant recipients, and the rate of patients with AML among the CB transplant recipients was significantly higher than that among the BM transplant recipients. Overall rates of high-risk patients were 58% for BMT and 60% for CBT. While the shorter time from diagnosis to transplantation in CB transplant recipients was not significant (P = .06), that from donor search to transplantation was significantly shorter among CB transplant recipients (median, 2.8 months; range, 0.7 to 36.3 months versus median, 10.8 months; range, 4.4 to 52.1 months; P < .01). The 6 possible matches between the recipient and the donor were scored serologically for HLA-A and -B and genetically for HLA-DRB1 alleles and the results showed 39 (87%) matched grafts in BMT patients and no complete matches in CBT patients. Although the number of leukocytes for CBT was 1 log lower than in BMT, 64 of 68 (94%) cord blood grafts contained more than 2.0 × 107 cells per kilogram. The median number of CD34+ progenitor cells was 0.9 × 105/kg (range, 0.2 × 105 to 9.0 × 105/kg) before freezing of cord blood grafts.
Engraftment, hematopoietic recovery, and GVHD
Three patients (4%) died within 28 days of CBT and primary graft failure occurred in 5 of 65 (8%) patients, as described in Table 2. Two patients, one with ALL in first remission and one with CML in second chronic phase at transplantation, had autologous hematologic recovery 52 days and 29 days after transplantation and survived in CR for more than 5 years and almost 1 year, respectively, without receiving any therapy for the disease. Another patient, with AML, had primary induction failure at transplantation, relapsed on day 55, and died 12 months after transplantation due to persistent leukemia and bacterial pneumonia. A second CBT was performed after the confirmation of engraftment failure in a patient with Philadelphia-positive ALL in first remission at transplantation, and she has been in CR for 6 months. There was one early death (2%) at day 26 due to multiple organ failure in the BMT group, but no patients had primary graft failure.
Early deaths and primary graft failures in CB transplant recipients
Patient no. . | No. of cells before freezing, × 107/kg . | No. of HLA matches* . | Diagnosis . | Outcomes . |
|---|---|---|---|---|
| Early deaths | ||||
| 292 | 1.63 | 5/6 | AML (Rel) | Death due to MOF at d27 |
| 372 | 2.25 | 4/6 | ALL (CR2) | Death due to bacterial pneumonia at d13 |
| 377 | 3.66 | 4/6 | ALL (Rel) | Death due to VOD at d18 |
| Primary engraftment failures | ||||
| 314 | 2.71 | 3/6 | ALL (CR1) | Autologous recovery at d52, and survival in CR for 1651 d |
| 362 | 2.43 | 4/6 | AML (PIF) | Relapse at d54, and death at d368 due to bacterial pneumonia |
| 422 | 2.27 | 4/6 | CML (BC) | Death due to fungal pneumonia at d48 |
| 427 | 2.10 | 3/6 | CML (CP2) | Autologous recovery at d29, and survival in molecular CR for 322 d |
| 439 | 3.44 | 5/6 | ALL (CR1) | Second CBT at d33, survival in remission for 182 d |
Patient no. . | No. of cells before freezing, × 107/kg . | No. of HLA matches* . | Diagnosis . | Outcomes . |
|---|---|---|---|---|
| Early deaths | ||||
| 292 | 1.63 | 5/6 | AML (Rel) | Death due to MOF at d27 |
| 372 | 2.25 | 4/6 | ALL (CR2) | Death due to bacterial pneumonia at d13 |
| 377 | 3.66 | 4/6 | ALL (Rel) | Death due to VOD at d18 |
| Primary engraftment failures | ||||
| 314 | 2.71 | 3/6 | ALL (CR1) | Autologous recovery at d52, and survival in CR for 1651 d |
| 362 | 2.43 | 4/6 | AML (PIF) | Relapse at d54, and death at d368 due to bacterial pneumonia |
| 422 | 2.27 | 4/6 | CML (BC) | Death due to fungal pneumonia at d48 |
| 427 | 2.10 | 3/6 | CML (CP2) | Autologous recovery at d29, and survival in molecular CR for 322 d |
| 439 | 3.44 | 5/6 | ALL (CR1) | Second CBT at d33, survival in remission for 182 d |
AML indicates acute myelogenous leukemia; Rel, relapse; MOF, multi-organ failure; ALL, acute lymphoblastic leukemia; CR2, second remission; VOD, veno-occlusive disease; CR1, first remission; PIF, primary induction failure; CML, chronic myelogenous leukemia; BC, blastic crisis; and CP2, second chronic phase.
HLA-A and HLA-B antigens were identified by serologic typing and HLA-DRB1 alleles were determined by high-resolution molecular typing
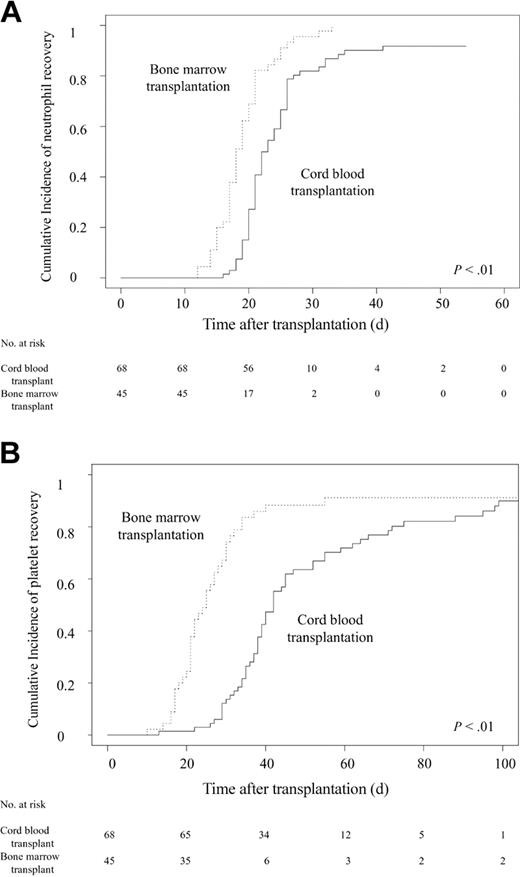
Patients receiving CB transplants had significantly slower neutrophil (Figure 1A) and platelet (Figure 1B) recovery in multivariate analysis (Table 3), in contrast with almost comparable recoveries in hematopoietic engraftment with longer-term followup. The overall myeloid engraftment rates on day 42 were 92% (95% confidence interval [CI], 85%-99%) for CBT and 100% for BMT. Platelet counts of more than 20 × 109/L (20 000 cells/mm3) on day 100 were 90% (95% CI, 82%-95%) and 91% (95% CI, 82%-100%) and platelet counts of more than 50 × 109/L (50 000 cells/mm3) at day 180 were 89% (95% CI, 82%-88%) and 89% (95% CI, 80%-100%) for CBT and BMT, respectively.
Cumulative incidences of neutrophil and platelet recovery after transplantation. (A) Probability of neutrophil recovery to more than 0.5 × 109/L (500 cells/mm3). The speed of recovery was significantly slower in CB transplant recipients than in BM transplant recipients (P < .01). The overall myeloid engraftment rates were 92% (95% CI, 85%-99%) in the CBT group and 100% in the BMT group on day 42. (B) Probability of platelet recovery to more than 20 × 109/L (20 000 cells/mm3). The speed of recovery was significantly slower in CB transplant recipients than in BM transplant recipients (P < .01). Platelet counts of more than 20 × 109/L (20 000/mm3) on day 100 were 90% (95% CI, 82%-95%) and 91% (95% CI, 82%-100%) for the CBT and BMT groups, respectively.
Cumulative incidences of neutrophil and platelet recovery after transplantation. (A) Probability of neutrophil recovery to more than 0.5 × 109/L (500 cells/mm3). The speed of recovery was significantly slower in CB transplant recipients than in BM transplant recipients (P < .01). The overall myeloid engraftment rates were 92% (95% CI, 85%-99%) in the CBT group and 100% in the BMT group on day 42. (B) Probability of platelet recovery to more than 20 × 109/L (20 000 cells/mm3). The speed of recovery was significantly slower in CB transplant recipients than in BM transplant recipients (P < .01). Platelet counts of more than 20 × 109/L (20 000/mm3) on day 100 were 90% (95% CI, 82%-95%) and 91% (95% CI, 82%-100%) for the CBT and BMT groups, respectively.
The results of multivariate analysis of time to engraftment and GVHD
. | Bone marrow transplantation, n = 45 . | Cord blood transplantation, n = 68 . | Hazard ratio (95% CI)* . | P . |
|---|---|---|---|---|
| Absolute neutrophil count greater than 0.5 × 109/L | 0.18 (0.11-0.30) | < .01 | ||
| No. of patients to achieve (%) | 45 (100) | 60 (88) | ||
| Median | 18 | 22 | ||
| Range | 12-33 | 16-41 | ||
| Platelet count greater than 20 × 109/L | 0.48 (0.29-0.81) | < .01 | ||
| No. of patients to achieve (%) | 42 (93) | 55 (81) | ||
| Median | 25 | 40 | ||
| Range | 10-172 | 13-99 | ||
| Platelet count greater than 50 × 109/L | 0.44 (0.28-0.69) | < .01 | ||
| No. of patients to achieve (%) | 38 (84) | 55 (81) | ||
| Median | 28 | 48 | ||
| Range | 16-113 | 30-263 | ||
| Acute GVHD | ||||
| No. of evaluable patients† | 45 | 60 | ||
| Grade no. (%) | ||||
| 0 | 6 (13) | 5 (8) | ||
| I | 9 (20) | 25 (42) | ||
| II | 18 (40) | 26 (43) | ||
| III | 2 (4) | 3 (5) | ||
| IV | 10 (22) | 1 (2) | ||
| II-IV | 30 | 30 | 0.61 (0.37-1.01) | .05 |
| III-IV | 12 | 4 | 0.09 (0.01-0.58) | .01 |
| Requiring steroid therapy for acute GVHD | ||||
| Patients treated with steroid | 21 | 13 | 0.33 (0.16-0.65) | < .01 |
| Chronic GVHD | ||||
| No. of evaluable patients‡ | 35 | 54 | ||
| Limited + extensive | 26 | 42 | 0.67 (0.35-1.26) | .21 |
| Extensive | 14 | 13 | 0.60 (0.28-1.28) | .18 |
. | Bone marrow transplantation, n = 45 . | Cord blood transplantation, n = 68 . | Hazard ratio (95% CI)* . | P . |
|---|---|---|---|---|
| Absolute neutrophil count greater than 0.5 × 109/L | 0.18 (0.11-0.30) | < .01 | ||
| No. of patients to achieve (%) | 45 (100) | 60 (88) | ||
| Median | 18 | 22 | ||
| Range | 12-33 | 16-41 | ||
| Platelet count greater than 20 × 109/L | 0.48 (0.29-0.81) | < .01 | ||
| No. of patients to achieve (%) | 42 (93) | 55 (81) | ||
| Median | 25 | 40 | ||
| Range | 10-172 | 13-99 | ||
| Platelet count greater than 50 × 109/L | 0.44 (0.28-0.69) | < .01 | ||
| No. of patients to achieve (%) | 38 (84) | 55 (81) | ||
| Median | 28 | 48 | ||
| Range | 16-113 | 30-263 | ||
| Acute GVHD | ||||
| No. of evaluable patients† | 45 | 60 | ||
| Grade no. (%) | ||||
| 0 | 6 (13) | 5 (8) | ||
| I | 9 (20) | 25 (42) | ||
| II | 18 (40) | 26 (43) | ||
| III | 2 (4) | 3 (5) | ||
| IV | 10 (22) | 1 (2) | ||
| II-IV | 30 | 30 | 0.61 (0.37-1.01) | .05 |
| III-IV | 12 | 4 | 0.09 (0.01-0.58) | .01 |
| Requiring steroid therapy for acute GVHD | ||||
| Patients treated with steroid | 21 | 13 | 0.33 (0.16-0.65) | < .01 |
| Chronic GVHD | ||||
| No. of evaluable patients‡ | 35 | 54 | ||
| Limited + extensive | 26 | 42 | 0.67 (0.35-1.26) | .21 |
| Extensive | 14 | 13 | 0.60 (0.28-1.28) | .18 |
CI indicates confidence interval; GVHD, graft-versus-host disease.
The hazard ratio is for cord blood transplantation compared with bone marrow transplantation
Acute GVHD was evaluated in patients surviving 21 days or longer after transplantation with evidence of engraftment
Chronic GVHD disease was evaluated in patients surviving 100 days or longer after transplantation with engraftment
Although GVHD prophylaxis was assigned according to the protocol in “Conditioning regimen, GVHD prophylaxis, and supportive care,” the tapering rate of immunosuppressant drugs differed among individual patients due to variations in GVHD severity, renal function, and primary disease risk. Consequently, immunosuppressants for CB transplant recipients tended to be discontinued faster than those for BM transplant recipients (Figure 2A). The cumulative incidence of terminating immunosuppressant drugs on day 180 were 40% (95% CI, 28%-52%) among CB transplant recipients and 16% (95% CI, 5%-26%) among BM transplant recipients. Despite the rapid tapering of prophylactic drugs for GVHD and the high degree of HLA disparity among CB transplant recipients, the cumulative incidence of grades II to IV acute GVHD tended to be lower than among BM transplant recipients, and the cumulative incidence of grades III and IV (Figure 2B) acute GVHD was significantly lower in multivariate analysis (hazard ratio, 0.09; 95% CI, 0.01-0.58; P = .01; Table 3). The cumulative incidence of requiring steroids for treating acute GVHD among CB transplant recipients was significantly lower than among BM transplant recipients (hazard ratio, 0.33; 95% CI, 0.16-0.65; P < .01; Figure 2C; Table 3).
Kinetics of immune suppressant use after transplantation. (A) Cumulative incidence of terminating cyclosporine A and FK506. The values on day 180 were 40% (95% CI, 28%-52%) among CB transplant recipients and 16% (95% CI, 5%-26%) among BM transplant recipients. Panel B shows the cumulative incidence of acute GVHD grades III to IV. The values for grades III and IV acute GVHD on day 100 were 6% (95% CI, 0%-19%) for CBT and 27% (95% CI, 15%-42%) for BMT. (C) Estimated cumulative incidences of requiring steroid therapy in patients after CBT and BMT.
Kinetics of immune suppressant use after transplantation. (A) Cumulative incidence of terminating cyclosporine A and FK506. The values on day 180 were 40% (95% CI, 28%-52%) among CB transplant recipients and 16% (95% CI, 5%-26%) among BM transplant recipients. Panel B shows the cumulative incidence of acute GVHD grades III to IV. The values for grades III and IV acute GVHD on day 100 were 6% (95% CI, 0%-19%) for CBT and 27% (95% CI, 15%-42%) for BMT. (C) Estimated cumulative incidences of requiring steroid therapy in patients after CBT and BMT.
Chronic GVHD affected 42 of 54 CB transplant and 26 of 35 BM transplant recipients surviving more than 100 days. Thirteen CB transplant and 14 BM transplant recipients developed extensive GVHD. There was no significant difference between CB transplant and BM transplant recipients in chronic GVHD incidence, although the cumulative incidence of extensive type GVHD among CB transplant recipients tended to be lower than that among BM transplant recipients (Table 3).
TRM, relapse, and DFS
The 1-year cumulative incidence of TRM was 9% (95% CI, 2%-16%) among CB transplant recipients and 29% (95% CI, 15%-42%) among BM transplant recipients (Figure 3A). In the multivariate analysis, as shown in Table 4, the hazard ratio for TRM was significantly lower after CBT. In contrast, there was no apparent difference between the risk of relapse in both groups (Table 4; Figure 3B). One HLA-mismatched graft and primary disease other than AML were significantly poor factors for TRM.
Outcomes among CB transplant and BM transplant recipients. (A) Probability of TRM. The 1-year cumulative incidence of TRM was 9% (95% CI, 2%-16%) among CB transplant recipients and 29% (95% CI, 15%-42%) among BM transplant recipients (P = .02). (B) Cumulative incidence of relapse. The 2-year probabilities of relapse among recipients were 16% (95% CI, 7%-25%) after CBT and 25% (95% CI, 12%-37%) after BMT. The difference between the 2 groups was not significant. (C) Kaplan-Meier estimate of DFS. The 2-year probabilities of DFS were 74% (95% CI, 63%-85%) after CBT and 44% (95% CI, 30%-59%) after BMT (P < .01).
Outcomes among CB transplant and BM transplant recipients. (A) Probability of TRM. The 1-year cumulative incidence of TRM was 9% (95% CI, 2%-16%) among CB transplant recipients and 29% (95% CI, 15%-42%) among BM transplant recipients (P = .02). (B) Cumulative incidence of relapse. The 2-year probabilities of relapse among recipients were 16% (95% CI, 7%-25%) after CBT and 25% (95% CI, 12%-37%) after BMT. The difference between the 2 groups was not significant. (C) Kaplan-Meier estimate of DFS. The 2-year probabilities of DFS were 74% (95% CI, 63%-85%) after CBT and 44% (95% CI, 30%-59%) after BMT (P < .01).
Multivariate analysis of graft source and factors associated with TRM, relapse, and DFS
Outcome and variable . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| TRM | ||
| Graft source | .02 | |
| Cord blood | 0.32 (0.12-0.86) | |
| Bone marrow | 1.0 | |
| HLA compatibility | < .01 | |
| 1AMM | 8.22 (2.96-22.8) | |
| Other than 1AMM | 1.0 | |
| Relapse | ||
| Graft source | .73 | |
| Cord blood | 0.76 (0.16-3.56) | |
| Bone marrow | 1.0 | |
| Disease status | < .01 | |
| High-risk disease | 22.3 (5.07-97.9) | |
| Standard risk | 1.0 | |
| Diagnosis of primary disease | < .05 | |
| Other than AML | 3.25 (1.01-10.4) | |
| AML | 1.0 | |
| DFS | ||
| Graft source | < .01 | |
| Cord blood | 0.27 (0.14-0.51) | |
| Bone marrow | 1.0 | |
| Disease status | < .01 | |
| High-risk disease | 6.15 (2.70-14.0) | |
| Standard risk | 1.0 |
Outcome and variable . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| TRM | ||
| Graft source | .02 | |
| Cord blood | 0.32 (0.12-0.86) | |
| Bone marrow | 1.0 | |
| HLA compatibility | < .01 | |
| 1AMM | 8.22 (2.96-22.8) | |
| Other than 1AMM | 1.0 | |
| Relapse | ||
| Graft source | .73 | |
| Cord blood | 0.76 (0.16-3.56) | |
| Bone marrow | 1.0 | |
| Disease status | < .01 | |
| High-risk disease | 22.3 (5.07-97.9) | |
| Standard risk | 1.0 | |
| Diagnosis of primary disease | < .05 | |
| Other than AML | 3.25 (1.01-10.4) | |
| AML | 1.0 | |
| DFS | ||
| Graft source | < .01 | |
| Cord blood | 0.27 (0.14-0.51) | |
| Bone marrow | 1.0 | |
| Disease status | < .01 | |
| High-risk disease | 6.15 (2.70-14.0) | |
| Standard risk | 1.0 |
TRM indicates transplantation-related mortality; 1AMM, one antigen mismatch; and DFS, disease-free survival.
DFS (Figure 3C) significantly improved among CB transplant compared with BM transplant recipients in multivariate analysis (Table 3). Higher risk of disease had a significant impact on the relapse and DFS results. Causes of death differed between CB transplant and BM transplant recipients: 10 of 24 deaths were GVHD related after BMT, whereas none of 16 deaths after CBT was GVHD related (Table 5). The major cause of death in CB transplant recipients was relapse in 10 of 16 patients.
Cause of death
. | Bone marrow transplantation, n = 24 . | Cord blood transplantation, n = 16 . |
|---|---|---|
| Relapse/refractory disease (%) | 9 (38) | 10 (63) |
| Infection (%) | 3 (13) | 3 (19) |
| GVHD ± infection (%) | 10 (42) | 0 |
| Organ failure ± infection (%) | 2 (8) | 3 (19) |
. | Bone marrow transplantation, n = 24 . | Cord blood transplantation, n = 16 . |
|---|---|---|
| Relapse/refractory disease (%) | 9 (38) | 10 (63) |
| Infection (%) | 3 (13) | 3 (19) |
| GVHD ± infection (%) | 10 (42) | 0 |
| Organ failure ± infection (%) | 2 (8) | 3 (19) |
GVHD indicates graft-versus-host disease.
Discussion
Hematologic recovery and GVHD incidence in CBT patients were similar to previous results on this type of allogeneic transplantation.4-7 In contrast, TRM and DFS for our CB transplant recipients were significantly better than previously reported.1-3,9,10,22 They were also superior to TRM and DFS in our BMT group, which were almost the same as previously reported.1-3,22,23 Significant delays in neutrophil and platelet engraftment rates occurred after CBT; however, overall hematopoietic engraftment rates were almost the same for both grafts, as reported,13 and early death and primary engraftment failure rates were only 4% and 8%, respectively, in our CBT series. These encouraging CBT results reflected the availability of grafts containing sufficient cell numbers6,7 ; the median cell number was 2.5 × 107 cells/kg and only 4 of 68 patients received cord blood containing less than 2.0 × 107 cells per kilogram. The shorter time from donor search to transplantation and low steroid therapy requirements for GVHD might be significant factors contributing to this improvement. Although the positive CMV antigenemia rate and incidence of preemptive ganciclovir therapy among CB transplant recipients tended to be higher than among BM transplant recipients,24 no clinical CMV infection was observed in any recipients after CBT (data not shown), probably because steroids were not required in most cases. A significantly lower incidence of infection as a cause of death in CBT patients was also observed in our series. On the other hand, previously published literature pointed to infection as a major cause of death.10,14,25
Despite a high frequency of HLA-mismatched grafts and faster tapering of GVHD prophylactic immunosuppressants in the CBT group, the risk of developing acute GVHD was lower than that in the BMT group, which was significant for grades III and IV severe acute GVHD, as described in other reports.12,26 The lower incidences of acute GVHD in both BM transplant and CB transplant recipients in our series compared with other reports could be correlated to the higher frequency of the gene that induces higher interleukin-10 production in the Japanese population27 or are assumed to reflect a lower degree of diversity for HLA and minor histocompatibility antigens among Japanese. Notably, there were no GVHD-related deaths after CBT, in contrast to more than 40% among BMT patients. Moreover, the probability of requiring steroid therapy to treat severe acute GVHD after CBT was significantly lower than that after BMT. Patients with acute GVHD of grades II to IV were generally considered for treatment with corticosteroid therapy; however, the decision to start the treatment in individual patients was flexible and determined by the physician depending on the condition of the patient. It is still uncertain why the majority of CB transplant recipients with severe acute GVHD could be successfully controlled without steroid therapy, resulting in good clinical outcomes regarding TRM and DFS. We used the Glucksberg-Seattle criteria20 for acute GVHD grading according to organ involvement, which have been shown to have a strong association with only BMT outcomes.2,28-30 However, acute GVHD after CBT might have occurred via a different pathophysiologic pathway and clinical outcomes could not be predicted using the same grading system as for BMT. In addition to immaturity and naivety,31-36 fewer cytotoxic and suppressor subsets,37 sustained functional CD40L expression,38 and enhanced apoptosis39-41 have also been reported in cord blood T cells. We are now analyzing immunologic reconstitution after CBT, particularly the T-cell maturation process.
The probability of relapse after CBT was almost equal to that of BMT, although slightly lower, suggesting that the graft-versus-tumor effect might be preserved, as previously reported.12,25 Notably, although more than half the patients in both graft groups belonged to the high-risk disease category, 2-year relapse rates of 22% after CBT and 30% after BMT were better than previously reported.1,3,12,25,42 Outcomes of AML patients were apparently good in our study; however, the ratio of advanced and standard AML patients was not especially different compared with other malignancies, and there were no particularly good prognostic factors in the AML group. The safety of the G-CSF–combined preparative regimen for myeloid malignancies has been confirmed17-19 and might contribute to reducing the risk of posttransplantation relapse.
In summary, we demonstrated that adult patients with hematologic malignancies receiving CB transplants from HLA partially mismatched unrelated donors had a lower risk of TRM and higher probability of DFS than HLA-matched unrelated BM transplant recipients. Although a larger-sized, randomized trial is needed, these results are encouraging in terms of CBT for adults requiring hematopoietic stem cell transplantation, if no family donor is available.
Prepublished online as Blood First Edition Paper, July 27, 2004; DOI 10.1182/blood-2004-03-1001.
Supported by The Kobayashi Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to doctor Pablo Rubinstein for critical review of the manuscript; the medical and nursing staff of the Hematopoietic Stem Cell Transplant Program at the Research Hospital, Institute of Medical Science, University of Tokyo for taking care of the patients; the Tokyo CBB, Hokkaido CBB, Hyogo CBB, Tokai CBB, Metro Tokyo Red Cross CBB, Chushikoku CBB, and the Caitlin Raymond International Registry for processing the cord blood units, especially to Drs Nobukatsu Watanabe and Tokiko Nagamura in Tokyo CBB for processing the cord blood grafts; and Ms Maki Ooiwa-Monna for her invaluable data management efforts.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal