Abstract
Idiopathic pneumonia syndrome (IPS) is a significant cause of mortality after allogeneic bone marrow transplantation (allo-BMT), and tumor necrosis factor-α (TNF-α) is a significant effector molecule in this process. However, the relative contribution of donor-versus host-derived TNF-α to the development of IPS has not been elucidated. Using a lethally irradiated parent → F1 mouse IPS model, we showed that 5 weeks after transplantation allo-BMT recipients developed significant lung injury compared with syngeneic controls, which was associated with increased bronchoalveolar lavage (BAL) fluid levels of TNF-α, elevated numbers of donor-derived TNF-α-secreting T cells, and increased pulmonary macrophage production of TNF-α to lipopolysaccharide (LPS) stimulation. Allo-BMT with TNF-α-/- donor cells resulted in significantly reduced IPS severity, whereas utilization of TNF-α-deficient mice as BMT recipients had no effect on IPS. We next determined that TNF-α secretion from both donor accessory cells (monocytes/macrophages) and T cells significantly contributed to the development of IPS. Importantly, the absence of donor T-cell-derived TNF-α resulted in a significant decrease in inflammatory chemokine production in the lung and near complete abrogation of IPS. Collectively, these data demonstrate that donor TNF-α is critical to the development of IPS and reveal a heretofore unknown mechanism for T-cell-derived TNF-α in the evolution of this process. (Blood. 2004;104:586-593)
Introduction
Allogeneic bone marrow transplantation (allo-BMT) is an accepted therapy for a number of malignant and nonmalignant diseases. Pulmonary toxicity, and specifically diffuse lung injury, is a major complication after allo-BMT and occurs in 25% to 55% of patients.1-6 Infectious microorganisms directly contribute to approximately one half of all cases of lung toxicity, but in the remaining 50%, no evidence of infection is found. Acute (within 4 months), noninfectious, diffuse lung injury that occurs after BMT has been defined as idiopathic pneumonia syndrome (IPS).1 Although recent studies have reported a lower incidence and an earlier time to onset of IPS than that originally described, the typical clinical course involving the rapid onset of pulmonary failure resulting in death remained unchanged, emphasizing the critical nature of this complication.4,7,8 Risk factors for IPS include myeloablative versus nonmyeloablative conditioning regimens, the use of total body irradiation, acute graft versus host disease (GVHD), and older recipient age,3,4,8-12 but the pathophysiology of this disorder remains enigmatic.
Experimental models have uncovered roles for allospecific donor T-cell responses13-16 and the production of inflammatory cytokines.14,17-21 With respect to the latter, previous work has uncovered a causal relationship between tumor necrosis factor-α (TNF-α) and IPS17,18,22 ; both hyporesponsiveness of donor cells to lipopolysaccharide (LPS) stimulation18 and the neutralization of TNF-α17 result in decreased lung injury after BMT. However, the importance of donor-versus host-derived TNF-α to the development of IPS has not been fully elucidated.
The recently demonstrated importance of TNF-α expression by alloreactive donor T cells to the development of systemic GVHD23 led us to hypothesize that TNF-α production from both donor monocytes and macrophages and donor T cells would contribute to the development of IPS. Data generated using lethally irradiated mouse IPS models and animals deficient in TNF-α support this hypothesis and demonstrate that IPS after allogeneic BMT is dependent upon donor-rather than host-derived TNF-α; both donor accessory cells (macrophages/monocytes) and T cells significantly contribute to IPS severity. Our data specifically reveal that TNF-α produced by donor T lymphocytes infiltrating the lung early after BMT regulates pulmonary chemokine expression and the subsequent recruitment of inflammatory cells that contributes to the progression of disease severity.
Materials and methods
Mice and bone marrow transplantation
Female B6D2F1 (H-2bxd), C57BL/6 (H-2b), B6.C-H2bm1/ByJ (H-2b), B6.C-H2bm12KhEgJ, TNF-α+/+ B6129SF2/J (H-2b), and TNF-α-/- B6.129S6-TnftmGk1(H-2b)24 were purchased from Jackson Laboratories (Bar Harbor, ME) or from the Frederick Cancer Research and Development Center (National Cancer Institute, Frederick, MD). Animals used for BMT and in vitro experiments were between 10 and 14 weeks old. All experiments were approved by the University of Michigan Committee on the Use and Care of Animals (UCUCA).
Mice underwent transplantation according to a standard protocol as described previously.25 When using the haploidentical B6 → B6D2F1 system, B6D2F1 recipients received 11 Gy of total body irradiation (TBI) (137Cs source) prior to transplantation with 5 × 106 bone marrow (BM) cells supplemented with 2 × 106 splenic T cells from either syngeneic (B6D2F1) or allogeneic C57BL/6, TNF-α+/+ B6129SF2/J, or TNF-α-/- B6.129-Tnf<tmGk1>donors. T-cell purification was performed by magnetic bead separation using CD4 and CD8 MicroBeads and the autoMACS system (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol, with more than 85% of cells obtained being positive for CD4 or CD8 surface antigens (data not shown). Percentages of purified CD4+ and CD8+ T cells did not significantly differ between donors. Mice undergoing transplantation were cared for as previously described.26
In experiments using the B6 → bm1 or bm1 → B6 BMT system (isolated major histocompatibility complex [MHC] class I mismatch), host mice received 11 Gy TBI followed by the infusion of 5 × 106 BM and 1.8 × 106 to 2.0 × 106 autoMACS-purified (more than 85% purity) CD8+ T cells, whereas in the B6 → bm12 or the bm12 → B6 BMT system (isolated MHC class II mismatch), bm12 mice received 9 Gy TBI and 0.25 × 106 autoMACS-purified (more than 90% purity) CD4+ T cells. These changes in transplantation parameters were made to ensure that sufficient animals would be available for analysis at time points when significant lung injury is present.
Bronchoalveolar lavage (BAL) and cytospin
At the time of analysis, mice were killed by exsanguinations, and bronchoalveolar lavage (BAL) was performed as previously described.27 After centrifugation, supernatants were frozen for subsequent analysis of cytokine content, and cell pellets were washed and counted. In some experiments, aliquots of cell suspensions were stained with fluorescent antibodies to cell surface antigens and analyzed by fluorescence-activated cell sorter (FACS) analysis or used for cytospin analysis.
For cytospin analysis, cells were stained using the Diff-Quik procedure (Dade Behring, Newark, DE) and differentiated by morphologic criteria into lymphocytes, macrophages/monocytes, and neutrophils as previously described.28 Cellular differentials were determined for each sample by counting 300 total cells and were performed in triplicate. Percentages for each sample were then obtained by averaging the 3 results.
Cell surface phenotype and intracellular cytokine analysis
To analyze cell surface phenotype, cells were stained with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (MoAbs) to CD4, CD8, CD45.1, CD45.2, CD11b, GR-1; with phycoerythrin (PE)-conjugated MoAbs to CD4, CD8, F4/80, interferon-γ (IFN-γ), TNF-α; or with allophycocyanin (APC)-conjugated MoAbs to CD4, CD8 for flow cytometric analysis as previously described.18 Intracellular cytokine staining was performed using a commercially available kit (BD Pharmingen, San Diego, CA; no. 559311) according to the manufacturer's protocol. All MoAbs were purchased from BD Pharmingen. Three-color flow cytometric analysis of 1 × 104 cells was performed using a FACSCalibur (BD Biosciences, San Jose, CA). The FACScan was calibrated using FITC-, PE-, and APC-conjugated nonspecific immunoglobulin G (IgG) antibodies.
Lung histopathology
In animals undergoing transplantation, pulmonary toxicity was determined by examination of lung histopathology 5 weeks after BMT as previously described.27 Hematoxylin and eosin-stained lung sections from individual mice were coded without reference to mouse type or prior treatment regimen and independently examined by C.L. to establish an index of injury. Photographs of histopathology were taken with an Olympus BX40 microscope (40x/0.65 lens) (Olympus Corporation, Tokyo, Japan) as digital images (camera: JVC-QX5HDU; Victor Company of Japan, Yokohama, Japan) using imaging manager and acquisition software (Leica-IM50; Leica Microsystems, Bannockburn, IL) and further processed with Adobe Photo-shop 6.0 (Adobe, San Jose, CA).
Cell culture, analysis of cytokine production, proliferative response, and CTL function
All culture media reagents were purchased from Gibco (Gaithersburg, MD). To determine TNF-α production of pulmonary macrophages after transplantation in mice, lung cells were harvested 5 to 6 weeks after BMT as previously described18 and suspended in 10% fetal calf serum (FCS) Dulbecco modified Eagle medium (DMEM) supplemented with 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acid, 0.02 mM β-mercaptoethanol, and 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 7.75). Cytospin analysis was performed to assess percentages of monocytes/macrophages for each animal. Lung cells were plated at 5 × 104 monocytes/macrophages per well in flat-bottomed 96-well Falcon plates (Lincoln Park, NJ); stimulated in vitro with LPS in concentrations of 5, 10, 50, and 100 ng/mL; and supernatants collected 4 hours later and analyzed for TNF-α levels as described below.
To measure proliferation of T cells in response to alloantigen, TNF-α+/+ or TNF-α-/- splenic T cells (0.25 × 105, 0.5 × 105, 1 × 105) were cultured in flat-bottomed 96-well Falcon plates for 120 hours in the presence of 1 × 105 irradiated (20 Gy) naive allogeneic (B6D2F1) or syngeneic (B6129SF2/J) peritoneal cells at 37°C in a humidified incubator supplemented with 7.5% CO2. After 72 hours, supernatant was obtained and analyzed for IFN-γ by enzyme-linked immunosorbent assay (ELISA). Proliferative response was measured by a 1205 Betaplate reader (Wallac, Turku, Finland) after 120 hours by incorporation of [3H]thymidine (3.7037 × 104 Bq) for the last 24 hours of incubation. To measure proliferation of T cells in response to mitogen, TNF-α+/+ or TNF-α-/- splenic T cells were cultured in flat-bottomed 96-well Falcon plates at 1 × 105 T cells ± 1.5 μg/mL concanavalin A (ConA; Sigma, St Louis, MO). Proliferative response was measured after 72 hours by incorporation of [3H]thymidine (3.7037 × 104 Bq) for the last 24 hours of incubation. Cytotoxic T lymphocyte (CTL) assays were performed according to previously published methods for a 51Cr release assay.26 The P815 (H-2d) mouse mastocytoma cell line as well as the EL-4 cell line (H-2b) were used as allogeneic and syngeneic target cell line, respectively, and percentages of specific lysis for different effector-target ratios were calculated as described.26
Lung chemokine analysis
Lungs were harvested 7 days after transplantation and immediately snap-frozen in liquid nitrogen. At time of analysis, lung samples were homogenized in 2 mL buffer solution (1 × phosphate-buffered saline [PBS], 1% Nonidet P-40 [NP-40], 0.5% Na-deoxycholate, 0.1% sodium dodecyl sulfate [SDS], and 1 tablet of Complete Protease Inhibitor Cocktail [Roche Diagnostics, Basel, Switzerland]), centrifuged at 3000 rpm for 20 minutes, and supernatant was harvested. Total protein concentration in the supernatant was determined by using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA) before chemokines were measured by ELISA as described below. Chemokine concentration in the supernatant was normalized to picograms per milligram of total protein.
Serum cytokine analysis and splenic T-cell expansion after BMT
Animals were exsanguinated 7 days after BMT, and blood samples were collected in 1.5 mL Eppendorf tubes and centrifuged at 5000 rpm for 5 minutes. Serum was harvested for subsequent analysis for TNF-α and IFN-γ by ELISA. Then, spleens were harvested at the same time point, and single-cell suspensions were generated from individual animals. Splenocytes were subsequently counted and stained for CD4, CD8, IFN-γ, and TNF-α.
Measurement of cytokine and chemokine protein levels by ELISA
Concentrations of specific cytokines and chemokines were measured in BAL fluid, serum, and supernatants from homogenized lungs or cell culture using ELISA kits for IFN-γ (OptEIA; BD Pharmingen), TNF-α (Quantikine M; R&D Systems, Minneapolis, MN, for cell culture supernatants and serum; BioSource no. KMC3012, BioSource, Camarillo, CA, for BAL fluid samples), RANTES (regulated on activation normal T cells expressed and secreted), macrophage inflammatory protein-1α (MIP-1α), monokine induced by interferon gamma (MIG), (Quantikine M), and monocyte chemoattractant protein-1 (MCP-1) (BioSource no. KMC1012). Assays were performed according to the manufacturer's protocol. Assay sensitivity was less than 7.5 pg/mL for IFN-γ, less than 3.0 pg/mL (BioSource) or less than 5.0 pg/mL (R&D Systems) for TNF-α, less than 2.0 pg/mL for RANTES, less than 1.5 pg/mL for MIP-1α, less than 9 pg/mL for MCP-1, and less than 3.0 pg/mL for MIG. ELISA plates were read by microplate reader (Bio-Rad).
Statistical considerations
All values are expressed as the mean ± SEM. Statistical comparisons between groups were completed using the parametric independent sample t test if there were 5 or more animals per group and using the Mann-Whitney test if there were fewer than 5 animals per group. The Wilcoxon rank test was used for analyzing survival data.
Results
Donor accessory cells and T cells in the lung are significant producers of TNF-α after allogeneic BMT
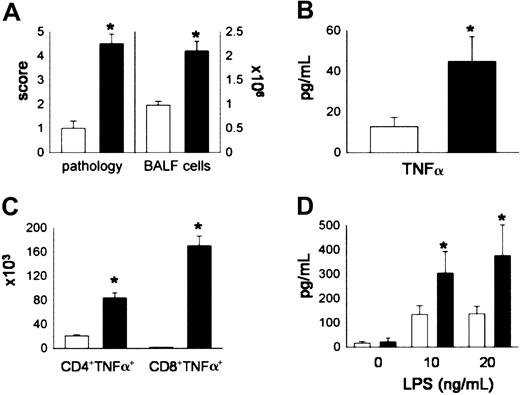
Lethally irradiated B6D2F1 mice received either syngeneic (B6D2F1) or allogeneic (C57BL/6) BMT as described in “Materials and methods.” The severity of IPS was assessed at week 5 after BMT, a time point at which repopulation of the lung by donor T cells and monocytes/macrophages is complete.28 Mice receiving syngeneic BMT maintained normal histology, whereas recipients of allo-BMT developed significant lung injury that included mononuclear cell infiltration around both vascular and bronchial structures and an acute pneumonitis involving the alveolar and interstitial spaces (data not shown). Using a semiquantitative scoring system, we found that significant pulmonary damage was present in allo-BMT recipients compared with syngeneic controls (Figure 1A). Lung histopathology after allo-BMT correlated with increasing numbers of total cells (Figure 1A) and with elevations in TNF-α levels (Figure 1B) in the bronchoalveolar space. To determine the cellular source of BAL fluid (BALF) TNF-α, BALF CD4+ and BALF CD8+ T cells were analyzed by flow cytometry for the presence of intracellular TNF-α as described in “Materials and methods.” We found that both CD4+ and CD8+ T cells produced TNF-α and, as shown in Figure 1C, the numbers of TNF-α-secreting CD4+ and CD8+ T cells were significantly increased in the BALF after allogeneic BMT. Furthermore, when pulmonary macrophages were obtained from animals undergoing transplantation and stimulated in vitro with LPS, cells from allo-BMT recipients produced significantly higher amounts of TNF-α compared with syngeneic controls. Collectively, these data demonstrate that donor-derived T cells and monocytes/macro phages present in the lungs after allo-BMT are capable of producing significant amounts of TNF-α and likely contribute to elevated BALF levels of this cytokine.
Donor T cells and donor accessory cells in the lung are significant producers of TNF-α after allogeneic BMT. Lethally irradiated B6D2F1 mice received BMT from either syngeneic (B6D2F1, □) or allogeneic (B6, ▪) donors as described in “Materials and methods.” Animals were analyzed at week 5 for (A) lung histopathology and BAL fluid cellularity, (B) BAL fluid TNF-α levels, (C) numbers of TNF-α-secreting T cells, and (D) TNF-α production by pulmonary macrophages upon restimulation with LPS for 4 hours in vitro. Data are presented as mean ± SEM and are from 1 of 2 comparable experiments; n = 5 per group; *P < .05, black bar versus open bar.
Donor T cells and donor accessory cells in the lung are significant producers of TNF-α after allogeneic BMT. Lethally irradiated B6D2F1 mice received BMT from either syngeneic (B6D2F1, □) or allogeneic (B6, ▪) donors as described in “Materials and methods.” Animals were analyzed at week 5 for (A) lung histopathology and BAL fluid cellularity, (B) BAL fluid TNF-α levels, (C) numbers of TNF-α-secreting T cells, and (D) TNF-α production by pulmonary macrophages upon restimulation with LPS for 4 hours in vitro. Data are presented as mean ± SEM and are from 1 of 2 comparable experiments; n = 5 per group; *P < .05, black bar versus open bar.
TNF-α production by donor but not host cells is critical to the development of IPS
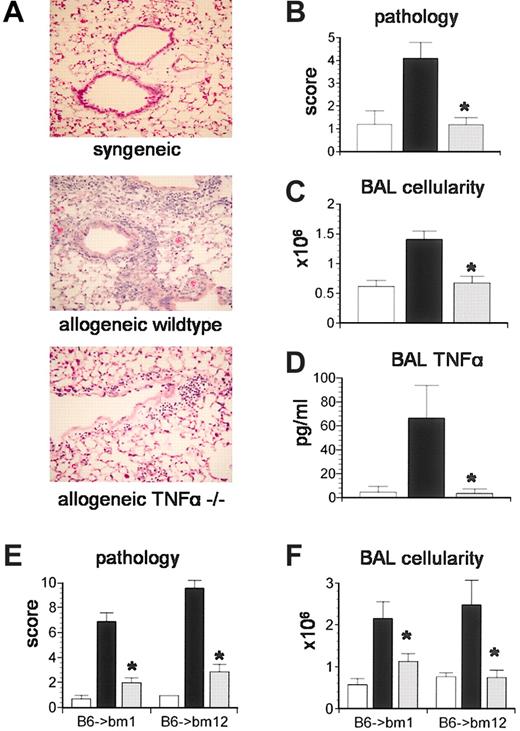
We next investigated the importance of TNF-α production by donor cells in the development of IPS. Lethally irradiated B6D2F1 mice received BMT from either syngeneic (B6D2F1) or allogeneic TNF-α+/+ or TNF-α-/- donors as described in “Materials and methods.” Recipients were analyzed 5 weeks after transplantation for lung histopathology and BAL fluid cellularity. As shown in Figure 2, recipients of allogeneic TNF-α-/- donor cells developed significantly less lung injury compared with allogeneic controls and, impressively, lung injury was not statistically different from that observed after syngeneic BMT (Figure 2A-B). BAL fluid cellularity and TNF-α levels correlated with changes in lung histopathology in all groups (Figure 2C-D). The severity of IPS was also reduced when TNF-α-/- donors were used in strain combinations with isolated mismatches in either MHC class I (B6 → bm1) or class II (B6 → bm12) antigens, demonstrating that the reduction in lung injury was not a strain-specific phenomenon (Figure 2E-F).
TNF-α production by donor cells is critical to the development of IPS.Lethally irradiated B6D2F1 mice received BMT from either syngeneic (B6D2F1, □), allogeneic wild-type (B6129SF2/J, ▪), or allogeneic TNF-α-/- (B6.129S6-TnftmGk1, ▦) donors as described in “Materials and methods.” Animals were analyzed at week 5 for (A-B) lung histopathology (hematoxylin and eosin; magnification × 200), (C) BAL fluid cellularity, and (D) BAL fluid TNF-α levels. Data are presented as mean ± SEM and are from 1 of 2 comparable experiments; n = 5 to 9 per group; *P < .05. In a second set of experiments, bm1 or bm12 mice received BMT from either syngeneic (bm1 or bm12, □), allogeneic wild-type (B6129SF2/J, ▪), or allogeneic TNF-α-/- (B6.129S6-TnftmGk1, ▦) donors as described in “Materials and methods.” (E) Lung histopathology and (F) BAL fluid cellularity were decreased after TNF-α-/- BMT in both systems. Data are presented as mean ± SEM and are from 1 of 2 comparable experiments; n = 4 to 7 per group; *P < .05, ▦ versus ▪.
TNF-α production by donor cells is critical to the development of IPS.Lethally irradiated B6D2F1 mice received BMT from either syngeneic (B6D2F1, □), allogeneic wild-type (B6129SF2/J, ▪), or allogeneic TNF-α-/- (B6.129S6-TnftmGk1, ▦) donors as described in “Materials and methods.” Animals were analyzed at week 5 for (A-B) lung histopathology (hematoxylin and eosin; magnification × 200), (C) BAL fluid cellularity, and (D) BAL fluid TNF-α levels. Data are presented as mean ± SEM and are from 1 of 2 comparable experiments; n = 5 to 9 per group; *P < .05. In a second set of experiments, bm1 or bm12 mice received BMT from either syngeneic (bm1 or bm12, □), allogeneic wild-type (B6129SF2/J, ▪), or allogeneic TNF-α-/- (B6.129S6-TnftmGk1, ▦) donors as described in “Materials and methods.” (E) Lung histopathology and (F) BAL fluid cellularity were decreased after TNF-α-/- BMT in both systems. Data are presented as mean ± SEM and are from 1 of 2 comparable experiments; n = 4 to 7 per group; *P < .05, ▦ versus ▪.
We next determined the contribution of TNF-α production from host cells in the development of IPS. Lethally irradiated TNF-α+/+ and TNF-α-/- mice received allogeneic BMT from MHC class I disparate bm1 donors as described in “Materials and methods.” Significant lung histopathology associated with increases in BALF cellularity and BALF TNF-α was observed in both allogeneic groups (Figure 3A-C). Identical findings were also seen when using a class II disparate system (bm12 → B6) wherein cytokine release is known to play a significant role in development of both systemic and target organ GVHD29 (Figure 3D-E). Taken together, these data show that the capacity for donor, but not host cells, to make TNF-α is critical to the development of IPS.
Host cell-derived TNF-α does not significantly contribute to the development of IPS after allogeneic BMT. bm1 donor bone marrow and T cells were given to either lethally irradiated syngeneic (bm1, □), allogeneic wild-type (B6129SF2/J, ▪), or allogeneic TNF-α-/- (B6.129-TnfS6tmGk1,) recipient mice, and lung injury after transplantation was assessed as described in “Materials and methods.” (A) Lung histopathology, (B) BAL fluid cellularity, and (C) BAL fluid TNF-α levels were equivalent in both allogeneic groups and significantly increased compared with syngeneic controls. (D-E) Identical results were also found when using a class II disparate system (bm12 → B6; syngeneic [bm12, □], allogeneic wild-type [B6129SF2/J, ▪], or allogeneic TNF-α-/- [B6.129S6-TnftmGk1, group; *P < .05, □ versus ▪ and.] recipients). Data are presented as mean ± SEM; n = 5 to 8 per
Host cell-derived TNF-α does not significantly contribute to the development of IPS after allogeneic BMT. bm1 donor bone marrow and T cells were given to either lethally irradiated syngeneic (bm1, □), allogeneic wild-type (B6129SF2/J, ▪), or allogeneic TNF-α-/- (B6.129-TnfS6tmGk1,) recipient mice, and lung injury after transplantation was assessed as described in “Materials and methods.” (A) Lung histopathology, (B) BAL fluid cellularity, and (C) BAL fluid TNF-α levels were equivalent in both allogeneic groups and significantly increased compared with syngeneic controls. (D-E) Identical results were also found when using a class II disparate system (bm12 → B6; syngeneic [bm12, □], allogeneic wild-type [B6129SF2/J, ▪], or allogeneic TNF-α-/- [B6.129S6-TnftmGk1, group; *P < .05, □ versus ▪ and.] recipients). Data are presented as mean ± SEM; n = 5 to 8 per
Production of TNF-α by donor accessory cells significantly contributes to the development of IPS
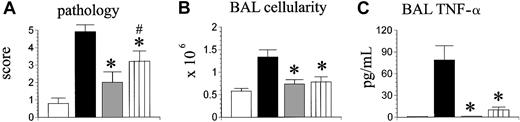
Previous data from our group demonstrated an important role for donor monocytes and macrophages in the development of IPS; allo-BMT with cells lacking a functional toll-like receptor-4 (tlr-4) resulted in decreased lung injury and BALF TNF-α levels.18 We hypothesized that the inability of donor accessory cells to produce TNF-α would result in a similar phenotype after allo-BMT. To test this hypothesis, B6D2F1 mice received syngeneic BMT or allo-BMT from either TNF-α+/+ or TNF-α-/- donors, and a third experimental group received allogeneic TNF-α-/- BM cells mixed with TNF-α+/+ T cells. Animals were analyzed on day 35 after transplantation for IPS severity. As shown in Figure 4A-C, the severity of IPS in recipients of allogeneic TNF-α-/- BM cells and TNF-α+/+ T cells was reduced in comparison with wild-type allo-BMT controls, but significant lung histopathology remained compared with syngeneic controls.
The inability of donor accessory cells to produce TNF-α results in reduced IPS severity. Lethally irradiated B6D2F1 mice underwent transplantation as described in Figure 2 (syngeneic □, allogeneic wild-type ▪, allogeneic TNF-α-/- ). A third allogeneic group received allogeneic TNF-α-/- bone marrow cells mixed with allogeneic TNF-α+/+ T cells (hatched bar). Lung injury was assessed 35 days after transplantation by lung histopathology (A), BAL cellularity (B), and BAL fluid TNF-α levels(C). Data are presented as mean ± SEM and are from 1 of 2 comparable experiments; n = 4 to 8 per group; *P < .05, gray and hatched bars versus black bar; #P < .05, ▥ versus □.
). A third allogeneic group received allogeneic TNF-α-/- bone marrow cells mixed with allogeneic TNF-α+/+ T cells (hatched bar). Lung injury was assessed 35 days after transplantation by lung histopathology (A), BAL cellularity (B), and BAL fluid TNF-α levels(C). Data are presented as mean ± SEM and are from 1 of 2 comparable experiments; n = 4 to 8 per group; *P < .05, gray and hatched bars versus black bar; #P < .05, ▥ versus □.
The inability of donor accessory cells to produce TNF-α results in reduced IPS severity. Lethally irradiated B6D2F1 mice underwent transplantation as described in Figure 2 (syngeneic □, allogeneic wild-type ▪, allogeneic TNF-α-/- ). A third allogeneic group received allogeneic TNF-α-/- bone marrow cells mixed with allogeneic TNF-α+/+ T cells (hatched bar). Lung injury was assessed 35 days after transplantation by lung histopathology (A), BAL cellularity (B), and BAL fluid TNF-α levels(C). Data are presented as mean ± SEM and are from 1 of 2 comparable experiments; n = 4 to 8 per group; *P < .05, gray and hatched bars versus black bar; #P < .05, ▥ versus □.
). A third allogeneic group received allogeneic TNF-α-/- bone marrow cells mixed with allogeneic TNF-α+/+ T cells (hatched bar). Lung injury was assessed 35 days after transplantation by lung histopathology (A), BAL cellularity (B), and BAL fluid TNF-α levels(C). Data are presented as mean ± SEM and are from 1 of 2 comparable experiments; n = 4 to 8 per group; *P < .05, gray and hatched bars versus black bar; #P < .05, ▥ versus □.
Donor T cell-derived TNF-α is critical to the development of IPS
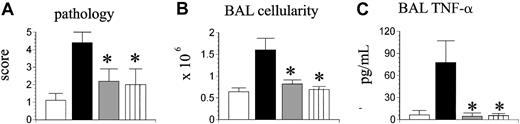
The presence of residual lung injury in mice receiving TNF-α-/- BM cells and TNF-α+/+ T cells suggested that donor T-cell production of TNF-α might also contribute to IPS. To investigate this possibility, B6D2F1 mice next received allo-BMT from wild-type (TNF-α+/+) or TNF-α-/- donors, and some mice now received a mixture of allogeneic TNF-α+/+ BM cells and TNF-α-/- T cells. Syngeneic controls were again included. As shown in Figure 5, transplantation of TNF-α-/- T cells had a significant impact on IPS severity regardless of the phenotype of the coadministered BM cells; not only was lung histopathology significantly decreased in recipients of TNF-α+/+ BM and TNF-α-/- T cells compared with wild-type allogeneic controls, but all parameters of lung injury were reduced down to levels that were not statistically different from those measured in syngeneic controls.
Donor T cell-derived TNF-α critically contributes to the development of IPS. Lethally irradiated B6D2F1 mice underwent transplantation as described in Figure 2 (syngeneic, ▥; allogeneic wild-type, ▪; allogeneic TNF-α-/-, ▦). A third allogeneic group received allogeneic TNF-α+/+ bone marrow cells mixed with allogeneic TNF-α-/- T cells (▥). Lung injury was assessed 35 days after transplantation by lung histopathology (A), BAL cellularity (B), and BAL fluid TNF-α levels (C). Data are presented as mean ± SEM and are from 1 of 2 comparable experiments; n = 4 to 8 per group; *P < .05, ▦ and ▥ versus ▪.
Donor T cell-derived TNF-α critically contributes to the development of IPS. Lethally irradiated B6D2F1 mice underwent transplantation as described in Figure 2 (syngeneic, ▥; allogeneic wild-type, ▪; allogeneic TNF-α-/-, ▦). A third allogeneic group received allogeneic TNF-α+/+ bone marrow cells mixed with allogeneic TNF-α-/- T cells (▥). Lung injury was assessed 35 days after transplantation by lung histopathology (A), BAL cellularity (B), and BAL fluid TNF-α levels (C). Data are presented as mean ± SEM and are from 1 of 2 comparable experiments; n = 4 to 8 per group; *P < .05, ▦ and ▥ versus ▪.
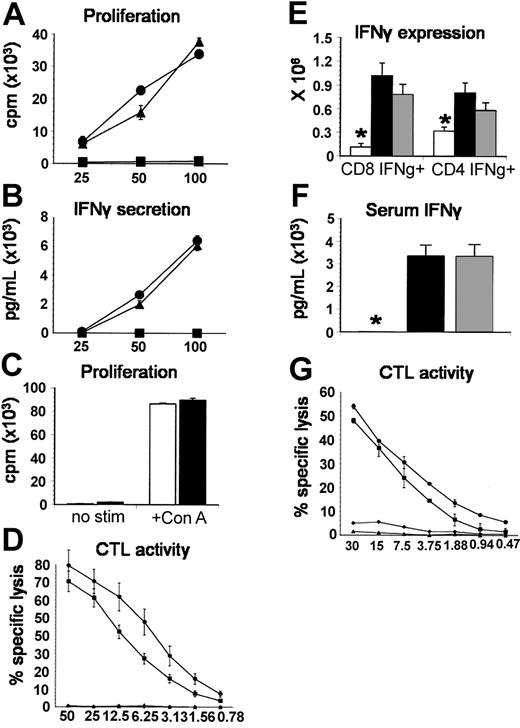
TNF-α-deficient T cells do not differ from TNF-α+/+ T cells in allospecific responses and cytolytic function in vitro or in vivo
In light of the aforementioned contribution of accessory cell-derived TNF-α, complete abrogation of IPS after the transfer of TNF-α-/- T cells was unexpected. We therefore determined whether this effect was solely attributed to the intrinsic absence of TNF-α production or whether TNF-α-/- T cells displayed other defects in alloreactivity and cytotoxicity. We compared TNF-α-/- T cells with wild-type T cells in their in vitro capacity to (1) proliferate and secrete IFN-γ during a 5-day mixed lymphocyte culture with B6D2F1 stimulator cells or after coculture with ConA (proliferation only) and (2) effectively lyse chromium-labeled allogeneic target cells in a CTL assay and found no differences between groups (Figure 6A-D). To exclude intrinsic defects that may only become evident in vivo, the expansion of IFN-γ-secreting splenic T cells, splenic T-cell cytotoxic function, and serum IFN-γ levels were measured 7 days following allo-BMT with either TNF-α-/- or TNF-α+/+ donors. As shown in Figure 6E-G, no significant differences between allogeneic groups were identified. Collectively, these data demonstrate that T cells from TNF-α-/- mice are capable of responding to alloantigen with the same vigor as wild-type T cells and therefore suggest that reduction in IPS after allo-BMT is not secondary to defects in alloresponsiveness.
TNF-α-deficient T cells do not differ from TNF-α+/+ T cells in allospecific responses and cytolytic function in vitro or in vivo. (A) Allospecific proliferation and (B) IFN-γ production were assessed in vitro during a mixed lymphocyte reaction (MLR) with TNF-α+/+ (▴) or TNF-α-/- (▪) T cells and allogeneic B6D2F1 stimulators or with TNF-α+/+ T cells and syngeneic B6 stimulators (▪) as described in “Materials and methods.” (C) Proliferation of TNF-α+/+ (▪) or TNF-α-/- (□) T cells with or without ConA stimulation. (D) Alloantigen-specific cytotoxic function of T cells after in vitro priming (bulk MLR) was determined by a chromium release assay using P-815 (H2d) and EL-4 (H2b) target cells as described (▪, TNF-α+/+ → P-815; ▪, TNF-α-/- → P-815; ▴, TNF-α+/+ → EL-4; ♦, TNF-α-/- → EL-4). All data presented are from 1 experiment representative of 2. To assess donor T-cell function in vivo, B6D2F1 mice received BMT from syngeneic (□), allogeneic wild-type (▪), or allogeneic TNF-α-/- (▦) donors as described in Figure 2. (E) The expansion of IFN-γ-secreting splenic CD4+ and CD8+ T cells and (F) serum IFN-γ levels were determined 7 days after BMT, and no differences were seen between allogeneic groups. (G) Cytotoxic function of splenic T cells after in vivo priming was determined using the chromium release assay described above 7 days after BMT with TNF-α+/+ donors (▪ P-815 targets or ▴ EL-4 targets) or TNF-α-/- donors (▪ P-815 targets, ♦ EL-4 targets). Data are presented as mean ± SEM; *P < .05, □ versus ▪ and ▦.
TNF-α-deficient T cells do not differ from TNF-α+/+ T cells in allospecific responses and cytolytic function in vitro or in vivo. (A) Allospecific proliferation and (B) IFN-γ production were assessed in vitro during a mixed lymphocyte reaction (MLR) with TNF-α+/+ (▴) or TNF-α-/- (▪) T cells and allogeneic B6D2F1 stimulators or with TNF-α+/+ T cells and syngeneic B6 stimulators (▪) as described in “Materials and methods.” (C) Proliferation of TNF-α+/+ (▪) or TNF-α-/- (□) T cells with or without ConA stimulation. (D) Alloantigen-specific cytotoxic function of T cells after in vitro priming (bulk MLR) was determined by a chromium release assay using P-815 (H2d) and EL-4 (H2b) target cells as described (▪, TNF-α+/+ → P-815; ▪, TNF-α-/- → P-815; ▴, TNF-α+/+ → EL-4; ♦, TNF-α-/- → EL-4). All data presented are from 1 experiment representative of 2. To assess donor T-cell function in vivo, B6D2F1 mice received BMT from syngeneic (□), allogeneic wild-type (▪), or allogeneic TNF-α-/- (▦) donors as described in Figure 2. (E) The expansion of IFN-γ-secreting splenic CD4+ and CD8+ T cells and (F) serum IFN-γ levels were determined 7 days after BMT, and no differences were seen between allogeneic groups. (G) Cytotoxic function of splenic T cells after in vivo priming was determined using the chromium release assay described above 7 days after BMT with TNF-α+/+ donors (▪ P-815 targets or ▴ EL-4 targets) or TNF-α-/- donors (▪ P-815 targets, ♦ EL-4 targets). Data are presented as mean ± SEM; *P < .05, □ versus ▪ and ▦.
TNF-α from infiltrating donor T cells increases the pulmonary expression of inflammatory chemokines early after allo-BMT
TNF-α is known to enhance chemokine expression and leukocyte migration to sites of inflammation.30 In addition, mRNA and protein levels of chemokines are increased in the lung within the first 2 weeks after allo-BMT.28,31,32 We hypothesized that pulmonary chemokine expression in this context is regulated in part by TNF-α produced from donor T cells infiltrating the lung early after BMT. To test this hypothesis, B6D2F1 mice received BMT as described before. Whole lungs were harvested on days +7 and +14 and analyzed for chemokine levels of the CCR5 ligands RANTES and MIP-1α, the CCR2 ligand MCP-1, and the CXCR3 ligand MIG as described in “Materials and methods.” As shown in Figure 7A, protein levels of each cytokine/chemokine tested were elevated in the lung after allo-BMT from TNF-α+/+ donors compared with syngeneic controls. By contrast, allo-BMT with TNF-α-/- T cells resulted in significant alterations in lung chemokine levels. Specifically, protein levels of RANTES and MIG were decreased in the lung as early as day 7, whereas levels of all chemokines were reduced by day 14 (Figure 7A). BMT with TNF-α-/- BM and wild-type TNF-α+/+ T cells had no effect on chemokine expression, demonstrating that T-cell and not accessory cell TNF-α regulates pulmonary chemokine expression at these early time points (data not shown). We next determined whether down-regulation of pulmonary chemokine expression early after BMT altered the recruitment of inflammatory cells to the lungs at later time points. The analysis of BALF cell types 5 weeks after BMT with TNF-α+/+ BM and TNF-α-/- T cells in comparison with TNF-α+/+ controls revealed that early modulation of chemokine expression was linked to a significant decrease in the numbers of donor CD4+ and CD8+ T cells. In addition, the number of F4/80+ cells coexpressing CD11b was significantly reduced, confirming a reduction in newly recruited macrophages to the alveolar space.33,34 By contrast, numbers of GR-1+ neutrophils did not differ between allogeneic groups.
Donor T cell-derived TNF-α is important for the induction of inflammatory chemokines in the lung and the subsequent recruitment of donor effector cells during IPS. Lethally irradiated B6D2F1 mice underwent transplantation as described in Figure 5 (syngeneic, □; allogeneic wild-type, ▪; allogeneic TNF-α-/-,, allogeneic TNF-α+/+ BM cells mixed with TNF-α-/-T cells, [hatched bar]). (A) Protein levels of RANTES, MIP-1α, MIG, and MCP-1 were determined in the lung on day 7 and 14 after BMT as described in “Materials and methods.” Data are presented as mean ± SEM; day 7 data are from 1 experiment, day 14 data are from 1 experiment representative of 2; n = 6 per group. (B) Reduced chemokine levels early after transplantation are associated with decreased BALF CD4+, CD8+, and F4/80+ CD11b+ cell numbers by week 5. **P < .01; *P < .05, and hatched bar versus ▪.
Donor T cell-derived TNF-α is important for the induction of inflammatory chemokines in the lung and the subsequent recruitment of donor effector cells during IPS. Lethally irradiated B6D2F1 mice underwent transplantation as described in Figure 5 (syngeneic, □; allogeneic wild-type, ▪; allogeneic TNF-α-/-,, allogeneic TNF-α+/+ BM cells mixed with TNF-α-/-T cells, [hatched bar]). (A) Protein levels of RANTES, MIP-1α, MIG, and MCP-1 were determined in the lung on day 7 and 14 after BMT as described in “Materials and methods.” Data are presented as mean ± SEM; day 7 data are from 1 experiment, day 14 data are from 1 experiment representative of 2; n = 6 per group. (B) Reduced chemokine levels early after transplantation are associated with decreased BALF CD4+, CD8+, and F4/80+ CD11b+ cell numbers by week 5. **P < .01; *P < .05, and hatched bar versus ▪.
Discussion
Idiopathic pneumonia syndrome is a frequently fatal complication after allo-BMT. The pathophysiology of IPS is complex and involves the recruitment of donor T-cell and accessory cell effectors and the production of inflammatory cytokines.13-15,17,21,22,28 In particular, TNF-α is increased in the BAL fluid during experimental and clinical IPS.21,35 Neutralization of TNF-α significantly reduces the severity of lung injury after BMT in mice,17 and similar therapy in humans has been associated with improvements in pulmonary dysfunction.7 However, the cellular source and whether donor- or host-derived TNF-α is critical in the development of IPS have not been fully examined. We now show that TNF-α production by both donor BM-derived accessory cells and mature T cells significantly contributes to the development of IPS. Importantly, TNF-α production by donor T cells regulates pulmonary chemokine expression early after BMT and the subsequent recruitment of donor effector cells to the lung.
Previous work has demonstrated that macrophages are important effector cells in the development of IPS.14,18,28,31 In some models, host macrophages appear to contribute to injury incurred within days after the transfer of allogeneic donor T cells.14,31 By contrast, work from our laboratory has underscored a significant role for donor-derived macrophages in the development of pulmonary injury that develops along a time course that more consistently reflects clinical disease. Specifically, we have shown that hyporesponsiveness of donor cells to LPS stimulation reduces acute lung injury after allogeneic BMT.18 Our current data confirm and extend these findings; allogeneic BMT with TNF-α-/- BM and TNF-α+/+ T cells resulted in a significant reduction in lung injury compared with animals receiving both TNF-α+/+ BM and TNF-α+/+ T cells. Moreover, the use of TNF-α-/- mice as BMT recipients had no effect on the severity of IPS, confirming that TNF-α produced by donor macrophages is a critical effector molecule during the development of lung injury
Donor T cells also contribute to experimental IPS. Host antigen-specific T-cell effectors home to the pulmonary interstitium and cause damage either directly by using the Fas-Fas ligand (Fas-FasL) cytolytic pathway36,37 or indirectly by producing Th1 cytokines like IFN-γ and enhancing the activation and functional status of pulmonary macrophages.13-15 In one study, increased MHC class II expression on pulmonary macrophages was directly attributed to the influx of alloreactive T cells.15 In addition, the infusion of allospecific CD4+ T-cell clones into nonirradiated recipients primed host alveolar macrophages to produce increased amounts TNF-α in response to LPS stimulation in vitro.14 Our data support a link between donor T-cell infiltration into the lung and enhanced pulmonary macrophage production of TNF-α at a time point after BMT when macrophages are of donor origin.28
Although a role for donor T-cell-derived TNF-α has recently been implicated in the development of systemic GVHD,23 the specific contribution of TNF-α from this cellular source to the development lung injury after allo-BMT has not been studied. Our results show that donor T-cell-derived TNF-α is critical in the development of IPS. TNF-α-secreting CD4+ and CD8+ T cells are elevated in the BALF after allo-BMT, and mice undergoing transplantation with allogeneic TNF-α+/+ bone marrow and TNF-α-/- T cells develop significantly less severe IPS compared with animals receiving TNF-α+/+ T cells. Surprisingly, the absence of TNF-α in the donor T-cell fraction alone was sufficient to reduce lung injury to levels observed after syngeneic BMT. The complete abrogation of lung injury seen after TNF-α-/- BMT could have been attributed in part to intrinsic defects in allospecific responses of TNF-α-/- T cells. However, our data confirm and extend previous reports23,38 and demonstrate that TNF-α-/- T cells do not differ from TNF-α+/+ T cells with respect to proliferation, IFN-γ production, and CTL activity in vitro or in their ability to expand, secrete IFN-γ, or to generate CTLs in response to host antigens in vivo following allo-BMT. The perforin/granzyme and Fas-FasL pathways account for most of the functional activity of CTLs. The chromium release assays used in our experiments primarily assess perforin-mediated cytolytic function of activated T cells.39 The perforin/granzyme and Fas-FasL pathways account for most of the functional activity of CTLs, but T cells deficient in both of these pathways still exhibit residual cytolytic activity, which has been ascribed to TNF-α.40 In particular, TNF-α has been directly implicated in the development of endothelial cell apoptosis in several systems,41-43 and we have found that TNF-α also contributes to apoptosis of the pulmonary vascular endothelium that accompanies the development of experimental IPS.44 Although our results strongly suggest that the reduction in lung injury after BMT with TNF-α-/- T cells is not secondary to decreased allospecific T-cell expansion and IFN-γ secretion, it remains possible that an impairment of direct TNF-α-mediated cytotoxicity contributes in part to this protective effect.
TNF-α is also a potent inducer of chemokine secretion and acts synergistically with IFN-γ to enhance the expression of several of these proteins, including the CCR2 ligand MCP-1, the CXCR3 ligands MIG and IP-10, as well as the CCR5/CCR1 ligands RANTES and MIP-1α.45-52 Furthermore, the expression of each of these inflammatory chemokines is increased in the lung during the development of IPS,28,31,32 and we have recently found that the CCR2 ligand MCP-1 is important for the recruitment of monocytes, macrophages, and T cells during the development of pulmonary toxicity after BMT.28 The data reported herein demonstrate that BMT with TNF-α-/- T cells and TNF-α+/+ BM not only resulted in a reduction of MCP-1 expression but also of MIG, RANTES and MIP-1α within the first 2 weeks of transplantation. This effect was directly associated with a significant reduction in leukocyte migration into the lung at later time points after BMT. These findings are in accord with the observations of Enelow and colleagues53-55 who demonstrated that CD8+ T-cell recognition of MHC class I-restricted viral epitopes on alveolar epithelial cells can induce TNF-α-mediated MCP-1 secretion with subsequent recruitment of inflammatory cells to the lung.
The observation that TNF-α produced by host tissues is not causally related to the development of IPS was unexpected and warrants further discussion. As noted above, previous reports have indicated a role for BMT conditioning and specifically TBI in the development of clinical and experimental IPS.8,56 Radiation is able to stimulate TNF-α production in a variety of cells,57,58 including both alveolar and peritoneal macrophages,59,60 and peripheral TNF-α levels in the BMT recipients are increased after high-dose TBI.61 Moreover, clinical reports have also shown that increased serum levels of TNF-α immediately after conditioning are associated with the development of both GVHD and IPS after clinical BMT.62,63 Despite these associations, however, a direct mechanistic link between host TNF-α and IPS (or GVHD) has not been shown. Although TNF-α released from host tissues may contribute to danger signals that facilitate the very early recruitment of alloreactive donor T cells to target organs including the lung, it is possible, and perhaps even likely, that other inflammatory mediators also contribute to this early proinflammatory milieu61,64 and continue to do so even when TNF-α is absent.
Collectively, our data demonstrate that the development of IPS is mediated by both donor T-cell- and monocyte/macrophage-derived TNF-α and suggest that donor-derived TNF-α contributes to the pathophysiology of IPS during both early and more advanced stages of disease. Although donor macrophage-derived TNF-α is a critical effector molecule in the development of pulmonary injury after allogeneic BMT, this function is superseded by the role of donor T cell-derived TNF-α early in the evolution of IPS. TNF-α secreted by donor T cells regulates the chemokine milieu in the lung within the first 2 weeks after BMT. The enhanced expression of chemokines including MCP-1 at these early time points contributes directly to the subsequent recruitment of donor-derived monocytes and macrophages. Once recruited, these cells release TNF-α, which promotes progressive pulmonary injury either by its direct cytotoxic effects41-43,65 or by facilitating the sustained recruitment of inflammatory leukocytes or both. Accordingly, the inability of donor T cells to secrete TNF-α results in a significant reduction in pulmonary chemokine expression and completely abrogates the influx of F480+/CD11b+ cells into the lung and the overall severity of IPS at later time points. Similar results were obtained following direct neutralization of MCP-1,28 thereby strengthening the mechanistic link between donor T-cell TNF-α secretion, chemokine (MCP-1) expression, and leukocyte recruitment to the lung.
These findings underscore the contribution of TNF-α to the development of IPS and support the use of agents that block TNF-TNF receptor (TNF-TNFR) interactions as novel strategies to prevent or treat this serious complication after BMT. Indeed, such strategies have been successful in reducing IPS and GVHD in animal models17,22,60,66-68 and are currently being studied after clinical BMT.7,69,70 As with any form of systemic immunosuppres sion, inhibition of TNF-α could result in a loss of the graft versus leukemia (GVL) effect as observed in some preclinical reports23,67,71 but not in others.68 However, the tempo of disease progression once IPS is diagnosed and unacceptably high mortalityrates, coupled with a relatively short (weeks versus months) anticipated duration of treatment, all support TNF-α neutralization as an attractive therapeutic strategy for IPS.
Prepublished online as Blood First Edition Paper, April 6, 2004; DOI 10.1182/blood-2003-12-4259.
Supported by grants 5K12HD028820-12, R01 HL072258-01, and R01 HL55162-05. G.C.H. is a Deutsche Krebshilfe e.V. Scholar. K.R.C. is an Amy Strelzer-Manasevit Scholar of the National Marrow Program, a fellow of the Robert Wood Johnson Medical Minority Faculty Development Program, and the recipient of a Translational Research Award from the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 3. Host cell-derived TNF-α does not significantly contribute to the development of IPS after allogeneic BMT. bm1 donor bone marrow and T cells were given to either lethally irradiated syngeneic (bm1, □), allogeneic wild-type (B6129SF2/J, ▪), or allogeneic TNF-α-/- (B6.129-TnfS6tmGk1,) recipient mice, and lung injury after transplantation was assessed as described in “Materials and methods.” (A) Lung histopathology, (B) BAL fluid cellularity, and (C) BAL fluid TNF-α levels were equivalent in both allogeneic groups and significantly increased compared with syngeneic controls. (D-E) Identical results were also found when using a class II disparate system (bm12 → B6; syngeneic [bm12, □], allogeneic wild-type [B6129SF2/J, ▪], or allogeneic TNF-α-/- [B6.129S6-TnftmGk1, group; *P < .05, □ versus ▪ and.] recipients). Data are presented as mean ± SEM; n = 5 to 8 per](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/2/10.1182_blood-2003-12-4259/6/m_zh80140464170003.jpeg?Expires=1769082576&Signature=G1H8CEEpVxSaSCFsMCKioF6YgViIltq7mrp6GmNkTi2pUCzFmyjf7pLWw9TQqkJw-VoL7y0QNK~ebK-pn7u7PFgWb~yRcOhB22~ATBbbi~d2T84N6EqOlNEP5IDVUkjpo7pKUMfEVm9trmkBUc9LhYNH4nCLq9~VaihtQkk2DEcndfIBMZPosxbYPCAjBUckn~DTJEMA9lKRf6b-hvPjh99YV-ViSfhVMSM125A-Z9gNIuuBtGnl791-KjVpAnZe14Xu-pDa~N9AgFn8rgZML7rXDDaNi0zDkCm86eiJYqonj1fgNIZo8SxdFHLXbC8rUOCEjXhAAOGwVyfksTroIA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Donor T cell-derived TNF-α is important for the induction of inflammatory chemokines in the lung and the subsequent recruitment of donor effector cells during IPS. Lethally irradiated B6D2F1 mice underwent transplantation as described in Figure 5 (syngeneic, □; allogeneic wild-type, ▪; allogeneic TNF-α-/-,, allogeneic TNF-α+/+ BM cells mixed with TNF-α-/-T cells, [hatched bar]). (A) Protein levels of RANTES, MIP-1α, MIG, and MCP-1 were determined in the lung on day 7 and 14 after BMT as described in “Materials and methods.” Data are presented as mean ± SEM; day 7 data are from 1 experiment, day 14 data are from 1 experiment representative of 2; n = 6 per group. (B) Reduced chemokine levels early after transplantation are associated with decreased BALF CD4+, CD8+, and F4/80+ CD11b+ cell numbers by week 5. **P < .01; *P < .05, and hatched bar versus ▪.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/2/10.1182_blood-2003-12-4259/6/m_zh80140464170007.jpeg?Expires=1769082576&Signature=AsaUrTKRQC8RkUFr1lviUMRjkbeU8OL3dOYBOOxBUMdqDzK2dk9DHaOLS~RqwywRYvHFrudMRn7ljqBaT9EW0d1MGTw2k1hrOOS50kdn9BgKY6Nffvr~ACT0lququ5O4QmVywip~i1qaM66blirziCzTPJlyHmOiqyWZTdMuSTl13J5~Yu9SPj1md99l52v85panWoefM-KowmzN24rgBQIktPitwwK4rT8V-kGQ2pescW1rXx3nrRFhALExqBNgBr3NF1HO5rUsWLiOQ~ArexRgArDRn0DY4QPk3HRT4fSnnraJW4B7Azw1~eA8EEAyrMAuZ0FZnGEJ-7-oB9IgEg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal