Abstract
The in vitro oxidation of low-density lipoprotein (LDL) by hypochlorous acid produces a modified form (HOCl-LDL) capable of stimulating platelet function. We now report that HOCl-LDL is highly effective at inducing platelet function, causing stable aggregation and α-granule secretion. Such stimulation depended on the presence of low levels of primary agonists such as adenosine diphosphate (ADP) and thrombin, or others like epinephrine (EPI) and macrophage-derived chemokine (MDC, CCL22). Agonist levels, which by themselves induced little or reversible aggregation, caused strong stable aggregation when combined with low levels of HOCl-LDL. Platelet activation by HOCl-LDL and ADP (1 μM) caused P-selectin (CD62P) exposure, without serotonin or adenosine triphosphate (ATP) secretion. Intracellular calcium levels rose slowly (from 100 to 200 nM) in response to HOCl-LDL alone and rapidly when combined with ADP to about 300 nM. p38 mitogen-activated protein kinase (MAPK) became phosphorylated in response to HOCl-LDL alone. This phosphorylation was not blocked by the protein kinase C (PKC) inhibitor bisindolylmaleimide, which reduced the extent of aggregation and calcium increase. However, the p38 MAPK inhibitor SB203580 blocked platelet aggregation and phosphorylation of p38 MAPK. These findings suggest that HOCl-LDL exposed during atherosclerotic plaque rupture, coupled with low levels of primary agonists, can rapidly induce extensive and stable thrombus formation. (Blood. 2004;104:380-389)
Introduction
Elevated plasma levels of native low-density lipoprotein (n-LDL) represent a well-recognized risk factor for stroke, heart attack, and atherosclerosis.1,2 This relationship has been linked to increased amounts of oxidized low-density lipoprotein (ox-LDL) present in atherosclerotic plaques.3,4 The atherogenic properties of ox-LDL include its ability to degrade the endothelial surface glycocalyx through the action of oxygen-derived free radicals5 and to ox-LDL interactions with platelets, stimulating their function.6 Such platelet activation and stimulation of aggregation may help precipitate cardiovascular incidents that could lead to both heart attack and stroke. Therefore, understanding the mechanisms by which ox-LDL influences platelet function is of special interest.
There have been a number of studies investigating the effects of ox-LDL on platelets.7-12 In general, ox-LDL increases platelet reactivity; however, functional consequences and potential mechanisms and receptors remain controversial. Much of the debate concerns the in vitro methods used to oxidize n-LDL and their biologic relevance for evaluating the signaling pathways, receptors, and functional outcomes. n-LDL has often been oxidized in the presence of low levels of copper.7,13,14 Copper-oxidized LDL by itself can cause strong aggregation and dense-granule secretion in washed platelets7-9 However, hypochlorous acid (HOCl)-LDL has been shown to possess similar functional properties as copper-oxidized LDL but with important differences15 and may well be closest to oxidized species formed in vivo.16 HOCl-LDL is most effective in washed platelets but will induce aggregation when used at high concentrations in platelet-rich plasma.17 There is good evidence that myeloperoxidase secreted by macrophages and other cells may be involved in the in vivo oxidation of LDL by generation of hypochlorous acid.18,19 Cell-associated lipoxygenase is also implicated in LDL oxidation by formation of reactive aldehydes.2 n-LDL itself can potentiate platelet function,20-24 complicating analyses of the roles of n-LDL versus ox-LDL in the pathogenesis of atherosclerosis.
The identity and importance of the receptors and signaling pathways involved in ox-LDL activation of platelets remain unclear. The glycoprotein scavenger receptor CD36, or GPIV, has been suggested as the primary receptor for ox-LDL6,25-28 ; however, the C-type lectin LOX-1 has been identified as a potential receptor for ox-LDL on activated platelets and may be involved in aggregate stabilization.8,29-32 The role of this receptor in platelets, however, has yet to be clarified, and the signaling pathways activated by either receptor are not fully characterized. LOX-1 is also present on endothelial cells and may well be involved during inflammation and platelet endothelial cell interactions.33-35
For these reasons, we have focused on the functional effects of HOCl-LDL interactions with blood platelets. An important aim was to determine whether HOCl-LDL stimulates platelet function under conditions approaching those in vivo in which low levels of platelet agonists such as adenosine diphosphate (ADP), epinephrine, and chemokines might be present.36-38
Materials and methods
Materials
n-LDL was purchased from Sigma (St Louis, MO) and Calbiochem (San Diego, CA). The P2Y12 receptor antagonist AR-C69931MX was from Astra-Zeneca (Campbell, CA). The protein kinase C inhibitor, bisindolylmaleimide I, and a highly specific inhibitor of p38 MAPK (mitogen-activated protein kinase), SB 203580, were from Calbiochem. Apyrase (grade VII), indomethacin, prostacyclin (PGI2), epinephrine, A2P5P (P2Y1 receptor antagonist), and other chemicals, including fatty acid-free albumin, were all obtained from Sigma. Lipid peroxidation assay kits were purchased from Oxis Chemicals (Portland, OR), and the Bradford protein assay kit was from BioRad (Hercules, CA). Indo-1, am cell permeant and calcium calibration buffer were obtained from Molecular Probes (Eugene, OR). Control cell extract and polyclonal antibodies against phospho-p38 MAPK (Thr180/Tyr182) and p38 MAPK were purchased from Cell Signaling (Beverly, MA).
Preparation of washed platelets
Platelet-rich plasma (PRP) was prepared by 3 short sequential centrifugations (at 350g) of human venous blood, anticoagulated with acid-citratedextrose (ACD). The blood was obtained with informed consent from volunteers, under protocols approved by the University of Virginia Human Investigation Committee. PRP was then subjected to centrifugation at 620g for 20 minutes in the presence of ACD, apyrase, prostacyclin, and indomethacin, according to procedures developed in our laboratory.39 The final pellet was suspended in an Eagle/HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer containing 1.5 mg/mL fibrinogen. Platelet yields were 80% to 90% of the count in PRP with final platelet concentrations typically being about 3.2 × 108/mL. Washed platelets were stored at room temperature. Platelet samples were warmed to 37°C for 10 minutes prior to experimental testing to restore their discoid shape and to minimize any activation changes that may occur at room temperature.40,41
Oxidation of low-density lipoproteins
n-LDL was oxidized with hypochlorous acid following the general procedures described by Volf et al.16 Procedural variations were as follows: LDL oxidation was carried out in Microfuge tubes in an Eppendorf-heated block for 15 minutes at 23°C, directly on fresh n-LDL, as supplied, and in a borate buffer (100 mM Na2B4O4, 50 mM NaCl, pH 7.3), at a 34200:1 molar ratio of NaOCl to LDL, with the molecular weight of the apo B100 protein taken as 513 kDa.42 The extent of LDL oxidation achieved was determined by quantifying malondialdehyde (MDA) production, which was always close to 6 nmol MDA equivalents/mg protein. This value is in the middle range of MDA content in LDL oxidized by copper for 30 minutes to 12 hours at 37°C.43 The amount of MDA in the starting n-LDL material was 0.78 nmol/mg LDL protein, and preparations were stored under argon at 4°C and used within 1 week. For the extent of oxidation experiments (Figure 2D), reaction times with hypochlorous acid were varied from 5, 10, 15, and up to 30 minutes.
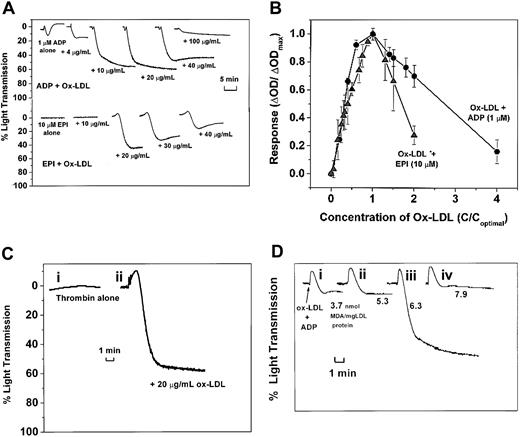
HOCl-LDL potentiates platelet aggregation according to its amount and extent of oxidation. (A) ADP (1 μM) and epinephrine (10 μM) top traces, ADP combined with HOCl-LDL at 0, 4, 10, 20, 40, and 100 μg/mL; bottom traces, epinephrine combined with HOCl-LDL at 0, 10, 20, 30, and 40 μg/mL. (B) Relative aggregatory effects of HOCl-LDL combined with 1 μM ADP (•) or 10 μM EPI (▴) (n = 3 donors). (C) Thrombin (i) alone (0.01 U/mL) or (ii) +HOCl-LDL at 20 μg/mL. (D) Effect of varying the extent of LDL oxidation: 1 μM ADP + 20 μg/mL LDL oxidized with HOCl for (i) 5, (ii) 10, (iii) 15, and (iv) 30 minutes (described in “Materials and methods”). MDA values are given as nmol/mg LDL protein.
HOCl-LDL potentiates platelet aggregation according to its amount and extent of oxidation. (A) ADP (1 μM) and epinephrine (10 μM) top traces, ADP combined with HOCl-LDL at 0, 4, 10, 20, 40, and 100 μg/mL; bottom traces, epinephrine combined with HOCl-LDL at 0, 10, 20, 30, and 40 μg/mL. (B) Relative aggregatory effects of HOCl-LDL combined with 1 μM ADP (•) or 10 μM EPI (▴) (n = 3 donors). (C) Thrombin (i) alone (0.01 U/mL) or (ii) +HOCl-LDL at 20 μg/mL. (D) Effect of varying the extent of LDL oxidation: 1 μM ADP + 20 μg/mL LDL oxidized with HOCl for (i) 5, (ii) 10, (iii) 15, and (iv) 30 minutes (described in “Materials and methods”). MDA values are given as nmol/mg LDL protein.
Platelet aggregation assays
Optical aggregometry low-shear aggregation. Two-channel aggregometers were used (Chronolog, Havertown, PA). Samples (500 μL) of washed platelets, prewarmed to 37°C for 10 minutes under static conditions, were normally first exposed to low levels of ADP, thrombin, or epinephrine and then immediately challenged with HOCl-LDL. HOCl-LDL was added no later than 5 seconds after agonist addition. Aggregation studies were conducted at a shear stress of about 1 dyne/cm2, corresponding to a stirring rate of 1000 rpm.44 When testing the effect of inhibitors, platelet samples were incubated with the appropriate inhibitor for 10 minutes at 37°C under static conditions.
Single-particle counting. Loss of single platelet particles was determined by single-particle counting as described.45 Samples of 700 μL washed platelets were prewarmed to 37°C for 10 minutes in the optical aggregometer. Once challenged with agonists, 50-μL samples were removed at appropriate times and quenched immediately in a 0.05% glutaraldehyde/0.15 M NaCl solution. The volume of 20 μL of each sample was diluted in 10 mL saline, and the number of single particles was counted in a resistive-particle counter (Particle Data, Elmhurst IL; or Coulter Z2, Hialeah FL).
Platelet assay for serotonin/ATP secretion
Platelets were labeled by incubating PRP with 0.2 μCi (0.0074 MBq) [2-14C] serotonin for 20 minutes at 37°C, then following the procedures outlined earlier for preparing washed preparations. Final platelet suspensions were prepared without fibrinogen. Subsequent procedures and estimation of the extent of secretion were as described previously.46 Potential secretion of dense-granule adenosine triphosphate (ATP) was assessed by a standard luciferase procedure in a Chronolog lumi-aggregometer.
P-selectin exposure
Surface exposure of P-selectin was determined by using flow cytometry. Washed platelets resuspended in Eagle buffer, without fibrinogen, at 1.0 × 108 platelets/mL, were stimulated for 30 seconds under stirring conditions. Subsequent treatments and analysis were performed as described previously.37
Immunoblotting, p38MAP kinase activation
Platelet stimulation with ox-LDL was stopped by the addition of 4 × sodium dodecyl sulfate (SDS) sample buffer. Lysates were centrifuged and boiled for 3 minutes. Samples (whole-cell lysates from 5 × 106 platelets/lane) were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. These were blocked in TTBS buffer (5% bovine serum albumin [BSA], 0.1% Tween-20, 50 mM Tris (tris(hydroxymethyl)aminomethane), pH 7.4, 145 mM NaCl) for 1 hour at 4°C and incubated overnight with an antiphospho-specific rabbit immunoglobulin G (IgG) that recognized phosphorylated form of p38 MAPK (Thr180/Tyr182) or with polyclonal anti-p38 MAPK antibody. After washing, the blots were developed by using enhanced chemiluminescence (ECL) system (Amersham, Piscataway, NJ). Amounts of dual-phosphorylated or total p38 MAPK were determined from band densities using ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Intracellular platelet calcium levels ([Ca2+]i)
PRP was incubated at 37°C for 30 minutes with 5 U/mL apyrase, 0.3 μg/mL prostacyclin, 1 μg/mL indomethacin, extra ACD (1:20 initial PRP volume), and 2.5 μM INDO-am and centrifuged for 20 minutes at 350g. Platelet pellets were resuspended in ACD buffer containing 5 U/mL apyrase and 3% BSA and centrifuged for final suspension in Eagle minimal essential medium containing 10 mM HEPES, 5 mM sodium bicarbonate, 3% BSA, no fibrinogen, 1.8 mM calcium. The INDO-loaded platelets were prewarmed for 15 minutes at 37°C before they were added to a 3-mL fluorescence cuvette with magnetic stirrer. Agonists were added to the stirred suspensions, and fluorescence emission intensity at 400 and 480 nm was continuously analyzed in an Aminco-Bowman SLM 8000 spectrofluorometer (Urbana, IL). When appropriate, inhibitors were added 5 minutes before the agonist. Excitation was at 335 nm, and free calcium ([Ca2+]i) was calculated from the 400:480 ratio.47
Scanning electron microscopy
Prewarmed platelets were normally exposed to HOCl-LDL for 30 seconds at 37°C in the absence or presence of the primary agonist (ADP) under low-shear conditions as described earlier, before they were quenched in 0.05% glutaraldehyde/0.15 M NaCl. Samples involving the protein kinase C inhibitor bisindolylmaleimide were preincubated with the inhibitor for 10 minutes. Subsequent procedures, dehydration, and critical-point drying were standard,40,48 and coated specimens were examined in a JEOL 6400 scanning electron microscope (JEOL, Tokyo, Japan) with automated image digitization and archiving.
Statistics
Where appropriate, mean values ± standard errors (SEs) are shown, and Student paired t test was used to assess the significance of differences between experimental situations. Statistics were performed on experimental results from different donors with n greater than or equal to 3 donors.
Results
HOCl-LDL induced stable aggregation in the presence of low levels of ADP
We first tested whether low levels of ADP influence the ability of HOCl-LDL to activate platelet aggregation. Both classic light-scattering methods were used as well as single-particle counting, which is highly sensitive for detecting early aggregation.39 Figure 1A shows that low levels of HOCl-LDL alone did not activate platelets as detected by light scattering, but when combined with ADP (1 μM) caused vigorous, irreversible aggregation. This low dose of ADP usually initiated minimal aggregation on its own detected optically, which was completely reversed within 1 minute. Figure 1B depicts the time-dependent loss of platelet singlets in the same low-shear assay. Single-particle counting revealed that HOCl-LDL alone caused a slow loss of platelets, leading to about a 30% decrease in platelet singlets by 8 minutes. The loss of singlets (70% to 80%) caused by the combination of ADP and HOCl-LDL was fast and complete within 30 seconds. There are always some 10% to 20% of single platelets in a normal preparation, which fail to aggregate and can be regarded as “effete” cells.39 Addition of ADP alone induced a very rapid, transient loss of singlets at 5 seconds of about 55%, a time when no aggregation was sensed by light scattering (Figure 1A). This early singlet loss was not enhanced by the presence of HOCl-LDL. However, stable aggregation was promoted such that about 70% singlet loss occurred by 30 seconds.
HOCl-LDL rapidly potentiates platelet aggregation in the presence of low levels of ADP. Washed platelets were exposed under low shear to ox-LDL alone (20 μg/mL, □), ADP alone (1 μM, ○), and dual addition of HOCl-LDL and ADP (▴). Aggregation was assessed by light scattering (A) and single-particle counting (B). Platelet singlet counts were taken at 0, 5, 30 seconds, 4 minutes, and 8 minutes after agonist challenge. *P < .05 (n = 5).
HOCl-LDL rapidly potentiates platelet aggregation in the presence of low levels of ADP. Washed platelets were exposed under low shear to ox-LDL alone (20 μg/mL, □), ADP alone (1 μM, ○), and dual addition of HOCl-LDL and ADP (▴). Aggregation was assessed by light scattering (A) and single-particle counting (B). Platelet singlet counts were taken at 0, 5, 30 seconds, 4 minutes, and 8 minutes after agonist challenge. *P < .05 (n = 5).
By using optical aggregometry, we found that high levels of fatty acid-free serum albumin (12 mg/mL) partially inhibited HOCl-LDL-induced aggregation compared with the standard preparation of washed platelets that contained 3 mg/mL albumin. Indomethacin (1-4 μg/mL) also partially inhibited HOCl-LDL and ADP-induced aggregation (data not shown).
Epinephrine, thrombin, and the chemokine MDC potentiate platelet activation by HOCl-LDL
Epinephrine enhances platelet aggregation induced by primary platelet agonists, such as ADP and thrombin,36,49 and may stabilize aggregates by slowing their disaggregation.50 Epinephrine alone (10 μM) caused minimal aggregation, whereas the combination of the 2 agonists resulted in stable aggregation (Figure 2A-B). There was a lag phase of about 1 minute after stimulation, followed by an activation phase that was complete within 1 minute after the onset of aggregation. A low level of thrombin (0.01 U/mL) failed to induce aggregation but caused strong stable aggregation when in combination with HOCl-LDL (Figure 2C).
The chemokines macrophage-derived chemokine (MDC) and stromal derived factor-1 (SDF-1) are effective in stimulating platelet aggregation in the presence of another agonist.37,51,52 We therefore investigated the ability of HOCl-LDL to initiate aggregation when combined with the chemokines MDC and SDF-1α. MDC (0.5 μg/mL) by itself caused reversible aggregation similar to 1 μM ADP (data not shown). However, the combination of HOCl-LDL and MDC induced significant and stable aggregation that varied from 20% to 50% between donors. SDF-1 did not cause aggregation in washed platelets acting alone or when combined with HOCl-LDL (data not shown).
Effect of HOCl-LDL concentration and oxidation state on platelet aggregation
The ability of HOCl-LDL to activate platelets was tested at different protein concentrations. HOCl-LDL can activate washed platelets in a dose-dependent manner.16 This finding was confirmed but with additional and distinctive observations. First, as described earlier (Figure 1A), HOCl-LDL acting alone at low concentrations either failed or only weakly induced macroaggregation as sensed by light scattering. Second, there was a range over which HOCl-LDL together with a constant level of coagonist induced maximal and stable aggregation. Figure 2A-B illustrates this phenomenon with low levels of ADP or epinephrine as coagonists. As HOCl-LDL levels were raised past the optimal value, less aggregation was seen. We also observed this biphasic effect when HOCl-LDL was combined with a low level (0.01 U/mL) of thrombin (data not shown).
The activation threshold seen with epinephrine as coagonist (Figure 2A) varied somewhat between donors. Therefore, the data were normalized to the HOCl-LDL level where maximal aggregation was seen, to determine an HOCl-LDL concentration-response relationship between donors. Figure 2B summarizes the relationship between the dose of HOCl-LDL and response as detected by the change in optical density after 5 minutes, for concentrations of ADP and EPI equal to 1 μM and 10 μM respectively, for different donors. Data are also presented as the ratio of the change in optical density at a given concentration of HOCl-LDL to the change in optical density found at the optimal HOCl-LDL concentration for each individual donor. The values for HOCl-LDL concentration are reported as the ratio of the concentration of HOCl-LDL to the optimal concentration of HOCl-LDL for each individual donor.
Oxidation may make LDL more or less active for inducing aggregation.4,7,53 We observed this phenomenon in terms of increasing MDA levels in the HOCl-LDL preparations induced by longer times of HOCl-induced oxidation for a constant amount of protein. Native LDL was exposed to NaOCl for 5, 10, 15, and 30 minutes (Figure 2Di-iv). A maximum in the extent of aggregation was seen in the HOCl-LDL preparation containing 6.3 nmol MDA/mg LDL protein, whereas 25% more LDL oxidation failed to induce significant aggregation.
Effect of fibrinogen on HOCl-LDL-induced platelet aggregation
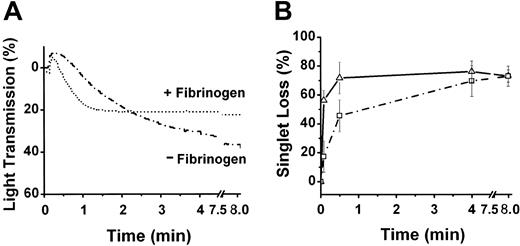
It is well known that the concentration of fibrinogen can influence ADP-induced aggregation under varying shear conditions.54 However, the necessity of extracellular fibrinogen for HOCl-LDL-induced aggregation is unknown. Light-scattering experiments revealed that external fibrinogen was not necessary for HOCl-LDL to induce aggregation in the presence of low levels of ADP (Figure 3A-B). However, preparations without added fibrinogen reacted more slowly (about 4-fold) than those with fibrinogen at early times. This was clearly evident by the difference in single-particle counts between 0 to 5 seconds, when only 18% loss of singlets occurred in the absence of extracellular fibrinogen compared with 58% in its presence. After 4 minutes, however, the singlet losses were identical for both situations.
External fibrinogen and HOCl-LDL induced aggregation. Washed platelets were prepared with (1.5 mg/mL, ▵) or without (□) added fibrinogen and then challenged with the combination of 1 μM ADP and 20 μg HOCl-LDL/mL. Aggregation was assessed either by light scattering (A) or by single-platelet disappearance (B; means ± SD from 3 platelet preparations).
External fibrinogen and HOCl-LDL induced aggregation. Washed platelets were prepared with (1.5 mg/mL, ▵) or without (□) added fibrinogen and then challenged with the combination of 1 μM ADP and 20 μg HOCl-LDL/mL. Aggregation was assessed either by light scattering (A) or by single-platelet disappearance (B; means ± SD from 3 platelet preparations).
HOCl-LDL does not induce serotonin or ATP secretion from dense granules
The ability of HOCl-LDL and ADP to induce major stable aggregation in the absence of added fibrinogen suggested that secretion of both dense and α-granule contents from internal granules had occurred. Copper ox-LDL has been shown to induce serotonin secretion after several minutes.55 Therefore, the ability of HOCl-LDL to induce dense-granule secretion was tested and minimal secretion occurred (Table 1). The combination of HOCl-LDL and ADP also failed to induce significant secretion. Potential secretion of ATP from dense granules was evaluated, using luciferase, and the combination of HOCl-LDL and ADP did not cause any increase in luminescence over 10 minutes, whereas positive controls with thrombin lead to major increases (data not shown).
Serotonin secretion induced by HOCI-LDL
Agonist . | Serotonin secretion, % . |
|---|---|
| Thrombin, 1 U/mL | 70.0 |
| ADP, 1 μM | 2.2 |
| HOCI-LDL, 20 μg/mL | 2.2 |
| HOCI-LDL + ADP, 1 μM | 4.0 |
Agonist . | Serotonin secretion, % . |
|---|---|
| Thrombin, 1 U/mL | 70.0 |
| ADP, 1 μM | 2.2 |
| HOCI-LDL, 20 μg/mL | 2.2 |
| HOCI-LDL + ADP, 1 μM | 4.0 |
Washed platelets were challenged with agonist under low shear as described in “Materials and methods.” Activated platelets were quenched with glutaraldehyde after 30 seconds and tested for serotonin secretion. The extent of secretion is presented as a percentage of the total radiolabeled serotonin above nonactivated control platelets. The data are means of 2 experiments
HOCl-LDL increases P-selectin exposure on the platelet surface
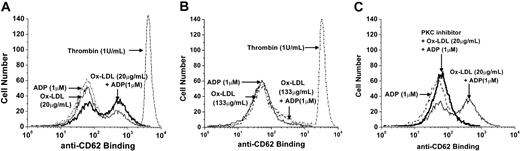
Because HOCl-LDL did not cause dense-granule secretion, we suspected that release from α-granules might still occur because we had seen this “phenomenon” during chemokine-stimulated platelet aggregation.37 Therefore, experiments were performed to measure surface exposure of P-selectin as an indication of α-granule secretion. Flow cytometry experiments revealed that HOCl-LDL indeed increased surface exposure of P-selectin. Figure 4A shows the presence of a small population of platelets with increased P-selectin exposure after exposure to HOCl-LDL alone, which increased with the combination of HOCl-LDL and ADP. ADP alone did not cause an increase in P-selectin exposure on the platelet surface. The large peak in fluorescence elicited by thrombin is presented for reference. Surprisingly perhaps, a high dose of HOCl-LDL (133 μg/mL) decreased the exposure of P-selectin to control levels with or without the presence of ADP (Figure 4B) and reflects the poor stimulation of aggregation that occurred (Figure 2A-B). The PKC inhibitor, bisindolylmaleimide, almost completely blocked P-selectin exposure elicited by both HOCl-LDL (20 μg/mL) and HOCl-LDL plus ADP (Figure 4C).
HOCl-LDL causes exposure of platelet P-selectin. Washed platelets were exposed to ADP (1 μM), HOCl-LDL (20 μg/mL or 133 μg/mL); (A) 1 μM ADP, 20 μg/mL HOCl-LDL, and their combination, as well as to thrombin (1U/mL); (B) 1 μM ADP, 133 μg/mL HOCl-LDL, and their combination, and to thrombin (1 U/mL); (C) 1 μM ADP and 20 μg/mL HOCl-LDL, with the latter combination in the presence of the PKC inhibitor bisindolylmaleimide at 3 μg/mL.
HOCl-LDL causes exposure of platelet P-selectin. Washed platelets were exposed to ADP (1 μM), HOCl-LDL (20 μg/mL or 133 μg/mL); (A) 1 μM ADP, 20 μg/mL HOCl-LDL, and their combination, as well as to thrombin (1U/mL); (B) 1 μM ADP, 133 μg/mL HOCl-LDL, and their combination, and to thrombin (1 U/mL); (C) 1 μM ADP and 20 μg/mL HOCl-LDL, with the latter combination in the presence of the PKC inhibitor bisindolylmaleimide at 3 μg/mL.
ADP-receptor antagonists inhibited platelet aggregation induced by HOCl-LDL and ADP
Several ADP receptors have been identified on the platelet surface.56-58 Specifically, the receptors P2Y1 and P2Y12 that are coupled to the G-proteins Gαq and Gαi, respectively, are necessary for full ADP activation.56 Therefore, we investigated which receptor is involved in the HOCl-LDL-ADP activation by using the inhibitors for P2Y1 and P2Y12, A2P5P and AR-C69931MX, respectively.52 Platelets were incubated with either inhibitor or saline for 10 minutes prior to activation. Platelet counts were taken after 4 minutes, after which time no further loss in singlets occurred. AR-C69931MX reduced the singlet loss caused by HOCl-LDL and ADP from 80.3% to 37.4%, whereas A2P5P diminished it even further to values close to those caused by HOCl-LDL alone (Table 2).
Influence of ADP-receptor antagonists on HOCI-LDL aggregation
HOCI-LDL . | HOCI-LDL + ADP . | HOCI-LDL + ADP + AR-C69931 . | HOCI-LDL + ADP + A2P5P . |
|---|---|---|---|
| 26.5 ± 6.8 | 80.3 ± 4.6 | 37.4 ± 7.4 | 19.7 ± 6.0 |
HOCI-LDL . | HOCI-LDL + ADP . | HOCI-LDL + ADP + AR-C69931 . | HOCI-LDL + ADP + A2P5P . |
|---|---|---|---|
| 26.5 ± 6.8 | 80.3 ± 4.6 | 37.4 ± 7.4 | 19.7 ± 6.0 |
Washed platelets were exposed to HOCI-LDL alone (20 μg/mL) or to the combination of 1 μM ADP and HOCI-LDL, with or without 10 minutes of preincubation with the ADP receptor antagonists AR-C69931 or A2P5P.52 Aggregation data are expressed as means ± SEs from 3 platelet preparations for the percentage loss of platelet singlets detected by resistive-particle counting.
Effects of the PKC inhibitor, bisindolylmaleimide, and the p38 MAPK inhibitor, SB203580, on the ability of HOCl-LDL to induce platelet aggregation
The ability of HOCl-LDL to increase the surface exposure of P-selectin (Figure 4A) suggested activation of protein kinase C. Therefore, the effect of the PKC inhibitor, bisindolylmaleimide, was investigated. It strongly reduced the extent of platelet aggregation induced by HOCl-LDL and ADP (1.0 μM), without influencing the stability of such aggregation (Figure 5A). An apparent “shape change” caused by HOCl-LDL alone detected by light scattering was also prevented by the inhibitor. Single-particle counting revealed that bisindolylmaleimide nearly completely abolished aggregation due to HOCl-LDL alone (data not shown). The inhibitor did not influence the potent but unstable response caused by 10 μM ADP. Shown in Figure 5B is the ability of the p38 MAPK inhibitor SB203580 to cause almost total inhibition of aggregation induced by HOCl-LDL with or without added ADP.
Effect of the PKC inhibitor, bisindolylmaleimide, and the p38 MAPK inhibitor, SB203580, on HOCl-LDL-induced aggregation. The effect of bisindolylmaleimide is shown in panel A and SB203580 is shown in panel B. ADP (10 μM); bisindolylmaleimide (PKCI, 3 μg/mL) + ADP (10 μM); HOCl-LDL alone (20 μg/mL); bisindolylmaleimide (3 μg/mL) + HOCl-LDL alone (20 μg/mL); HOCl-LDL (20 μg/mL) + 1 μM ADP; (f) HOCl-LDL (20 μg/mL) + 1 μM ADP + bisindolylmaleimide (3 μg/mL).
Effect of the PKC inhibitor, bisindolylmaleimide, and the p38 MAPK inhibitor, SB203580, on HOCl-LDL-induced aggregation. The effect of bisindolylmaleimide is shown in panel A and SB203580 is shown in panel B. ADP (10 μM); bisindolylmaleimide (PKCI, 3 μg/mL) + ADP (10 μM); HOCl-LDL alone (20 μg/mL); bisindolylmaleimide (3 μg/mL) + HOCl-LDL alone (20 μg/mL); HOCl-LDL (20 μg/mL) + 1 μM ADP; (f) HOCl-LDL (20 μg/mL) + 1 μM ADP + bisindolylmaleimide (3 μg/mL).
The data illustrated in Figure 6A reveal that HOCl-LDL caused significant phosphorylation of p38 MAPK detected by a phosphospecific antibody that recognized phosphorylated Thr180/Tyr182. ADP alone failed to induce any phosphorylation. However, the combination HOCl-LDL and ADP did not induce extra phosphorylation, even though aggregation was markedly enhanced under these conditions (Figure 1A). Surprisingly, perhaps, high levels of HOCl-LDL combined with ADP further enhanced p38 MAPK phosphorylation, but this was associated with less aggregation (Figure 2B). The Western blots in Figure 6B show that p38 MAPK phosphorylation was not blocked by the PKC inhibitor bisindolylmaleimide, even though the extent of aggregation detected by light scattering was strongly inhibited (Figure 5A). As expected, SB203580 completely blocked p38 MAPK phosphorylation (data not shown).
HOCl-LDL causes phosphorylation of p38 MAPK. (A) Human platelets were activated with 1 and 20 μg/mL HOCl-LDL with or without 1 μM ADP for 30 seconds. Anisomycin-treated C6 glioma cells were a positive control for p38 MAP kinase activation. The activated and total p38 MAPK levels were determined by Western blot analysis using phosphospecific (upper panel) and total anti-p38 MAPK (lower panel) antibodies. (B) Platelets preincubated with the specific PKC inhibitor Bis for 5 minutes at 37°C were stimulated with HOCl-LDL (1 μg/mL, ox-LDL) for 30 seconds. Activated and total p38 MAPK levels were determined by Western blot as in panel A.
HOCl-LDL causes phosphorylation of p38 MAPK. (A) Human platelets were activated with 1 and 20 μg/mL HOCl-LDL with or without 1 μM ADP for 30 seconds. Anisomycin-treated C6 glioma cells were a positive control for p38 MAP kinase activation. The activated and total p38 MAPK levels were determined by Western blot analysis using phosphospecific (upper panel) and total anti-p38 MAPK (lower panel) antibodies. (B) Platelets preincubated with the specific PKC inhibitor Bis for 5 minutes at 37°C were stimulated with HOCl-LDL (1 μg/mL, ox-LDL) for 30 seconds. Activated and total p38 MAPK levels were determined by Western blot as in panel A.
Intracellular calcium changes induced by HOCl-LDL
We used the calcium-sensitive dye Indo-1 to study the ability of HOCl-LDL to increase intracellular calcium ([Ca2+]i). The traces in Figure 7A demonstrate a rapid and sustained increase of about 200 nM [Ca2+]i over about 2 minutes in the presence of 1 μM ADP, whereas ADP on its own only caused a similar initial transient increase. This ability of ADP has been noted in earlier studies by using continuous-flow methodology.47 HOCl-LDL on its own causes a slow increase in [Ca2+]i after an initial lag of about 25 seconds (Figure 7B), whereas bisindolylmaleimide almost completely prevented the increase (Figure 7C).The potential ability of SB203580, a specific inhibitor of p38 MAPK, on HOCl-LDL induced changes in [Ca2+]i could not be assessed because of strong autofluorescence interference by the inhibitor.
Increases in platelet intracellular calcium ([Ca2+]i) induced by HOCl-LDL. Platelets (108/mL) loaded with Indo-1 were stimulated in a plastic cuvette with stirring at 37°C in the presence of 1.8 mM extracellular Ca2+. The elevation of [Ca2+]i was measured as a fluorescence ratio (400:480) and calibrated using Ca2+ standards. (A) Platelets activated with 1 μM ADP for 5 seconds were stimulated with HOCl-LDL (ox-LDL, 1 μg/mL) or control buffer. (B) Platelets were stimulated with low (1 μg/mL) and high (20 μg/mL) doses of HOCl-LDL. (C) Platelets were preincubated with the PKC-specific inhibitor Bis (10 μM) or 0.1% (vol/vol) dimethyl sulfoxide (DMSO) for 5 minutes, before HOCl-LDL (ox-LDL, 1 μg/mL) was added. Traces are representative for 4 experiments (A-B) and 2 experiments (C). The arrowheads indicate the addition of agonists.
Increases in platelet intracellular calcium ([Ca2+]i) induced by HOCl-LDL. Platelets (108/mL) loaded with Indo-1 were stimulated in a plastic cuvette with stirring at 37°C in the presence of 1.8 mM extracellular Ca2+. The elevation of [Ca2+]i was measured as a fluorescence ratio (400:480) and calibrated using Ca2+ standards. (A) Platelets activated with 1 μM ADP for 5 seconds were stimulated with HOCl-LDL (ox-LDL, 1 μg/mL) or control buffer. (B) Platelets were stimulated with low (1 μg/mL) and high (20 μg/mL) doses of HOCl-LDL. (C) Platelets were preincubated with the PKC-specific inhibitor Bis (10 μM) or 0.1% (vol/vol) dimethyl sulfoxide (DMSO) for 5 minutes, before HOCl-LDL (ox-LDL, 1 μg/mL) was added. Traces are representative for 4 experiments (A-B) and 2 experiments (C). The arrowheads indicate the addition of agonists.
Platelet morphologic changes induced by HOCl-LDL
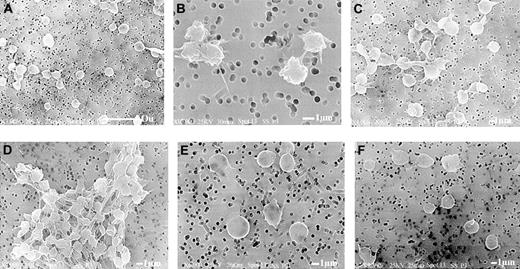
Platelets exposed to low levels of ADP experience morphologic shape changes and short-lived aggregation.59 Experiments were, therefore, performed to ascertain whether HOCl-LDL induces these changes and to evaluate aggregate morphology (Figure 8). Scanning electron micrographs reveal that HOCl-LDL by itself was very weak at inducing any shape change in washed platelets. Figure 8C shows the formation of some small loose aggregates, but when combined with ADP (1 μM), large stable aggregates were seen (Figure 8D). However, the platelets in the aggregate did not show significant “bleb” and pseudopodia formation normally associated with ADP or thrombin activation.48 Bisindolylmaleimide clearly prevented aggregate formation, giving single platelets that resembled control platelets (Figure 8E-F).59
Scanning electron micrographs reveal that HOCl-LDL alone induces formation of microaggregates; combination with ADP results in stable aggregate formation. Washed platelets were subjected to HOCl-LDL (20 μg/mL), ADP (1 μM), and the combination of the 2 agonists with or without the presence of PKC inhibitor for 30 seconds at 37°C as described for Figure 1 and then fixed with glutaraldehyde for subsequent processing and examination by scanning electron microscopy. (A) Control platelets, (B) ADP alone, (C) HOCl-LDL alone, (D) HOCl-LDL + ADP, (E) HOCl-LDL + bisindolylmaleimide (3 μg/mL), (F) HOCl-LDL + ADP + bisindolylmaleimide. The white bar represents 10 μm for panel A and 1 μm for panels B-F.
Scanning electron micrographs reveal that HOCl-LDL alone induces formation of microaggregates; combination with ADP results in stable aggregate formation. Washed platelets were subjected to HOCl-LDL (20 μg/mL), ADP (1 μM), and the combination of the 2 agonists with or without the presence of PKC inhibitor for 30 seconds at 37°C as described for Figure 1 and then fixed with glutaraldehyde for subsequent processing and examination by scanning electron microscopy. (A) Control platelets, (B) ADP alone, (C) HOCl-LDL alone, (D) HOCl-LDL + ADP, (E) HOCl-LDL + bisindolylmaleimide (3 μg/mL), (F) HOCl-LDL + ADP + bisindolylmaleimide. The white bar represents 10 μm for panel A and 1 μm for panels B-F.
Discussion
The results presented here show that HOCl-LDL was highly effective at inducing platelet aggregation when combined with low levels of primary agonists, whereas each agonist by itself caused little aggregation. This synergistic behavior was seen when HOCl-LDL was combined with ADP, thrombin, epinephrine, or the chemokine MDC (CCL22). Maximal aggregation was induced over a limited range of HOCl-LDL concentrations, and further increases in HOCl-LDL became less effective at initiating aggregation. A general observation was the ability of HOCl-LDL to stabilize macroaggregate formation as sensed by optical aggregometry. Platelet activation occurred in the absence of dense-granule secretion but was associated with P-selectin exposure. In addition, HOCl-LDL caused phosphorylation of p38 MAPK as well as increases in platelet [Ca2+]i.
The influence of ox-LDL on platelet function is controversial. This concerns whether oxidized lipid products11,60-62 or oxidized LDL protein are most involved.17,18,63 Other issues concern the relative roles of candidate receptors such as CD36 (GPIV),6,25,64,65 and LOX-1,29 as well as the relevance of how studies with in vitro oxidized LDL relate to in vivo situations. Some reports have shown that mildly oxidized LDL (mox-LDL) alone, prepared by LDL exposure to copper for 24 hours, can strongly activate platelets, causing macroaggregation as detected by light-scattering techniques.7 However, HOCl-LDL by itself can activate platelets, causing macroaggregation without the need for simultaneous addition of a primary agonist.16,17 We, therefore, expected that HOCl-LDL alone would induce strong platelet aggregation, but our results indicated a limited ability at low levels to induce macroaggregation (Figure 1A). However, single-particle counting, P-selectin exposure, p38 MAPK activation, and scanning electron microscopy revealed some platelet activation because of HOCl-LDL acting alone (Figures 1B, 4A, and 6C). In preparations analyzed over a 2-year period, we have found that levels of HOCl-LDL as low as 1 to 2 μg/mL were capable of inducing some aggregation in the absence of a coagonist. A consistent observation, however, for all preparations has been the ability of low levels of ADP to induce very strong aggregation when combined with low levels of HOCl-LDL (Figures 1 and 5B); whereas high levels of HOCl-LDL (60 to 100+ μg/mL) became less effective at stimulating aggregation.
The effectiveness of HOCl-LDL to induce stable, irreversible platelet aggregation suggested that granule secretion might occur. However, HOCl-LDL, with or without ADP (1 μM), did not cause any secretion of [2-14C] serotonin (Table 1) or ATP from dense granules (data not shown) over 10 minutes of exposure to HOCl-LDL and ADP, even though strong stable aggregation had occurred. Stimulation of α-granule secretion without parallel dense-granule secretion is unusual, but analogous to P-selectin exposure seen after chemokine activation of platelet aggregation in the presence of low levels of ADP.37 Our present results are in direct contrast with those of Volf et al17 who reported serotonin secretion in response to HOCl-LDL alone. Examination of their data reveals that only at more than 50% aggregation (detected by light scattering) did any serotonin secretion occur, never more than 50%. Another apparent difference concerns platelet responses to increasing concentrations of HOCl-LDL. We found that high levels (60-100 μg protein/mL, or greater), in combination with low levels of a primary agonist such as ADP, became less effective at stimulating aggregation than low levels of 1 to 40 μg/mL (Figures 1 and 2). The study of Volf et al17 involved only HOCl-LDL and did not reveal such biphasic responses. We also used a higher concentration of HOCl for oxidizing LDL. Other factors were different in vitro such as LDL oxidation temperatures, being 23°C in our studies to approach better physiologic conditions (“Materials and methods”), compared with 0°C for Volf et al.17 Lipid-phase separations in LDL are altered significantly with changing temperatures66,67 and may influence the nature and amount of lipid- and protein-oxidized species being generated.
The persistence of strong aggregation in experiments carried out in the absence of fibrinogen (Figure 3) may result from secretion of fibrinogen, fibronectin, von Willebrand factor (VWF), or thrombospondin. These α-granule proteins are likely to be secreted in parallel with P-selectin exposure on the platelet surface (Figure 4). The combination of HOCl-LDL and ADP increased P-selectin exposure compared with HOCl-LDL alone, whereas ADP by itself had no effect (Figure 4B-C), being the same as resting platelets (data not shown). Importantly, HOCl-LDL on its own induced significant P-selectin exposure, about two thirds that when combined with ADP (Figure 4A). This ability of HOCl-LDL to cause P-selectin exposure parallels data reported by Takahashi et al68 who used copper-oxidized LDL, which possess similar MDA levels as our HOCl-oxidized LDL (Figure 2D).43
Our finding that as levels of HOCl-LDL increased above optimal, less aggregation occurred was unexpected (Figure 2A-C). This was observed in terms of either more protein for a constant oxidation state (Figure 2A-C) or for greater oxidation, while maintaining a constant amount of protein (Figure 2D). There was a relatively narrow range of HOCl-LDL concentration where maximal aggregation occurred (Figure 2B). This finding parallels that reported by Weidtmann et al7 for copper-oxidized LDL, when 0.3 mg/mL ox-LDL induced minimal aggregation, whereas 0.4 mg/mL elicited maximal, irreversible aggregation. At intermediate doses of HOCl-LDL (40-60 μg/mL), light scattering revealed some reversal of aggregation (Figure 2A-B). The highest amount of HOCl-LDL (100 μg protein/mL) induced minimal aggregation and similar levels (133 μg/mL) caused very little P-selectin exposure (Figure 4B). It is interesting to note that the decline in reactivity with increasing concentrations of HOCl-LDL was weaker when it was combined with ADP than with epinephrine (Figure 2B). Epinephrine signals through the α2A-adrenergic receptor linked to Gzα and Gi2α,69,70 whereas ADP acts through 2 receptors with distinct G-protein coupling mechanisms.71,72
Signaling pathways activated by ox-LDL may be suggested by our results. It is known that coactivation of both Gq and Gi pathways provides for effective platelet aggregation.56,69,73 The action of HOCl-LDL is likely to involve primarily activation of Gαi, because the combination of HOCl-LDL and MDC was effective (data not shown). We have previously reported that MDC activates both Gq- and Gi-coupled receptors, causing increases in cytosolic platelet calcium and platelet aggregation, whereas SDF-1α activates only Gi.52 We used the ADP receptor inhibitors A2P5P and AR-C69931MX to block differentially the P2Y1 Gq- and P2Y Gi-coupled receptors, respectively. A2P5P was capable of reducing the loss of single platelets because of the combination of HOCl-LDL and ADP to levels caused by HOCl-LDL alone, whereas AR-C69931MX was less potent (Table 2). Observations from our HOCl-LDL and epinephrine studies also support this conclusion, specifically the presence of the lag phase prior to aggregation (data not shown). If HOCl-LDL involved the Gq pathway, rapid activation would be expected when combined with epinephrine, which activates Gi- and Gz-coupled receptors.70,73
Platelet activation by primary agonists often involves the p38 MAPK family74,75 and is linked to dual phosphorylation of the Thr180 and Tyr182 residues. Our data showed that low levels of HOCl-LDL on their own activated p38 MAPK (Figure 6). In addition, HOCl-LDL-induced irreversible platelet aggregation of ADP-stimulated platelets (Figure 5B) and associated activation of p38 MAPK were effectively blocked by platelet pretreatment with a specific inhibitor of p38 MAPK, SB203580. Hackeng et al76 reported that n-LDL induces p38 MAPK activation and that this may be involved in a slow process of platelet sensitization to other agonists. Stimulation of p38 MAP kinase by HOCl-LDL did not appear to involve protein kinase C, because platelet treatment with a specific PKC inhibitor had no influence on p38 MAPK phosphorylation (Figure 6B). MAPK activation by HOCl-LDL may occur directly or indirectly in human platelets. For example, only activated platelets expose the LOX-1 ox-LDL receptor on their surface,29 and it is unlikely that this receptor is directly involved in mediating MAP kinase activation. Several LDL and oxidized LDL receptors may function as signal-transduction receptors, and CD36 (GPIV) is a more likely candidate.24,25,27
The ability of fatty acid-free albumin and indomethacin to block partially HOCl-LDL-induced aggregation, also suggests a role for lipid mediators,61,77,78 whereas partial sensitivity to indomethacin indicates that PLA2 activation and TxA2 are not essential for HOCl-LDL stimulation of platelet function. These results suggest that several mechanisms exist which involve both initial activation pathways as well as events required for the stabilization of platelet aggregates.
The results from our aggregation studies reveal the importance of primary agonists. Low levels of ADP on their own induced some shape change and aggregation which were reversed within 30 seconds (Figure 1A-B), but, when combined with HOCl-LDL, aggregation became irreversible. Thus, HOCl-LDL appears to prevent activation of “disaggregation” pathways, a conclusion clearly evident from the single-particle counting data. These results also indicate that platelets must be challenged with ADP and HOCl-LDL nearly simultaneously, for maximal effect, because the extent of aggregation decreased as the interval between addition of ADP and HOCl-LDL increased. This desensitization was nearly complete within 2 minutes (data not shown). Desensitization of lipoprotein receptors in platelets has also been observed previously with n-LDL.79
The need for coactivation between some agonists is firmly established. For example, Steen et al36 have shown the need for simultaneous addition of ADP and epinephrine, and our laboratory has also demonstrated this phenomenon regarding activation by the chemokines thymus and activation-regulated chemokine (TARC), SDF-1α, and MDC with ADP or thrombin.37 Costimulation has significant implications regarding the linkage between inflammation and atherosclerosis. Chemokine release from endothelial cells, activated platelets, monocytes, and neutrophils may all contribute to ox-LDL's ability to activate platelet function.80-82 Tissue damage will lead to local appearance of ATP and ADP in millimolar to high micromolar concentrations in blood. The released ATP may play an important activating role by way of the P2 × 1 ATP-gated cation channel receptor on platelets.72,83-85 In addition, the presence of extracellular apyrases (CD39) on endothelial cells will generate ADP from any released ATP and further enhance platelet activation.86-88
The role of PKC in granule secretion and its activation by lipid mediators is well known.89 Also, phosphorylation of CD36 by ecto-PKC has been shown to inhibit thrombospondin while enabling collagen binding to the receptor and to prevent spontaneous aggregation.90 In binding to CD36, a major ox-LDL receptor,25,78,91 ox-LDL may inhibit the activity of these ectokinases, resulting in dephosphorylation of CD36 on its ectodomain. This action has been shown to enhance platelet aggregation.92 Inhibition of ecto-protein kinase C, coupled with exposure of additional GpIIb/IIIa receptors, fibrinogen, and thrombospondin from α-granules may well occur during ox-LDL stimulation. Studies have revealed that ox-LDL binding to the C-type lectin receptor or, LOX-1, may also be involved and LOX-1 can be surface exposed on activated platelets.29 In endothelial cells, LOX-1 binds ox-LDL with lower affinity than CD36, facilitates the endocytosis and degradation of ox-LDL and stimulates apoptosis.93 This makes LOX-1 a good candidate for involvement in platelet and endothelial cell responses to ox-LDL.34,35
In conclusion, low levels of LDL oxidized by hypochlorous acid were highly effective at activating platelet function when combined with low levels of other agonists, such as ADP, thrombin, epinephrine, and the inflammatory chemokine MDC. These observations may be relevant physiologically because hypochlorous acid is a natural oxidant and hypochlorite-modified proteins are found in plaque material.94,95 In addition, there are recent data showing strong correlations linking myeloperoxidase, oxidized species in plasma and in atherosclerotic lesions.96-99 Our data suggest that one of HOCl-LDL's major actions is to stabilize aggregates, potentially involving regulation of systems such as the ephrins and eph-kinases.100 This function, rather than the immediate activation caused by HOCl-LDL, may represent its most important in vivo action, causing the formation of stable thrombi after rupture of atherosclerotic plaques.
Prepublished online as Blood First Edition Paper, March 30, 2004; DOI 10.1182/blood-2003-08-2961.
Supported by the Carman Trust, by the Partners Research Fund of the University of Virginia Cardiovascular Research Center, and by the National Institutes of Health (HL HL07878 to L.G.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Kailo Schelegel of the Division of Nephrology in the School of Medicine of the University of Virginia for help with the flow-cytometric analyses, Linda Beggarly for blood drawing, Gary Manuel for ATP secretion experiments, and Kevin Lynch and Colleen McNamara for discussions during the project.




![Figure 7. Increases in platelet intracellular calcium ([Ca2+]i) induced by HOCl-LDL. Platelets (108/mL) loaded with Indo-1 were stimulated in a plastic cuvette with stirring at 37°C in the presence of 1.8 mM extracellular Ca2+. The elevation of [Ca2+]i was measured as a fluorescence ratio (400:480) and calibrated using Ca2+ standards. (A) Platelets activated with 1 μM ADP for 5 seconds were stimulated with HOCl-LDL (ox-LDL, 1 μg/mL) or control buffer. (B) Platelets were stimulated with low (1 μg/mL) and high (20 μg/mL) doses of HOCl-LDL. (C) Platelets were preincubated with the PKC-specific inhibitor Bis (10 μM) or 0.1% (vol/vol) dimethyl sulfoxide (DMSO) for 5 minutes, before HOCl-LDL (ox-LDL, 1 μg/mL) was added. Traces are representative for 4 experiments (A-B) and 2 experiments (C). The arrowheads indicate the addition of agonists.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/2/10.1182_blood-2003-08-2961/6/m_zh80140464070007.jpeg?Expires=1770039278&Signature=TksJTm7wZ~q2cX3~D9EpVME-KN27QOH1AHvtgmmLnlPZMPJ~UIyc3TD49iLs8tevKD~uOUJ9aBFVv6dvqf1q6JWkGOs2IFTI8P4HBEKXE-jycuwbXT8hhiNFKmLey~JrjKEi-lqhaWngfmT~mcwr0nm7T~by42JqxB8lBCmfALHbSWhtqZqV3X~TL7mU1xe-VXN7N600tR0uLjeeKjZQxa9VwDoi6TMYVfByhLjcmBZ36XPzyjoMso~tI~jgKPJjvjbciWyoNpOZSnw02joYIB7fs9884fbqAZxfF6iL8KzTz7nlXaTPeahJx1laah-FW8IT4lRdN5N80v86OPrIkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal