Abstract
Bone marrow transplantation (BMT) can cure myelodysplastic syndrome (MDS), although transplantation carries significant risks of morbidity and mortality. Because the optimal timing of HLA-matched BMT for MDS is unknown, we constructed a Markov model to examine 3 transplantation strategies for newly diagnosed MDS: transplantation at diagnosis, transplantation at leukemic progression, and transplantation at an interval from diagnosis but prior to leukemic progression. Analyses using individual patient risk-assessment data from transplantation and nontransplantation registries were performed for all 4 International Prognostic Scoring System (IPSS) risk groups with adjustments for quality of life (QoL). For low and intermediate-1 IPSS groups, delayed transplantation maximized overall survival. Transplantation prior to leukemic transformation was associated with a greater number of life years than transplantation at the time of leukemic progression. In a cohort of patients under the age of 40 years, an even more marked survival advantage for delayed transplantation was noted. For intermediate-2 and high IPSS groups, transplantation at diagnosis maximized overall survival. No changes in the optimal transplantation strategies were noted when QoL adjustments were incorporated. For low- and intermediate-1-risk MDS, delayed BMT is associated with maximal life expectancy, whereas immediate transplantation for intermediate-2- and high-risk disease is associated with maximal life expectancy. (Blood. 2004;104:579-585)
Introduction
Myelodysplastic syndromes (MDS) are clonal hematopoietic disorders characterized by ineffective hematopoiesis, marrow dysplasia, and variable rates of transformation to acute myelogenous leukemia (AML). The annual incidence in the United States is estimated to be between 3.5 and 12.6 in 100 000.1 Incidence rises with increasing age, with the median age at diagnosis in the seventh decade.1
Survival after diagnosis of MDS varies from a few months to several years. Among patients under the age of 60 years, median survival is 4.6 years; it is significantly lower for those diagnosed after the age of 60 years.2 There are numerous prognostic systems developed to try to predict outcomes for patients with MDS.3-9 The most widely used system, developed by the International MDS Risk Analysis Workshop (IMRAW), is the International Prognostic Scoring System (IPSS).2 The IPSS incorporates cytogenetics, bone marrow myeloblast percentage, and peripheral blood counts to predict survival.
Allogeneic stem cell transplantation currently is the only potentially curative therapy for MDS. However, since most patients with MDS are older than 60 years, few are candidates for myeloablative transplantation. Approximately 25% of patients with MDS are younger than 60 and may be considered for transplantation.2 Trials of stem cell transplantation demonstrate long-term survival rates between 25% and 70%.10-14 Despite advances in transplantation technology, there is still considerable morbidity and mortality associated with this approach. Treatment-related mortality and overall survival are influenced by age, French-American-British (FAB) classification subtype, cytogenetic abnormalities, the conditioning regimen used, and the duration of disease prior to transplantation.10-12,14-17 The IPSS is reported to be a useful predictor of transplantation outcome.18,19
The optimal timing of bone marrow transplantation from HLA-identical siblings for MDS is unknown. Many patients enjoy a long period after diagnosis without obvious disease progression. For these patients, the risks of immediate morbidity and mortality associated with transplantation are unacceptably high. Eventually, however, most patients with MDS develop symptomatic cytopenias, or their disease evolves to a more aggressive phenotype or transforms into AML, at which time stem cell transplantation is less likely to be successful. Prospective comparisons of different transplantation timing strategies are not available and are unlikely to be performed because of the relatively small numbers of patients affected and patient and physician treatment preferences. Consequently, physicians cannot reliably offer evidence-based recommendations to their patients regarding transplantation timing. To address this problem, we performed a decision analysis examining the optimal timing of bone marrow transplantation for MDS patients with HLA-identical sibling donors.
Decision analysis is a statistical technique used to aid medical decision making under conditions of uncertainty.20 The technique is flexible and allows estimation of outcome given multiple variations of initial testing conditions and assumptions. Treatment decisions are suggested based on the area under the survival curves, thus incorporating effects on both early and late mortality into the treatment decision. The technique has been applied widely to decision making in hematologic diseases.21-26
Patients and methods
Decision strategy
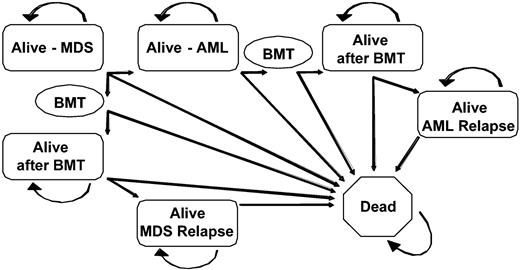
The primary decision examined in this study was the timing of myeloablative allogeneic stem cell transplantation from HLA-identical sibling donors in patients with MDS. Three possible transplantation strategies were tested: (1) transplantation at the time of diagnosis of MDS; (2) transplantation at the time of progression to AML; (3) transplantation at a fixed time interval after diagnosis. Analyses were performed for all 4 IPSS risk groups. Using the decision software package DATA v3.5 (TreeAge Software, Williams-town, MA), a Markov decision model comparing the 3 strategies was constructed (Figure 1).
Markov decision model. All patients began alive in the Alive-MDS state and were able to transition after each 6-month cycle to other health states. Patients could have remained in an alive state for any number of cycles without transitioning to another health state. The BMT state was transitory and all subjects entering the BMT state transitioned to another health state by the end of the cycle. MDS indicates myelodysplastic syndrome; AML, acute myelogenous leukemia; BMT, bone marrow transplantation.
Markov decision model. All patients began alive in the Alive-MDS state and were able to transition after each 6-month cycle to other health states. Patients could have remained in an alive state for any number of cycles without transitioning to another health state. The BMT state was transitory and all subjects entering the BMT state transitioned to another health state by the end of the cycle. MDS indicates myelodysplastic syndrome; AML, acute myelogenous leukemia; BMT, bone marrow transplantation.
A Markov decision model tracks survival outcomes as hypothetical subjects transition between clinically relevant health states within the model. Possible health states considered in this model included alive with MDS, alive after undergoing transplantation for MDS, alive after transplantation with relapsed MDS, alive with AML, alive after undergoing transplantation for AML, alive after transplantation with relapsed AML, and dead (due to MDS, AML, transplant-related complications including graft-versus-host disease (GVHD), disease relapse, or causes unrelated to MDS, AML, or transplantation). As Figure 1 demonstrates, the “dead” state is absorbing (there is no transition out of this state). The cycle length between state transitions was 6 months, which was felt to best represent the available data and clinical decision-making process. Transition rates between the permissible states were calculated using database information as described in “Data sources” and were allowed to vary based on primary data for each of the IPSS risk levels.
Quality-of-life adjustments were made by incorporating utilities into the decision model. Utilities are numerical representations of the perceived value of a given health state and are expressed as values between 0 (a health state equivalent to being dead) and 1 (perfect health). Analyses with and without adjustments for quality of life were performed independently.
Data sources
All outcome data were derived from prospectively collected databases. All statistical analyses were performed with the SAS v.8 software package (SAS, Cary, NC).
Nontransplantation MDS cohort. Data on the outcome of patients not undergoing transplantation were derived from the IMRAW database.2 The IMRAW group has been following a cohort of primary MDS patients for many years and represents the largest database of prospectively followed and reported MDS patients in the medical literature. Both published data2 and unpublished extended follow-up data were provided by the IMRAW group. Only individuals 60 years or younger at the time of entry to the IMRAW cohort were included in this analysis. Since proliferative chronic myelomonocytic leukemia was excluded by the IMRAW investigators, these cases were also excluded from this analysis. Of the initial 816 individuals in the inception cohort, 184 patients were eligible for analysis based on age and FAB subtype. Kaplan-Meier outcome curves of leukemia-free and overall survival were constructed for these 184 patients and were stratified by IPSS risk group at the time of diagnosis. From the life tables, 6-month leukemic transformation and MDS-related mortality rates were calculated and were transformed to transition probabilities for use in the Markov model.
MDS transplantation cohort. Two groups of patients were combined to construct the MDS transplantation cohort. The first group was composed of patients who received allogeneic bone marrow transplants from HLA-identical siblings between January 1, 1990, and December 31, 1999, and were registered with the International Bone Marrow Transplant Registry (IBMTR). The IBMTR is a voluntary research organization that prospectively collects patient data on approximately 40% to 50% of all transplantation procedures performed in the United States. The second group of patients included individuals who received allogeneic bone marrow transplants from HLA-identical siblings at the Fred Hutchinson Cancer Research Center (FHCRC) between January 1, 1990, and December 31, 1999. There was no overlap between these patient populations.
A total of 868 transplant recipients were considered for analysis. Of these, 127 subjects were younger than 18 years old, 107 received peripheral blood stem cell transplants, 142 had nonsibling, unrelated, or unknown donors, and 56 used T-cell depletion as GVHD prophylaxis. These 432 patients were therefore excluded from the analysis. Of the remaining 436 subjects, 260 individuals had sufficient data to calculate IPSS scores at the time of transplantation. All patients received ablative conditioning regimens and received cyclosporine or tacrolimus with methotrexate prophylaxis for GVHD. A total of 81.2% of the cases were from the IBMTR and the remaining cases from the FHCRC. In comparison with patients who were not included in the analysis due to insufficient data to calculate an IPSS score, patients included in the analysis were of similar ages (40.4 years vs 38.8 years, P = .12), had a similar sex distribution (55% male vs 56% male, P = .80), and had similar [pretransplantation hemoglobin levels (94 g/L vs 93 g/L; P = .76), white blood cell counts (4.7 × 109/L vs 4.3 × 109/L; P = .51), and platelet counts (91.3 × 109/L vs 94.5 × 109/L; P = .80). Patients included in the analysis had higher cytogenetic risk scores (Mantel-Haenszel P = .02), but had a lower proportion of marrow blasts prior to transplantation (8.2% vs 12.4%; P = .02) and a higher proportion of unknown MDS subtypes (10.2% vs. 3.5%; P = .03). Kaplan-Meier probabilities of relapse-free survival, overall survival, and survival following relapse were constructed. Relapse and transplant-related mortality rates were stratified by IPSS risk group at the time of transplantation, but mortality after relapse was not. From the life tables, 6-month relapse, relapse-free survival, and survival after relapse rates were generated and were transformed to transition probabilities for use in the Markov model.
AML transplantation cohort. A cohort of patients who underwent bone marrow transplantation from an HLA-identical sibling for AML developing from primary MDS was obtained from the IBMTR. A total of 1155 patients was reported to the IBMTR between January 1, 1990, and December 31, 1999, of whom 445 had underlying MDS. Of these 445 subjects, 53 were younger than 18 years old, 80 received peripheral blood stem cells, and 82 received allografts from nonsibling related donors or unrelated donors, leaving a total of 230 patients who were included this analysis. Patients who had received induction chemotherapy for AML were included in the analysis, regardless of remission status. Treatment-related or secondary MDS were excluded. Kaplan-Meier outcome curves measuring relapse-free survival, overall survival, and survival following relapse were constructed. No stratification by original IPSS group was done. From the life tables, 6-month relapse, relapse-free survival, and survival after relapse rates were generated and were transformed to transition probabilities for use in the Markov model.
Age- and sex-matched controls. Mortality rates for age- and sex-matChed controls were obtained from US Census Bureau Information (1998) and included in the model.
Baseline testing conditions, assumptions, and sensitivity analyses. For the purposes of this analysis, a base case of a 35-year-old man with newly diagnosed MDS and an available HLA-matched sibling donor was used. Analyses for all 4 IPSS risk groups were performed independently. An annual discount rate of 3% was used for all survival outcomes. Quality-of-life (QoL) data for patients with MDS are lacking in the literature. Estimates from a similar decision analysis study of patients with AML were used to attempt to adjust for QoL in this study.27 Those that were not available from the literature were estimated using the available values as benchmarks. Quality-of-life values can be found in Table 1, along with the range of plausible values that were tested in sensitivity analyses. Other assumptions refer to the rate of transplantation for patients with leukemic transformation from MDS. It was assumed that up to 50% of individuals with leukemic transformation of MDS would not undergo transplantation due to death during induction chemotherapy, severe illness making ablative transplantation infeasible, or refusal of the procedure. This assumption was tested in sensitivity analyses. Other sensitivity analyses evaluated the influence of QoL estimates and recipient age on transplant-related outcomes.
Quality-of-life utilities
. | . | Range . | . | |
|---|---|---|---|---|
. | Utility . | Low . | High . | |
| Alive with MDS | 0.95 | 0.85 | 0.99 | |
| Alive after transplantation | 0.92 | 0.80 | 0.95 | |
| Alive with AML | 0.85 | 0.70 | 0.90 | |
| Alive with relapsed MDS/AML | 0.57 | 0.50 | 0.70 | |
. | . | Range . | . | |
|---|---|---|---|---|
. | Utility . | Low . | High . | |
| Alive with MDS | 0.95 | 0.85 | 0.99 | |
| Alive after transplantation | 0.92 | 0.80 | 0.95 | |
| Alive with AML | 0.85 | 0.70 | 0.90 | |
| Alive with relapsed MDS/AML | 0.57 | 0.50 | 0.70 | |
Utilities are numerical representations of the perceived value of a given health state and are expressed as values between 0 and 1. Utilities were incorporated into quality-adjusted calculations and were tested across the ranges shown in sensitivity analyses. MDS indicates myelodysplastic syndrome; AML, acute myelogenous leukemia.
Results
Patients
Baseline characteristics for subjects included in this analysis are found in Table 2. There were 184 individuals under the age of 60 years available for analysis from the IMRAW. Median follow-up for the entire cohort was 35.4 months (range, 1.4-206.7 months). There were 260 individuals included in the MDS transplantation cohort. Median follow-up of this cohort was 11.4 months (range, 0.1-131.6 months). In comparison to the nontransplantation cohort, the transplantation cohort was younger, and had a higher proportion of cases with more advanced MDS by FAB and IPSS criteria. The IBMTR MDS transplantation cohort was younger and had less-advanced IPSS scores than the FHCRC cohort.
Baseline characteristics of the registry data groups
. | Nontransplantation . | Transplantation . | . | . | Statistical differences . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | MDS IMRAW . | MDS IBMTR . | MDS FHCRC . | AML IBMTR . | P* . | P† . | P‡ . | ||||
| No. of patients | 184 | 193 | 67 | 230 | |||||||
| Age, y, median (range) | 49.8 (18-60) | 39.4 (18-59) | 45.6 (20-60) | 41.7 (18-60) | <.0001 | <.0001 | NS | ||||
| Male sex, % | 58.2 | 53.4 | 59.7 | 53.5 | NS | NS | NS | ||||
| FAB subtype, % | <.0001 | NS | NA | ||||||||
| RA | 51.6 | 30.57 | 38.81 | NA | |||||||
| RARS | 16.9 | 2.07 | 4.48 | NA | |||||||
| RAEB | 25.0 | 28.5 | 37.31 | NA | |||||||
| RAEB-t | 6.5 | 34.72 | 17.91 | NA | |||||||
| Unknown | 0 | 4.15 | 1.49 | NA | |||||||
| IPSS risk group, % | <.0001 | <.001 | NA | ||||||||
| Low | 31.0 | 9.84 | 2.99 | NA | |||||||
| Int-1 | 42.9 | 53.37 | 32.84 | NA | |||||||
| Int-2 | 20.1 | 17.62 | 46.27 | NA | |||||||
| High | 6.0 | 19.17 | 17.91 | NA | |||||||
. | Nontransplantation . | Transplantation . | . | . | Statistical differences . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | MDS IMRAW . | MDS IBMTR . | MDS FHCRC . | AML IBMTR . | P* . | P† . | P‡ . | ||||
| No. of patients | 184 | 193 | 67 | 230 | |||||||
| Age, y, median (range) | 49.8 (18-60) | 39.4 (18-59) | 45.6 (20-60) | 41.7 (18-60) | <.0001 | <.0001 | NS | ||||
| Male sex, % | 58.2 | 53.4 | 59.7 | 53.5 | NS | NS | NS | ||||
| FAB subtype, % | <.0001 | NS | NA | ||||||||
| RA | 51.6 | 30.57 | 38.81 | NA | |||||||
| RARS | 16.9 | 2.07 | 4.48 | NA | |||||||
| RAEB | 25.0 | 28.5 | 37.31 | NA | |||||||
| RAEB-t | 6.5 | 34.72 | 17.91 | NA | |||||||
| Unknown | 0 | 4.15 | 1.49 | NA | |||||||
| IPSS risk group, % | <.0001 | <.001 | NA | ||||||||
| Low | 31.0 | 9.84 | 2.99 | NA | |||||||
| Int-1 | 42.9 | 53.37 | 32.84 | NA | |||||||
| Int-2 | 20.1 | 17.62 | 46.27 | NA | |||||||
| High | 6.0 | 19.17 | 17.91 | NA | |||||||
MDS indicates myelodysplastic syndrome; AML, acute myelogenous leukemia; Tx, transplant; FAB, French-American-British Classification of Myelodysplastic Syndrome; RA, refractory anemia; RARS, refractory anemia with ringed sideroblasts; RAEB, refractory anemia with excess blasts; RAEB-t, refractory anemia with excess blasts in transformation; IMRAW, International MDS Risk Assessment Workshop; IBMTR, International Bone Marrow Transplant Registry; FHCRC, Fred Hutchison Cancer Research Center; NS, not significant; and NA, not applicable.
Probability of testing MDS IMRAW versus all transplantations.
Probability of testing MDS IBMTR versus MDS FHCRC.
Probability of testing MDS versus AML transplantation.
The AML cohort was composed of 230 patients whose median follow-up was 7.9 months (range, 0-115.2 months). There were no significant differences in age or sex distribution between the MDS and the AML transplantation cohorts.
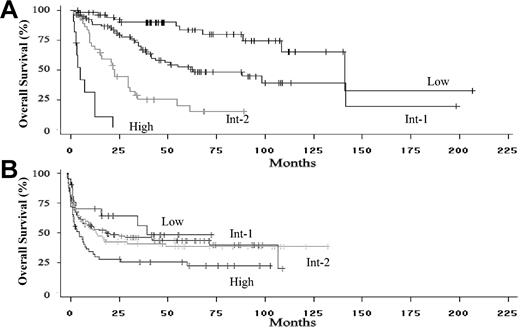
Median survival of the nontransplantation cohort was 62.9 months. When stratified by IPSS score, median survivals were 141.1, 62.9, 22.5, and 4.9 months for the low-, intermediate-1 (int-1)-, intermediate-2 (int-2)-, and high-risk groups, respectively (Figure 2A, P < .0001 for differences by log rank test). The 25% leukemic transformation time was 31.2 months for the entire cohort and 84.6, 19.2, and 2.7 months for the int-1-, int-2-, and high-risk groups, respectively. The 25% transformation rate was not reached in the low-risk group (P < .0001 for differences by log rank test).
Overall survival of patients included in the analysis. (A) Overall survival of the International MDS Risk Assessment Workshop patients who did not undergo stem cell transplantation, stratified by IPSS score at the time of diagnosis (P < .001 for differences in risk groups). (B) Overall survival of the IBMTR/FHCRC bone marrow transplantation cohort of patients, stratified by IPSS risk score at the time of transplantation (P < .001 for differences in risk groups).
Overall survival of patients included in the analysis. (A) Overall survival of the International MDS Risk Assessment Workshop patients who did not undergo stem cell transplantation, stratified by IPSS score at the time of diagnosis (P < .001 for differences in risk groups). (B) Overall survival of the IBMTR/FHCRC bone marrow transplantation cohort of patients, stratified by IPSS risk score at the time of transplantation (P < .001 for differences in risk groups).
Median survival for the MDS transplantation cohort was 14.0 months. When stratified by IPSS score, median survivals were 40.2, 20.5, 14.8, and 6.1 months for the 4 IPSS groups (Figure 2B; P = .04 by log rank test). There were no differences in survival outcomes between the IBMTR and FHCRC subgroups (P = .09). Median survival for the AML transplantation cohort was 9.9 months. Relapse was noted in 9.5%, 13.5%, 21.9%, and 44.4% of patients undergoing transplantation for low-, int-1-, int-2-, and high-risk MDS groups, respectively (overall 21%). Among patients undergoing transplantation for AML, 38.4% relapsed after transplantation.
Decision model
The discounted life expectancies for the strategies of immediate transplantation, transplantation at the time of AML progression, and transplantation at a fixed interval after diagnosis for each of the 4 IPSS risk groups are shown in Table 3. Fixed time intervals of 2, 4, 6, and 8 years after diagnosis were chosen for analysis.
Discounted life expectancy, in years, for alternative transplantation strategies
. | Transplantation at diagnosis . | Transplantation at a fixed time point . | . | . | . | Transplantation at AML progression . | |||
|---|---|---|---|---|---|---|---|---|---|
| Patients, by IPSS risk group . | . | 2y . | 4y . | 6y . | 8y . | . | |||
| All patients | |||||||||
| Low | 6.51 | 6.86 | 7.47 | 7.46 | 7.49* | 7.21 | |||
| Int-1 | 4.61 | 4.74 | 4.72 | 5.02 | 5.20* | 5.16 | |||
| Int-2 | 4.93* | 3.21 | 2.94 | 2.85 | 2.84 | 2.84 | |||
| High | 3.20* | 2.75 | 2.75 | 2.75 | 2.75 | 2.75 | |||
| Patients younger than 40 y | |||||||||
| Low | 5.62 | 6.63 | 7.53 | 8.32 | 9.00 | 10.21* | |||
| Int-1 | 2.48 | 4.04 | 5.37 | 6.53 | 7.49 | 10.21* | |||
| Int-2 | 1.65* | 1.48 | 1.51 | 1.52 | 1.53 | 1.53 | |||
| High | — | — | — | — | — | — | |||
. | Transplantation at diagnosis . | Transplantation at a fixed time point . | . | . | . | Transplantation at AML progression . | |||
|---|---|---|---|---|---|---|---|---|---|
| Patients, by IPSS risk group . | . | 2y . | 4y . | 6y . | 8y . | . | |||
| All patients | |||||||||
| Low | 6.51 | 6.86 | 7.47 | 7.46 | 7.49* | 7.21 | |||
| Int-1 | 4.61 | 4.74 | 4.72 | 5.02 | 5.20* | 5.16 | |||
| Int-2 | 4.93* | 3.21 | 2.94 | 2.85 | 2.84 | 2.84 | |||
| High | 3.20* | 2.75 | 2.75 | 2.75 | 2.75 | 2.75 | |||
| Patients younger than 40 y | |||||||||
| Low | 5.62 | 6.63 | 7.53 | 8.32 | 9.00 | 10.21* | |||
| Int-1 | 2.48 | 4.04 | 5.37 | 6.53 | 7.49 | 10.21* | |||
| Int-2 | 1.65* | 1.48 | 1.51 | 1.52 | 1.53 | 1.53 | |||
| High | — | — | — | — | — | — | |||
—indicates in sufficient data to perform analysis.
Dominant strategies.
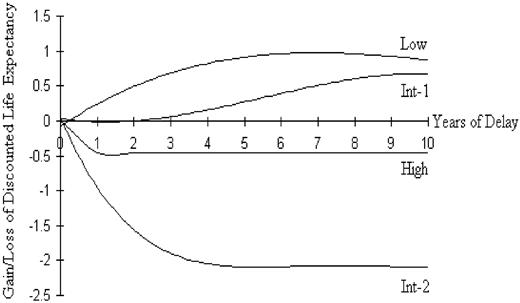
For low-risk and int-1-risk IPSS groups, transplantation at the time of leukemic progression was associated with a higher life expectancy than was the strategy of transplantation at the time of diagnosis. For both of these risk groups, however, transplantation at a fixed interval after diagnosis (but prior to the development of AML) was the strategy that maximized overall discounted life years. The gains in discounted life expectancy for delayed transplantation compared with transplantation at the time of diagnosis are seen in Figure 3. For the more-advanced IPSS risk groups (int-2 and high), the strategy that maximized discounted life expectancy was transplantation at the time of diagnosis. The decrement in life expectancy associated with delayed transplantation in these groups is shown in Figure 3.
Net benefit or loss of overall discounted life expectancy for the 4 IPSS risk groups are shown above and below the x-axis. A net benefit for delaying transplantation is noted for low and int-1 risk groups, whereas any delay in the time to transplantation is associated with a loss in survivorship in the higher risk groups.
Net benefit or loss of overall discounted life expectancy for the 4 IPSS risk groups are shown above and below the x-axis. A net benefit for delaying transplantation is noted for low and int-1 risk groups, whereas any delay in the time to transplantation is associated with a loss in survivorship in the higher risk groups.
Quality-adjusted discounted life expectancy was approximated using the utility values shown in Table 1; results are shown in Table 4. Adjustment for QoL did not change the preferred treatment strategy for any of the 4 IPSS risk groups; however, some strategies were influenced by the estimated QoL parameters (such as the strategy for low-risk disease). No decisions were sensitive to varying (between 25% and 75%) the estimate of the proportion of patients who would not undergo transplantation once MDS had progressed to AML.
Quality-adjusted discounted life expectancy, in years, for alternative transplantation strategies
. | Transplantation at diagnosis . | Transplantation at a fixed time point . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Patients, by IPSS risk group . | . | 2 years . | 4 years . | 6 years . | 8 years . | Transplantation at AML progression . | |||
| All patients | |||||||||
| Low | 5.99 | 6.37 | 6.98 | 7.00 | 7.05* | 6.83 | |||
| Int-1 | 4.23 | 4.41 | 4.42 | 4.72 | 4.89* | 4.87 | |||
| Int-2 | 4.53* | 2.99 | 2.75 | 2.65 | 2.65 | 2.65 | |||
| High | 2.94* | 2.53 | 2.53 | 2.53 | 2.53 | 2.53 | |||
| Patients younger than 40 y | |||||||||
| Low | 5.17 | 6.16 | 7.04 | 7.81 | 8.47 | 9.70* | |||
| Int-1 | 2.29 | 3.78 | 5.06 | 6.16 | 7.08 | 9.68* | |||
| Int-2 | 1.52* | 1.39 | 1.43 | 1.44 | 1.45 | 1.45 | |||
| High | — | — | — | — | — | — | |||
. | Transplantation at diagnosis . | Transplantation at a fixed time point . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Patients, by IPSS risk group . | . | 2 years . | 4 years . | 6 years . | 8 years . | Transplantation at AML progression . | |||
| All patients | |||||||||
| Low | 5.99 | 6.37 | 6.98 | 7.00 | 7.05* | 6.83 | |||
| Int-1 | 4.23 | 4.41 | 4.42 | 4.72 | 4.89* | 4.87 | |||
| Int-2 | 4.53* | 2.99 | 2.75 | 2.65 | 2.65 | 2.65 | |||
| High | 2.94* | 2.53 | 2.53 | 2.53 | 2.53 | 2.53 | |||
| Patients younger than 40 y | |||||||||
| Low | 5.17 | 6.16 | 7.04 | 7.81 | 8.47 | 9.70* | |||
| Int-1 | 2.29 | 3.78 | 5.06 | 6.16 | 7.08 | 9.68* | |||
| Int-2 | 1.52* | 1.39 | 1.43 | 1.44 | 1.45 | 1.45 | |||
| High | — | — | — | — | — | — | |||
—indicates insufficient data to perform analysis.
Dominant strategies.
When all above analyses were limited to patients younger than 40 years, the preferred treatment strategy for the low-risk and int-1risk IPSS groups was transplantation at progression to AML. All discounted life expectancy values were improved when compared with the data analyzed in its entirety. These analyses were insensitive to QoL parameters. For the int-2-risk group, transplantation at the time of diagnosis remained the preferred strategy. Analyses of the younger age cohort were limited by the small numbers of patients and clinical events in the nontransplantation group. There were too few high-risk patients under the age of 40 years who did not undergo transplantation to perform these analyses.
Discussion
Allogeneic hematopoietic stem cell transplantation is the only curative treatment available for patients with MDS. However, stem cell transplantation is associated with a significant risk of early morbidity and mortality, causing reluctance to use this strategy for first-line treatment of a disease with a variable natural history. The International MDS Risk Assessment Workshop developed a prognostic system to predict overall survival and transformation to acute leukemia in patients with newly diagnosed MDS treated with conventional supportive therapy. However, the scoring system did not address the difficult treatment decisions that patients with MDS face if they have HLA-matched siblings and could tolerate a myeloablative transplantation procedure.
Unfavorable prognostic variables that influence outcome after transplantation for MDS include older age, high marrow blast counts, marrow cytogenetics, and the use of induction chemotherapy prior to transplantation.13,15,17,28 All of these factors argue in favor of transplantation early after the diagnosis of MDS, regardless of IPSS risk group. In fact, one study demonstrated that transplantation early after the diagnosis of MDS was associated with the most favorable outcome.16 Despite these results, it remains unclear whether early transplantation for all patients with MDS leads to maximization of survival for the group as a whole. To address this question, we performed a decision analysis using clinical data from IMRAW, IBMTR and FHCRC.
This analysis demonstrated that life expectancy of patients with low-risk IPSS scores (low- and int-1-risk groups) who have HLA-identical siblings was higher when transplantation is delayed by some period but performed prior to the development of AML. This was particularly true for patients under the age of 40 years. Since the prognosis of low- and int-1-risk patients with supportive measures alone is excellent, with a median survival that exceeds a decade in the low-risk IPSS group, it seems reasonable to avoid the immediate risks of transplantation.2 The caveat, of course, is that it is not possible to perfectly predict the time of transformation to AML. For high-risk MDS (IPSS int-2 and high), transplantation soon after the diagnosis confers the best prognosis, since the rate of transformation to acute leukemia is high, with most patients progressing within the first year.
The treatment algorithms suggested here apply to large groups of patients; use in an individual patient must take into account many patient-specific factors. For example, individual patient risk-taking or risk-averting behaviors must be considered when suggesting a potentially life-threatening procedure such as bone marrow transplantation. Nonetheless, the suggested delay in transplantation for early-stage MDS seems to be contrary to popular clinical practice, as the IPSS int-1-risk group represented the largest subgroup of patients (46%) undergoing transplantation reported to the IBMTR between 1989 and 1997.28 In this analysis, these patients underwent transplantation a median of 6.2 months from the time of diagnosis.
Quality-of-life considerations are also important. Quality of life among patients with MDS may be influenced by factors such as recurrent infection and transfusion dependence although there are few data on this subject. Quality of life among transplant recipients has been assessed in several studies.29-32 In a prior decision analysis using physician surveys to estimate QoL, the utility of being alive after stem cell transplantation was estimated to be between 0.9 and 0.98 for patients with and without chronic GVHD.23 In our model, a utility of 0.92 was used based on the recent assumptions of Sung et al,27 but was tested over a broad range in sensitivity analyses.
In contrast, very few assessments of QoL among patients with MDS exist. A recent evaluation of transfusion-dependent patients with MDS demonstrated a significant correlation between serum hemoglobin and QoL measured via 3 independent validated tools.33 A trial of the DNA hypomethylating agent, azacytidine, demonstrated significant positive effects on QoL in patients with MDS.34,35 A direct integration of the QoL measures from these studies into the utilities used in our analysis was not performed for a variety of reasons, including the lack of a validated transformation algorithm for the QoL tools used and the distinct differences in the population surveyed (median ages 73 and 67 years vs 50 years).33-35 For the purposes of this analysis, it was assumed that the QoL of patients with MDS was at least equivalent to the posttransplantation state. The utility of 0.95 was used and was tested broadly in sensitivity analyses. When the QoL utilities were incorporated into the decision model, a delayed approach to transplantation remained the dominant strategy, however, some estimates were sensitive to the QoL estimates at the extremes of the ranges tested.
There are several potential sources of bias in this analysis. One factor to consider is selection bias in the time to transplantation in the IBMTR/FHCRC group. In this cohort, the median time to transplantation from diagnosis was 5.4 months. Regardless of the timing of transplantation in the model, the IBMTR/FHCRC cohorts were treated as a whole. This was felt to be acceptable, since within IPSS subgroups, no differences in outcome were noted when survival curves were stratified by time from diagnosis to transplantation.
Another potential source of bias is the good outcome of the IBMTR AML transplantation cohort. This group had a 5-year overall survival of 36.5%, which compares favorably with other reported series, but is consistent with other recent reports.13 Since the IBMTR does not record data on patients for whom stem cell transplantation was planned but could not be performed, we assumed that up to 50% of patients with AML that had developed from primary MDS would not proceed to transplantation for a variety of reasons. Patients could become ineligible for transplantation due to severe illness or death during AML induction chemotherapy or the unavailability of their stem cell donor. When the proportion of patients not proceeding to transplantation was varied widely, the results noted in Table 3 were not significantly different.
A third considerable source of bias is the modeling of transplantation outcome based on IPSS score at the time of transplantation, rather than at the time of diagnosis. The IPSS score was not designed to provide prognostic information at the time of transplantation nor at any time after diagnosis. However, studies suggest that this scoring system can provide useful prognostic information, when scores at diagnosis and at the time of transplantation are evaluated.18,28 If the data were analyzed using the IPSS score at diagnosis, the higher-risk transplantation subgroups would be comprised of heterogeneous mixtures of patients whose early MDS was progressing and those in whom advanced MDS was recently diagnosed. A final criticism of the design of this analysis is the inclusion of patients with refractory anemia with excess of blasts transformed (RAEB-t) according to the FAB classification of myeloid malignancies. The current World Health Organization (WHO) classification of myeloid diseases classifies patients with more than 20% marrow blasts as having AML.36 Despite this, the FAB classification and the IPSS remain broadly used clinical tools, whereas the newer WHO classification of MDS is not yet widely accepted.37,38 Transplantation outcomes for patients with RAEB and RAEB-t were not statistically different in this analysis (data not shown). Therefore, even using the newer diagnostic criteria of the WHO and excluding patients who would be classified as AML by the WHO, the results presented here remain valid.
The strategy that maximized survival for low-risk IPSS groups (low and int-1) in this study was transplantation at a fixed interval but prior to the development of AML for the entire cohort of patients tested. It is likely that the best individual estimate for this interval of time corresponds to important clinical events for the patient with MDS, such as the development of a new cytogenetic abnormality or the development of transfusion dependence. This likely corresponds to the migration from a lower to a higher IPSS risk category. The strategy of transplantation at the time of progression from one IPSS risk group to another is an attractive one for patients presenting with low-risk disease, however, we were unable to test this strategy with the available data. Since transplantation outcomes for early-stage disease may be superior to those for later-stage disease, this strategy makes sense if monitoring for progression is employed.13
Nontransplantation therapy for MDS is changing, with novel agents in development, the use of which may lead to transfusion independence and improved quality of life in patients with MDS. Similarly, novel transplantation technology may substantially reduce treatment-related morbidity and mortality and may reduce relapse rates after transplantation. Examples of transplantation innovations include the use of peripheral blood stem cells, which have been associated with reduced transplant-related mortality and increased survival in MDS, and nonmyeloablative conditioning regimens, which have been associated with reduced peritransplantation morbidity and mortality.39,40 The impact of these novel therapies and transplantation technologies on the decision to undergo early or delayed stem cell transplantation is unknown and were not evaluated in the present study. Also, although not evaluated in this study, suggestions regarding the timing of transplantation using unrelated histocompatible donors are likely to be similar to those in this study. Since outcomes with unrelated donors are generally not as favorable as with related donors,28,41 it is likely that delayed transplantation for patients with low-risk disease and unrelated donors is likely to provide the longest life expectancy. Last, since the MDS classification for adults does not adequately describe all the clinical syndromes that occur in children, this analysis should not be used in decision making for patients under the age of 18 years.
In summary, using decision analysis and prospectively collected registry data, we have shown that delayed transplantation for IPSS low and int-1 risk groups is associated with maximum discounted and quality-adjusted discounted life years. We hypothesize that the optimal timing of transplantation for this cohort is at the time of the development of a new cytogenetic abnormality, the appearance of a clinically important cytopenia, or the progression from one IPSS group to a higher risk group. For patients with int-2 and high IPSS risk scores, transplantation at the time of diagnosis is associated with maximization of discounted life years for the entire cohort of patients. These results should serve as a guide for treatment in the absence of randomized data, and may be useful as a starting point to begin discussion of treatment options with patients with MDS.
Prepublished online as Blood First Edition Paper, March 23, 2004; DOI 10.1182/blood-2004-01-0338.
Supported by Public Health Service grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; and grants from Allianz Life/Life Trac; American Cancer Society; American Red Cross; American Society of Clinical Oncology; Amgen Inc; Anonymous donation to the Medical College of Wisconsin; Aventis Pharmaceuticals; Baxter Healthcare Corp; Baxter Oncology; Berlex Oncology; Blue Cross and Blue Shield Association; The Lynde and Harry Bradley Foundation; Bristol Myers Squibb Oncology; Cedarlane Laboratories Ltd; Cell Pathways; CelMed Biosciences; Centocor Inc; Cubist Pharmaceuticals; Darwin Medical Communications Ltd; Dynal Biotech ASA; Edwards Lifesciences RMI; Endo Pharmaceuticals Inc; Enzon Pharmaceuticals Inc; Excess Inc; Fujisawa Healthcare Inc; Gambro BCT Inc; GlaxoSmithKline Inc; Human Genome Sciences; ICN Pharmaceuticals Inc; ILEX Oncology; The Kettering Family Foundation; Kirin Brewery Company; Ligand Pharmaceuticals Inc; Eli Lilly and Company; Nada and Herbert P. Mahler Charities; Merck & Company; Millennium Pharmaceuticals; Miller Pharmacal Group; Milliman USA Inc; Miltenyi Biotec; Irving I. Moskowitz Foundation; National Marrow Donor Program; NeoRx; Novartis Pharmaceuticals Inc; Novo Nordisk Pharmaceuticals; Orphan Medical Inc; Ortho Biotech Inc; Osiris Therapeutics Inc; PacifiCare Health Systems; Pall Medical; Pfizer US Pharmaceuticals; Pharmacia Corp; Pharmametrics; Pharmion Corp; Protein Design Labs; Roche Laboratories; SangStat Medical; Schering AG; StemCyte Inc; StemCell Technologies Inc; Stemco Biomedical; StemSoft Software Inc; SuperGen Inc; Sysmex; THERAKOS, a Johnson & Johnson Co; Unicare Life & Health Insurance; University of Colorado Cord Blood Bank; ViaCell Inc; ViaCor Biotechnologies; WB Saunders Mosby Churchill; and Zymogenetics Inc. C.S.C. is a Physician-Scientist of the Leukemia Research Foundation of America and was supported by a fellowship award from the Cancer and Leukemia Group B (CALGB).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal