Abstract
The phagocytosis of pathogens is a critical event in host defense, not only for clearance of the invading microorganism, but also for the subsequent immune response. We have examined Dectin-1, a proinflammatory nonopsonic receptor for β-glucans, and show that it mediates the internalization of β-glucan-bearing ligands, including yeast particles. Although requiring tyrosine phosphorylation and the cytoplasmic immunoreceptor tyrosine-based activation motif (ITAM)-like motif, uptake mediated by Dectin-1 was different from any previously reported phagocytic receptor and was not dependent on Syk-kinase in macrophages. Furthermore, intracellular trafficking of this receptor was influenced by the nature of the β-glucan ligand, which has significance for the biologic activity of these immunomodulatory carbohydrates. (Blood. 2004;104:4038-4045)
Introduction
Phagocytosis plays a critical role in innate immunity, both by facilitating the removal and killing of pathogens and by priming the adaptive immune response. The phagocytic process is initiated by the cross-linking of an array of dedicated surface receptors, some capable of direct recognition, the so-called pattern recognition receptors (PRRs), and others that recognize opsonins coating the pathogens. Of these receptors, the opsonic Fcγ (FcγRs) and complement receptors (CRs) are the best described and exhibit different phagocytic mechanisms and subsequent cellular responses that reflect important differences in their signaling pathways.1,2 Nonopsonic PRRs, such as the macrophage mannose receptor, scavenger receptors, and recently CEACAM3,3-5 have also been suggested to possess phagocytic capacity, but the mechanisms underlying these activities are less clear.
We have identified Dectin-1 as the major macrophage PRR for β-glucans, carbohydrate polymers that possess anti-infective and antitumorigenic properties in vivo.6-8 Dectin-1, which is also expressed on the surface of other innate immune cells, including neutrophils and dendritic cells,9 is a C-type lectinlike transmembrane receptor containing an immunoreceptor tyrosine-based activation motif (ITAM)-like motif in its cytoplasmic tail, which becomes tyrosine phosphorylated on ligand binding.10,11 We and others have shown this motif was required for proinflammatory cytokine production, in collaboration with the Toll-like receptors (TLRs), and for induction of the respiratory burst in response to β-glucan ligands, including fungal pathogens.11,12
The proinflammatory properties of Dectin-1 are similar to those of the ITAM-containing FcγRs, which also mediate the internalization of immune complexes.13 Phagocytosis by FcγR, after ligand binding, is thought to be initiated by src kinase-mediated tyrosine phosphorylation of the receptor ITAM domains leading to the recruitment of p72Syk, a protein tyrosine kinase that is required for subsequent cellular activation and ligand internalization.1,14 Although the exact downstream pathways leading from Fcγ and other receptors to actin polymerization and phagocytosis is currently unclear, other molecules, including phosphatidylinositol (PI)-3 kinase, protein kinase C (PKC), and the Rho guanosine triphosphatases (GTPases), are known to be involved.1
We determined if Dectin-1 could also internalize β-glucan ligands and show here that this receptor can convert “nonprofessional” phagocytes into efficient phagocytes for yeasts. Furthermore, we show that although this activity was dependent on the ITAM-like motif in the cytoplasmic tail, the mechanism of Dectin-1-mediated phagocytosis in macrophages is distinct from that mediated by any other receptor characterized, including the FcγR and CR. We have also followed the intracellular trafficking of Dectin-1 and show that it is dependent on the nature of the β-glucan ligand.
Materials and methods
Primary cells and cell lines
Thioglycollate-elicited peritoneal macrophages were isolated from C57BL/6 mice as previously described.9 Syk-deficient bone marrow-derived macrophages (BMDMs) were generated from chimeric mice, as described.15 In brief, fetal livers from CD45.2 Syk+/- × Syk+/- matings were removed from day 16.5 embryos and the fetal liver cell suspensions from Syk-/- and Syk-sufficient womb-mates were then used to reconstitute irradiated CD45.1 C57BL6/SJL recipients. Bone marrows were harvested 4 to 6 weeks after reconstitution and checked by fluorescence-activated cell sorting (FACS) for CD45.2 expression (data not shown). Animals were kept according to institutional guidelines. The generation of the Dectin-1 constructs and transduction of NIH 3T3 fibroblasts (American Type Culture Collection [ATCC], Manassas, VA; ATCC no. 1658) and RAW 264.7 macrophages (ATCC no. TIB 71) have been described previously.7,12
Cells were maintained in either RPMI (primary cells) or Dulbecco modified Eagle medium (DMEM; cell lines) with 10% heat-inactivated fetal calf serum (FCS), 50 IU/mL penicillin G, 50 μg/mL streptomycin, and 2 mM glutamine (RPMI medium), except for the BMDMs, which were cultured in RPMI medium supplemented with 15% (vol/vol) L cell-conditioned medium as a source of macrophage colony-stimulating factor (M-CSF).16 Where necessary, the medium was supplemented with 600 μg mL-1 G418 (Gibco, Carlsbad, CA) to maintain expression of transduced genes.
Phagocytosis and uptake assays
Opsonized, PKH2-labeled, sheep red blood cells (SRBCs; TCS Biosciences, Buckingham, United Kingdom) and fluorescein isothiocyanate (FITC)-labeled zymosan particles (Molecular Probes, Eugene, OR) were used for the phagocytic assays. The SRBCs were opsonized with subagglutinating concentrations of rabbit anti-SRBC IgG (Sigma, St Louis, MO), washed extensively, and then labeled with PKH2 (Sigma), as described by the manufacturer.
Phagocytosis was quantified as previously described.17 In brief, cells were seeded at either 3 × 105/well (NIH-3T3) or 5 × 105/well (RAW264.7 and BMDMs) in 12-well plates and incubated at 37°C for 24 hours. Where required, inhibitors were added 40 minutes prior to and then maintained throughout the assay, except for toxin B, which was added 120 minutes prior to the assay and chelerythrine, which was added 10 minutes prior to the assay (see “Inhibition assays”). After washing, labeled zymosan or opsonized SRBCs were added (10 particles/cell) and allowed to settle onto the cells for 1 hour at 4°C. The cells were then washed thoroughly, to remove unbound particles, and incubated at 37°C for a further 30 or 60 minutes to allow internalization of the bound particles. After cooling to 4°C the cells were blocked with phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and 5% heat-inactivated goat serum (FACS-blocking solution). External zymosan particles, stained with rabbit antizymosan antibody (Molecular Probes), or opsonized SRBCs were detected with an allophycocyanin (APC)-conjugated goat anti-rabbit antibody (Molecular Probes). The cells were then detached and fixed in 1% formaldehyde and analyzed by flow cytometry, performed according to conventional protocols, by gating on the FITC/PKH2+ cell populations, which have bound/internalized zymosan or SRBCs. The percentage of phagocytosis was determined by comparing the APC- (internalized particles) to the APC+ (noninternalized particles) cell populations. This FACS-based assay is sensitive to at least 0.5 particles/cell (data not shown). Cells treated with cytochalasin D to block phagocytosis were used as controls.
To examine the receptor recovery at the cell surface in primary cells, thioglycollate-elicited peritoneal macrophages were seeded at 2 × 105 cells/well in 24-well plates and allowed to adhere overnight. Where necessary, protein synthesis was blocked by the addition of 10 μg/mL cycloheximide (Sigma) 2 hours prior to the assay and then maintained throughout the assay. Inhibition of protein synthesis was confirmed by assessing [35S]-methionine incorporation (data not shown). Surface expressed Dectin-1 was quenched by the addition of 100 μg/mL unlabeled zymosan, glucan phosphate, or laminarin (Sigma) for 60 minutes at 4°C. After synchronous warming to 37°C for 10 minutes, to ensure complete quenching of surface receptor, unbound particles or soluble glucans were removed by thorough washing with ice-cold medium. The recovery of Dectin-1 to the cell surface, after incubation at 37°C for the indicated periods, was then measured by monitoring the recovery of cold zymosan-binding capacity, as described.8 Briefly, FITC-labeled zymosan was added (100 particles/cell) and allowed to bind to the cells for 60 minutes at 4°C. After washes to remove unbound particles, the cells were lysed in 3% Triton X-100, and the amount of FITC-zymosan bound by the cells quantified using a Titertek Fluoroscan II (Labsystems Group [UK], Basingstoke, United Kingdom). The role of Dectin-1 in the recovery of zymosan-binding capacity was confirmed by inhibition of this activity with 5 μg/mL 2A11.
Inhibition assays
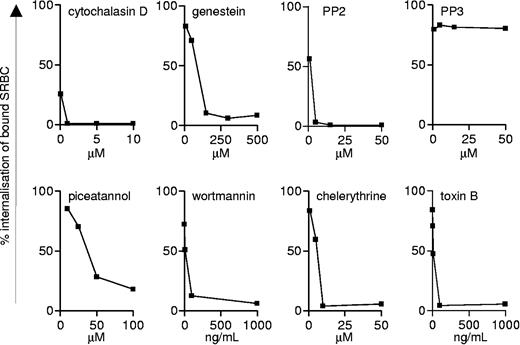
The following inhibitors were used in these studies: 5 μM cytochalasin D (Sigma), 15 μM PP2 (Calbiochem, San Diego, CA), 15 μM PP3 (Calbiochem), 50 μM piceatannol (Sigma), 100 ng/mL toxin B (Calbiochem), 150 μM genestein (Sigma), 10 μM chelerythrine (Calbiochem), or 100 ng/mL wortmannin (Sigma). The inhibitor concentrations used did not have adverse effects on cell viability, during the course of the assays, and were based on comparison with concentrations used elsewhere in the literature and from titration assays (Figure 1). Control cells were treated with the same concentration of the carrier in which the inhibitors had been dissolved.
Titration of inhibitors of Fcγ-mediated phagocytosis. The effects of various inhibitors on SRBC uptake in RAW264.7 cells was assessed to identify conditions that inhibited the components of the Fcγ uptake machinery, as described in “Dectin-1-mediated phagocytosis is Syk-independent in macrophages.”
Titration of inhibitors of Fcγ-mediated phagocytosis. The effects of various inhibitors on SRBC uptake in RAW264.7 cells was assessed to identify conditions that inhibited the components of the Fcγ uptake machinery, as described in “Dectin-1-mediated phagocytosis is Syk-independent in macrophages.”
To determine the contribution of the various Rho GTPases to Dectin-1-mediated phagocytosis, 1 × 106 NIH-3T3 cells expressing full-length hemagglutinin (HA)-tagged Dectin-1, were seeded in the wells of a 6-well plate. After overnight incubation, the cells were transfected with the various constructs using the GenePorter transfection reagent (Gene Therapy Systems, San Diego, CA), as described by the manufacturer, and assayed the following day. The myc-tagged dominant-negative constructs used in these assays (N19RhoA, N17Rac-1, and N17Cdc-42) have been described previously.2 An irrelevant myc-tagged EGF-TM7 protein18 was included as a control. To analyze the effects on phagocytosis, FITC-labeled zymosan was added to the transfected cells and incubated at 4°C for 1 hour. After thorough washing to remove unbound zymosan, the cells were incubated at 37°C for a further hour to allow internalization of the bound particles. After staining the zymosan, as described (see “Phagocytosis and uptake assays”), the cells were detached, fixed in 4% paraformaldehyde, and then blocked and permeabilized with 0.5% saponin (Sigma) in FACS-blocking solution. Expression of the myc-tagged proteins were then detected using mouse anti-myc (9E10; Abcam, Cambridge, United Kingdom) and R-phycoerythrin-conjugated goat anti-mouse IgG Fcγ (Jackson ImmunoResearch Laboratories, West Grove, PA). Phagocytosis was quantitated by FACS analysis, and the effect of the various dominant-negative constructs determined by gating on the myc+ cell populations.
Immunofluorescence microscopy
For immunofluorescence microscopy, cells were plated at 2.5 × 104 (NIH-3T3) or 5 × 104 (RAW264.7) cells per well, on glass coverslips, in 24-well plates. After synchronous incubation with FITC-labeled zymosan (5 particles/cell), the cells were fixed in 4% paraformaldehyde and permeabilized using 0.5% saponin in FACS-blocking solution. Cells were then stained with anti-LAMP-1 (ID4B; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City) and cyanine 3 (Cy3)-labeled goat anti-rat antibody (Jackson ImmunoResearch), or Alexa 488-conjugated anti-HA (HA.11; Covance, Princeton, NJ). Actin was detected with tetramethylrhodamine isothiocyanate (TRITC)-labeled phalloidin (Sigma). After mounting, the cells were observed by confocal laser scanning microscopy (Bio-Rad 1024, Hercules, CA) on a Nikon Diaphot 200 inverted microscope and images were processed using Adobe Photoshop version 6.0.
Immunoblotting
Immunoblotting was performed on whole-cell lysates and antigens detected with mouse anti-Syk (Abcam), or mouse anti-β actin (Sigma), and donkey ant-mouse horseradish peroxidase (HRP; (Jackson ImmunoResearch), as described previously.19 Blots were developed using the enhanced chemiluminescence (ECL) kit (Amersham, Uppsala, Sweden).
Statistics
Statistics were calculated in GraphPad Prism (version 3.00; GraphPad Software) using either one-way analysis of variance (ANOVA) with the Bonferroni multiple comparison test or paired 2-tailed t tests. P < .05 indicated significance (shown by an asterisk); NS indicates not significant.
Results
Dectin-1 is a phagocytic receptor for yeast
We have previously shown that Dectin-1 can recognize zymosan,7 a yeast-derived particle widely used to study phagocytosis.6 We wished to determine whether this receptor could also mediate internalization of these particles and examined the fate of fluorescently labeled zymosan after binding to transfected NIH-3T3 fibroblasts. Using confocal analysis, it was apparent that the transfected cells were capable of internalizing bound particles through distinctive actin-rich phagocytic cups that appeared to extend over and surround the particles (Figure 2A and Brown and Gordon7 ). Zymosan internalization required warming to 37°C and the ability to block uptake, but not binding, with cytochalasin D, demonstrated the actin-dependent nature of this process (Figure 2D).
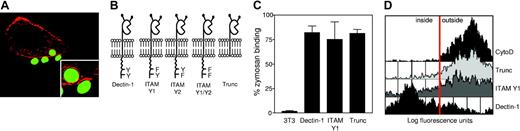
Zymosan phagocytosis by Dectin-1 is actin-dependent and requires the ITAM-like motif in the cytoplasmic tail of the receptor. (A) NIH-3T3 cells stably expressing full-length Dectin-1 bind and internalize FITC-labeled zymosan particles (green) through actin-rich phagocytic cups. Actin was visualized by staining with TRITC-labeled phalloidin (red) and phagocytosis was not synchronized. (B) Schematic representation of the constructs used in these experiments demonstrating the position of the various cytoplasmic tail mutations. (C) Comparative binding of FITC-labeled zymosan to NIH-3T3 fibroblasts transduced with selected constructs. (D) FACS-based analysis showing the extent of zymosan internalization by the indicated transductants. The red line indicates the threshold between cells with external or internalized zymosan, as determined by inhibition of uptake with cytochalasin D (CytoD). The data shown are representative of 3 independent experiments.
Zymosan phagocytosis by Dectin-1 is actin-dependent and requires the ITAM-like motif in the cytoplasmic tail of the receptor. (A) NIH-3T3 cells stably expressing full-length Dectin-1 bind and internalize FITC-labeled zymosan particles (green) through actin-rich phagocytic cups. Actin was visualized by staining with TRITC-labeled phalloidin (red) and phagocytosis was not synchronized. (B) Schematic representation of the constructs used in these experiments demonstrating the position of the various cytoplasmic tail mutations. (C) Comparative binding of FITC-labeled zymosan to NIH-3T3 fibroblasts transduced with selected constructs. (D) FACS-based analysis showing the extent of zymosan internalization by the indicated transductants. The red line indicates the threshold between cells with external or internalized zymosan, as determined by inhibition of uptake with cytochalasin D (CytoD). The data shown are representative of 3 independent experiments.
The conserved motif in the cytoplasmic tail of Dectin-1 is reminiscent of an ITAM sequence, which is known to be essential for FcγR-mediated phagocytosis.1 To explore this further, we generated a number of NIH-3T3 cell lines stably expressing recombinant Dectin-1 molecules containing mutations in the cytoplasmic tail and ITAM-like motif and examined the ability of these cells to internalize zymosan (Figure 2B). The recombinant receptors were expressed to similar levels (data not shown) and conferred equivalent zymosan-binding abilities to the cells (Figure 2C). Using a FACS-based assay, we observed that removal of the cytoplasmic tail or a mutation in the membrane proximal tyrosine almost completely blocked the ability of Dectin-1 to internalize the zymosan particles (Figure 2D). Mutation of the membrane distal tyrosine had no apparent effect on phagocytosis (data not shown; Figure 4). Thus on ligation, Dectin-1 actively induces cytoskeletal rearrangement and phagocytosis through the ITAM-like motif in its cytoplasmic tail.
Zymosan phagocytosis in RAW264.7 macrophages expressing various forms of HA-tagged Dectin-1. RAW264.7 macrophages expressing Dectin-1 lacking the cytoplasmic tail (Trunc) or mutations in the membrane proximal tyrosine of the cytoplasmic ITAM-like motif (Y1 and Y1/Y2) were unable to internalize bound zymosan. No effect on phagocytosis was observed with mutations in the membrane distal tyrosine (Y2). Cells expressing full-length Dectin-1 were pretreated with cytochalasin D to block internalization, as a control. Zymosan phagocytosis in these cells was synchronized and allowed to occur for 60 minutes. Dectin-1 was visualized by staining for the HA tag (red), using an APO oil immersion lens at 60× magnification, 1.4 NA.
Zymosan phagocytosis in RAW264.7 macrophages expressing various forms of HA-tagged Dectin-1. RAW264.7 macrophages expressing Dectin-1 lacking the cytoplasmic tail (Trunc) or mutations in the membrane proximal tyrosine of the cytoplasmic ITAM-like motif (Y1 and Y1/Y2) were unable to internalize bound zymosan. No effect on phagocytosis was observed with mutations in the membrane distal tyrosine (Y2). Cells expressing full-length Dectin-1 were pretreated with cytochalasin D to block internalization, as a control. Zymosan phagocytosis in these cells was synchronized and allowed to occur for 60 minutes. Dectin-1 was visualized by staining for the HA tag (red), using an APO oil immersion lens at 60× magnification, 1.4 NA.
Dectin-1-mediated phagocytosis requires protein tyrosine kinases and Rho GTPases
Because Dectin-1 becomes tyrosine phosphorylated on zymosan binding,11 we wondered if any of the signaling molecules known to be involved in ITAM-mediated (FcγR) phagocytosis were also involved in Dectin-1-mediated uptake. For this study, we examined the effects of pharmacologic inhibitors of specific signaling molecules on zymosan uptake in the transduced NIH-3T3 fibroblasts (Figure 3A). Using a FACS-based assay, we observed that the general tyrosine kinase inhibitor, genestein, and the specific Src family kinase inhibitor, PP2, but not its inactive analog, PP3, were able to inhibit Dectin-1-mediated zymosan phagocytosis. Furthermore, significant inhibition of Dectin-1-mediated uptake was observed using piceatannol, wortmannin, chelerythrine, and toxin B, which inhibit p72Syk, PI-3 kinase, PKC, and the Rho GTPase family, respectively (Figure 3A).
Dectin-1-mediated phagocytosis resembles that of FcγR in transduced NIH-3T3 fibroblasts. (A) The effect of pharmacologic inhibitors on Dectin-1-mediated zymosan uptake was determined using flow cytometry, as described in “Materials and methods.” Zymosan phagocytosis was synchronized and allowed to occur for 60 minutes before quantitation. DMSO (dimethyl sulfoxide) represents a solvent-alone control. (B) Dectin-1-expressing NIH-3T3 cells were transfected with the dominant-negative Rho-GTPase constructs N19RhoA, N17Rac-1, and N17Cdc42 24 hours prior to quantitation of phagocytic capacity by flow cytometry. A transfection control, without DNA, was included to demonstrate the effects of the transfection on particle uptake. The values shown are the mean ± SEM of data pooled from 3 independent experiments and are expressed relative to uptake in cells in medium only. *P < .05.
Dectin-1-mediated phagocytosis resembles that of FcγR in transduced NIH-3T3 fibroblasts. (A) The effect of pharmacologic inhibitors on Dectin-1-mediated zymosan uptake was determined using flow cytometry, as described in “Materials and methods.” Zymosan phagocytosis was synchronized and allowed to occur for 60 minutes before quantitation. DMSO (dimethyl sulfoxide) represents a solvent-alone control. (B) Dectin-1-expressing NIH-3T3 cells were transfected with the dominant-negative Rho-GTPase constructs N19RhoA, N17Rac-1, and N17Cdc42 24 hours prior to quantitation of phagocytic capacity by flow cytometry. A transfection control, without DNA, was included to demonstrate the effects of the transfection on particle uptake. The values shown are the mean ± SEM of data pooled from 3 independent experiments and are expressed relative to uptake in cells in medium only. *P < .05.
The Rho family of GTPases, in particular, are essential mediators of phagocytosis, involved in the reorganization of the actin cytoskeleton.20 Because the Rho inhibitor, toxin B, was effective at inhibiting Dectin-1-mediated phagocytosis (Figure 3A), we wished to define which particular Rho GTPase was involved in this process. Using dominant-negative (DN) constructs to selectively inhibit each molecule in turn, we observed that the myc-tagged DN Cdc42 construct (N17Cdc42) and the DN Rac-1 construct (N17Rac-1) had the most significant inhibitory activity on Dectin-1-mediated zymosan phagocytosis (Figure 3B). No effect was observed with the DN Rho (N19RhoA) or the control (TM-7) construct. The lack of inhibition by DN-Rho, and the relative degree of inhibition by the DN Rac-1 and Cdc42 constructs, was similar to that previously observed for FcγR-mediated phagocytosis.2 Thus, overall, these results suggest that Dectin-1-mediated phagocytosis in NIH-3T3 cells resembles that of FcγR.
Dectin-1-mediated phagocytosis is Syk-independent in macrophages
Given the apparent mechanistic similarity of Dectin-1 to that of the FcγR, we then directly compared phagocytosis mediated by these receptors. Because RAW264.7 macrophages have been previously used to study FcγR function, but only express low levels of Dectin-1, we used cells transduced to express higher levels of Dectin-1 for these experiments.12 Similar to the NIH-3T3 transductants, the ability of the transduced macrophages to internalize bound zymosan was dependent on the membrane proximal tyrosine within the ITAM-like motif in the cytoplasmic tail of Dectin-1, and mutation of the membrane distal tyrosine had no effect on internalization (Figure 4).
We next examined the effect of the various inhibitors on phagocytosis mediated by these receptors, using equivalent numbers of antibody opsonized SRBCs and zymosan particles per cell, as specific ligands for the FcγR and Dectin-1, respectively (Figure 5A). The inhibitors were used at concentrations that significantly affected specific components involved in Fcγ-mediated uptake (Figure 1), thereby allowing a direct assessment of their contribution to Dectin-1-mediated uptake. Although all the inhibitors tested, except piceatannol, had a significant inhibitory effect, they showed marked differences in their effects on particle uptake mediated by these 2 receptors. Inhibition of the src kinases, PI-3K, and the Rho GTPases almost completely blocked FcγR-mediated uptake, but had only a partial effect on Dectin-1 phagocytosis. More significantly, in contrast to what was observed in the NIH-3T3 cells, the Syk kinase inhibitor, piceatannol, had no effect on Dectin-1-mediated uptake in the RAW macrophages.
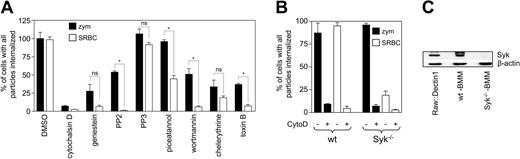
Comparison of Dectin-1 and FcγR-mediated phagocytosis in macrophages, demonstrating that Dectin-1-mediated phagocytosis is Syk-independent. (A) RAW264.7 cells expressing Dectin-1 were incubated with the various inhibitors, as indicated in “Materials and methods,” prior to synchronized uptake of equal numbers of particles of zymosan or antibody-opsonized SRBCs for 30 minutes. The amount of uptake was normalized relative to the DMSO solvent control to allow direct comparison between experiments, although there was no significant difference in uptake between the zymosan or antibody-opsonized SRBCs fed control cells (84.9 ± 5.1 versus 76.24 ± 12.33, respectively). The data presented were pooled from 3 independent experiments and are shown as the mean ± SEM. * indicates significant differences (P < .05) in uptake between the 2 receptors; ns indicates not significant. (B) Comparative phagocytosis of unopsonized zymosan and antibody opsonized SRBCs in wild-type (wt) and Syk deficient (Syk-/-) bone marrow-derived macrophages. Cytochalasin D (CytoD) was added to inhibit phagocytosis, as a control in these experiments. The figure shows the mean ± SEM of data pooled from 3 independent experiments. (C) Anti-p72Syk Western blot demonstrates the presence or absence of Syk in whole-cell lysates, as indicated. Zap70 was also not detected in any of the lysates (data not shown).
Comparison of Dectin-1 and FcγR-mediated phagocytosis in macrophages, demonstrating that Dectin-1-mediated phagocytosis is Syk-independent. (A) RAW264.7 cells expressing Dectin-1 were incubated with the various inhibitors, as indicated in “Materials and methods,” prior to synchronized uptake of equal numbers of particles of zymosan or antibody-opsonized SRBCs for 30 minutes. The amount of uptake was normalized relative to the DMSO solvent control to allow direct comparison between experiments, although there was no significant difference in uptake between the zymosan or antibody-opsonized SRBCs fed control cells (84.9 ± 5.1 versus 76.24 ± 12.33, respectively). The data presented were pooled from 3 independent experiments and are shown as the mean ± SEM. * indicates significant differences (P < .05) in uptake between the 2 receptors; ns indicates not significant. (B) Comparative phagocytosis of unopsonized zymosan and antibody opsonized SRBCs in wild-type (wt) and Syk deficient (Syk-/-) bone marrow-derived macrophages. Cytochalasin D (CytoD) was added to inhibit phagocytosis, as a control in these experiments. The figure shows the mean ± SEM of data pooled from 3 independent experiments. (C) Anti-p72Syk Western blot demonstrates the presence or absence of Syk in whole-cell lysates, as indicated. Zap70 was also not detected in any of the lysates (data not shown).
Because Syk is thought to be an essential kinase required for ITAM signaling,21 we next examined FcγR and Dectin-1-mediated phagocytosis in Syk-deficient BMDMs (Figure 5B). Using zymosan particles, whose uptake in these cells is mediated by Dectin-1 (data not shown and Brown et al8 ), as well as opsonized SRBCs, we observed a similar degree of uptake of each type of particle in wild-type cells, which could be completely inhibited with cytochalasin D (Figure 4B). As described previously, the Syk-/- macrophages were unable to mediate phagocytosis through the FcγR,14 but were fully competent in Dectin-1-mediated internalization. In addition, Syk-/- macrophages were still able to produce tumor necrosis factor α (TNF-α) in response to zymosan (data not shown). The presence or absence of Syk in these macrophages and in RAW264.7 cells was confirmed by Western blotting (Figure 5C). Thus from these data it appears that Dectin-1, while resembling FcγR in NIH-3T3 fibroblasts, uses different cellular machinery to induce phagocytosis and cellular activation in macrophages.
Intracellular trafficking of Dectin-1 is dependent on the nature of the β-glucan ligand
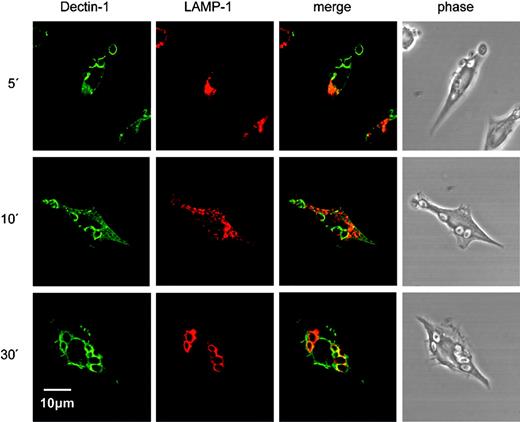
We next examined the intracellular fate of Dectin-1 after ligand binding and internalization in RAW264.7 macrophages expressing full-length HA-tagged Dectin-1. The zymosan phagosome is known to mature and fuse with lysosomes, so we followed the synchronous phagocytosis of zymosan over time along with the late endosomal/lysosomal marker, lysosomal membrane glycoprotein 1 (LAMP-122 ; Figure 6). The zymosan particles were observed to be rapidly internalized along with Dectin-1, which colocalized with LAMP-1 by 30 minutes. At time points beyond 30 minutes, the HA epitope, which is exposed to the phagolysosomal interior, became difficult to detect, which suggested that it was being degraded within this compartment. Thus, Dectin-1 is retained on the phagosomal membrane throughout its maturation to a phagolysosome.
Dectin-1 traffics to lysosomes after zymosan binding and internalization. A time course of confocal images following the synchronous phagocytosis of zymosan in RAW 264.7 cells stably expressing HA-tagged Dectin-1 is shown. Cells were fixed, permeabilized, and stained to visualize Dectin-1 (green) and the late endosomal/lysosmal compartment marker LAMP-1 (red), using an APO oil immersion lens at 60× magnification, 1.4 NA. Zymosan phagosomes started becoming LAMP-1+ by 10 minutes and showed extensive colocalization of Dectin-1 and LAMP-1 by 30 minutes. The images are representative of 3 separate time-course experiments.
Dectin-1 traffics to lysosomes after zymosan binding and internalization. A time course of confocal images following the synchronous phagocytosis of zymosan in RAW 264.7 cells stably expressing HA-tagged Dectin-1 is shown. Cells were fixed, permeabilized, and stained to visualize Dectin-1 (green) and the late endosomal/lysosmal compartment marker LAMP-1 (red), using an APO oil immersion lens at 60× magnification, 1.4 NA. Zymosan phagosomes started becoming LAMP-1+ by 10 minutes and showed extensive colocalization of Dectin-1 and LAMP-1 by 30 minutes. The images are representative of 3 separate time-course experiments.
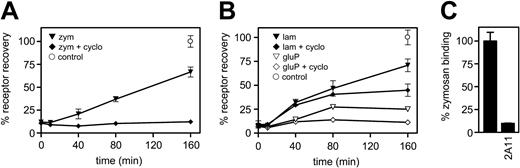
We next characterized receptor trafficking in thioglycollate-elicited macrophages, primary macrophages that express high levels of Dectin-1 at the cell surface.9 Because the anti-Dectin-1 antibody 2A11 is sensitive to fixation,8 we determined receptor trafficking indirectly, by analyzing Dectin-1 recovery at the cell surface, after ligand binding, in the presence or absence of the protein synthesis inhibitor, cycloheximide (Figure 7). The recovery of Dectin-1 at the cell surface was determined by measuring the capacity of the cells to bind fluorescently labeled zymosan, over time, and by FACS analysis with 2A11 (data not shown). When the cells were incubated with an excess of unlabeled zymosan, the receptor was rapidly internalized and then surface expression recovered over time (Figure 7A). In the presence of cycloheximide, however, no recovery of Dectin-1 surface expression was observed. Because some primary macrophages express other receptors that can recognize zymosan,9 we confirmed that the recovered zymosan-binding activity was due to Dectin-1, by blocking binding with 2A11 (Figure 7C and Brown et al8 ). Thus these data suggest that after zymosan binding and internalization, the recovery of Dectin-1 at the cell surface is due to new synthesis.
The intracellular trafficking of Dectin-1 depends on the nature of the β-glucan ligand. After overnight incubation, thioglycollate-elicited macrophages were pretreated with or without cycloheximide (cyclo) and then incubated with an excess of unlabeled (A) zymosan (zym) or (B) laminarin (lam) or glucan phosphate (gluP) for 60 minutes at 4°C. Cells were then incubated at 37°C for the times indicated, before the extent of Dectin-1 recovery at the cell surface was measured using cold FITC-labeled zymosan. The results are expressed as the percentage recovery of Dectin-1 versus control cells, at 160 minutes, that were not exposed to carbohydrate. Values are expressed as the mean ± SEM from triplicate samples and are representative of at least 3 independent experiments. Similar receptor kinetics were also observed by FACS analysis using 2A11 (data not shown). (C) To confirm that the recovered zymosan-binding capacity was due to Dectin-1, control cells from the final time point were treated with monoclonal antibody 2A11 to block the Dectin-1 lectin-binding site for 60 minutes prior to adding the FITC zymosan particles. The contribution of Dectin-1 was also confirmed in cells pretreated with zymosan, laminarin, and glucan phosphate (data not shown).
The intracellular trafficking of Dectin-1 depends on the nature of the β-glucan ligand. After overnight incubation, thioglycollate-elicited macrophages were pretreated with or without cycloheximide (cyclo) and then incubated with an excess of unlabeled (A) zymosan (zym) or (B) laminarin (lam) or glucan phosphate (gluP) for 60 minutes at 4°C. Cells were then incubated at 37°C for the times indicated, before the extent of Dectin-1 recovery at the cell surface was measured using cold FITC-labeled zymosan. The results are expressed as the percentage recovery of Dectin-1 versus control cells, at 160 minutes, that were not exposed to carbohydrate. Values are expressed as the mean ± SEM from triplicate samples and are representative of at least 3 independent experiments. Similar receptor kinetics were also observed by FACS analysis using 2A11 (data not shown). (C) To confirm that the recovered zymosan-binding capacity was due to Dectin-1, control cells from the final time point were treated with monoclonal antibody 2A11 to block the Dectin-1 lectin-binding site for 60 minutes prior to adding the FITC zymosan particles. The contribution of Dectin-1 was also confirmed in cells pretreated with zymosan, laminarin, and glucan phosphate (data not shown).
Dectin-1 is also the major receptor for soluble β-glucans on macrophages and is internalized after binding these polymers.8 Because it is thought that the immunomodulatory effects of soluble β-glucans are related to their molecular weight,6 we examined Dectin-1 trafficking after binding of 2 glucans with differing molecular weights, glucan phosphate and laminarin (156 000 versus 7700 g/mol, respectively23 ; Figure 6B). By examining the recovery of surface Dectin-1 in primary macrophages after incubation with excess soluble ligand, as before, we observed that after the addition of the small glucan, laminarin, the receptor returned to the cell surface, even in the presence of cycloheximide, suggestive of receptor recycling. In contrast, the levels of surface Dectin-1 did not greatly recover after the addition of the large glucan, glucan phosphate, even in the absence of cycloheximide, throughout the duration of the experiment. Thus these data suggest that the route of intracellular trafficking of Dectin-1 depends on the nature of the β-glucan ligand.
Discussion
We have shown that the cytoskeletal rearrangements triggered by Dectin-1 to induce particle internalization are dependent on a motif in the cytoplasmic tail of this receptor, which is strikingly similar to ITAM sequences found in a variety of activating immune receptors, including DAP12 and the T-cell, B-cell, and Fcγ receptors.21 The motif in the Dectin-1 tail (YxxxI/Lx7YxxL), which is conserved in the mouse, human, and monkey receptors, differs only slightly from the canonical ITAM sequence (YxxL/Ix7-12YxxL/I). We speculated that this would imply mechanistic similarity with FcγRs, and particularly to FcγRIIA, another single-chain receptor containing an ITAM in its cytoplasmic tail.13 Indeed, our initial analysis in NIH-3T3 cells suggested that Dectin-1 was using similar signaling pathways/molecules to mediate phagocytosis. Major differences, however, only became apparent when we directly compared these receptors in macrophages. This highlights an essential requirement to study these processes in the correct cellular context.
Dectin-1 becomes tyrosine phosphorylated after ligand binding,11 and we have shown that phagocytosis by this receptor was blocked by the nonspecific tyrosine kinase inhibitor, genestein. Tyrosine phosphorylation is an initiating event for FcγR-mediated, but not CR-mediated, phagocytosis and is mediated by the Src family kinases.1,24 At least 6 src kinases are known to be expressed in phagocytes and are essential for FcγR-mediated internalization (Figure 5 and Majeed et al25 and Garcia-Garcia and Rosales26 ). Consistent with previous observations of zymosan uptake in macrophages,25 we found that the Src kinases were only partially involved in Dectin-1-mediated uptake, and were not essential, because complete inhibition of uptake was not obtained with the src-kinase inhibitor PP2. This suggests that other kinases are involved in the phosphorylation of Dectin-1.
In FcγR, Syk is recruited to the phosphorylated ITAMs and is essential for subsequent ITAM-mediated signaling events in myeloid cells.21,24 Strikingly, in macrophages, Dectin-1-mediated phagocytosis did not require Syk, a finding that explains previous observations on the nature of zymosan uptake by macrophages deficient in this kinase.14,19 Although only the membrane proximal tyrosine was required for Dectin-1-mediated uptake, whereas both tyrosines are optimally required for FcγR-mediated phagocytosis, this lack of dependence on Syk is surprising, given that some FcγR-mediated internalization can occur with imperfect ITAM sequences.27,28 However, this does imply that the motif in the cytoplasmic tail of Dectin-1 does not function as a classic ITAM in macrophages. Although uptake in the transduced NIH-3T3 fibroblasts was blocked with piceatannol, suggesting a role for Syk in these cells, this inhibitory effect may have been due to inhibition of other unrelated kinases.29 Overall, these findings suggest that in macrophages the initiating events leading to Dectin-1-mediated phagocytosis are likely to involve novel signaling pathways or molecules or both.
We have also examined the contribution of other enzymes known to be involved in receptor-mediated phagocytosis, including PI-3 kinase, PKC, and the Rho GTPases. PI-3 kinase is a lipid kinase that is involved in a variety of signaling pathways and is required for FcγR- and CR-mediated phagocytosis in macrophages.30,31 In FcγR-mediated uptake, PI-3 kinase has also been shown to be required for pseudopod extension and phagosomal closure.31,32 Although inhibition of PI-3 kinase affected Dectin-1-mediated particle internalization, it did not completely block this process, suggesting that this enzyme was not essential for phagocytosis by this receptor. Because wortmannin sensitivity is normally relieved by smaller particles,32 and because SRBCs are slightly smaller than zymosan, the effect we observed is unlikely to be due to differences in particle size. This may be a unique feature of Dectin-1 in macrophages because PI-3 kinase-independent uptake has only been shown previously with Fcγ receptors in monocytes.33 PI-3 kinase has also recently been implicated in β-glucan-mediated immunomodulation in vivo,34 but the role of Dectin-1 in this response has yet to be established.
PKC comprises a large family of serine/threonine kinases that are required for phagocytosis by a number of receptors, including both CR and FcγR.35 PKC is also required by Dectin-1 because phagocytosis by this receptor was blocked by the PKC inhibitor, chelerythrine, consistent with previous reports that this enzyme is required for zymosan uptake by macrophages.22 Although the exact role of PKC in phagocytosis is unknown, one of its substrates, MARCKS (myristoylated, alanine-rich C-kinase substrate), possesses actin cross-linking activity that is involved in zymosan phagocytosis.22 Furthermore, because MARCKS-deficient macrophages appear to have partial defects in zymosan phagocytosis only, MARCKS may be specifically induced by Dectin-1.36 It is also tempting to speculate that PKC is involved in the Dectin-1-mediated induction of the NAPDH oxidase,11 as has been demonstrated for complement-opsonized zymosan in neutrophils.37
The Rho GTPases are important regulators of the actin cytoskeleton and are involved in many cellular processes, including phagocytosis.20 Two distinct internalization pathways have been described, which are controlled by different Rho GTPases and which depend on the surface receptor engaged: type I phagocytosis, which requires Cdc42 and Rac-1 and is used by the FcγR, and type II phagocytosis, which requires Rho and is used by CR.2 We were able to show that Dectin-1-mediated actin reorganization is dependent on these GTPases and that the receptor mediates type I phagocytosis in NIH-3T3 cells, requiring Rac-1 and Cdc42, but not Rho. Notably, the Rho GTPase inhibitor, toxin B, did not completely block Dectin-1-mediated uptake in RAW macrophages when compared to FcR, which may suggest that other mechanisms are also involved in these cells. Rac-1 has also been shown to be involved in TLR2-mediated nuclear factor κB (NF-κB) activation,38 but because toxin B does not inhibit zymosan-induced proinflammatory cytokine production in macrophages (data not shown), it is unlikely that Rac-1 is involved in the cooperation between TLR2/6 and Dectin-1.11,12
In addition to triggering uptake, the cytoplasmic domains of phagocytic receptors also determine their intracellular fate, although these targeting domains are better defined in endocytic receptors.39 Some receptors recycle, such as the macrophage mannose receptor and Dec-205, whereas others, such as the FcγR, traffic to lysosomes where they are degraded along with their cargo.3,40,41 We found that the intracellular fate of Dectin-1 depended on the nature of the β-glucan ligand, which is the first demonstration of a receptor with this characteristic. With the particulate ligand, zymosan, Dectin-1 trafficked to lysosomes inducing de novo synthesis of the receptor. On the other hand, little de novo synthesis was observed with the addition of the soluble glucans. Furthermore, the receptor was observed to recycle with laminarin, but not with glucan phosphate. Although the basic structures of these 2 carbohydrates are similar, glucan phosphate is considerably bigger than laminarin. It is unclear how these differences in polymer size induce the various intracellular trafficking pathways, but it may be indicative of a cellular mechanism by which the larger molecular weight glucan mediates their immunomodulatory activities in vivo.6 Furthermore, the retention of Dectin-1 within the cell also explains why glucan phosphate was much better than laminarin at inhibiting cellular responses to zymosan in vitro (data not shown and Brown et al12 ).
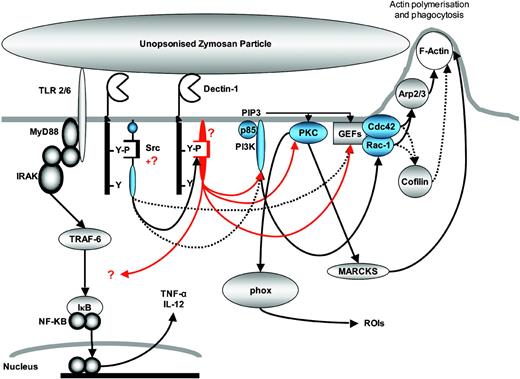
In conclusion, we have shown that Dectin-1 is a phagocytic receptor whose activities depend on an ITAM-like motif in the cytoplasmic tail. Based on the data presented here, we propose a model for Dectin-1-mediated phagocytosis and cellular activation shown schematically in Figure 8. Although Dectin-1-mediated uptake involves some known intracellular signaling molecules in macrophages, the initiating events are novel, and the overall mechanism appears different from any other phagocytic receptor known. Thus these data further demonstrate the complexity of phagocytic mechanisms present in macrophages.
Schematic representation of Dectin-1-mediated signaling in macrophages. Dectin-1 ITAM-like motif becomes phosphorylated on ligand binding and is likely to recruit a novel SH2-containing kinase that mediates many of the downstream signaling pathways. The signaling mediators examined in this report are shown in blue and putative novel mediators are shown in red. Red lines indicate proposed signaling pathways mediated by these intermediates and black lines indicate previously suggested (dotted lines) or established (solid lines) interactions.1,11,12,26,42
Schematic representation of Dectin-1-mediated signaling in macrophages. Dectin-1 ITAM-like motif becomes phosphorylated on ligand binding and is likely to recruit a novel SH2-containing kinase that mediates many of the downstream signaling pathways. The signaling mediators examined in this report are shown in blue and putative novel mediators are shown in red. Red lines indicate proposed signaling pathways mediated by these intermediates and black lines indicate previously suggested (dotted lines) or established (solid lines) interactions.1,11,12,26,42
Prepublished online as Blood First Edition Paper, August 10, 2004; DOI 10.1182/blood-2004-03-1140.
Supported by grants from the Medical Research Council, the Nuffield Dominions Trust, and the Wellcome Trust. G.D.B. is a Wellcome Trust Senior Research Fellow in Biomedical Science in South Africa.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank the staff of our animal facilities for the care of the animals used in these studies. We thank Dr Philip Taylor for critically reading this manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal