Comment on Offman et al, page 822
Just as life begins to return to normal, some solid organ transplant patients develop leukemia. Why?
Patients who have received cytotoxic therapy with chemotherapy drugs and/or radiotherapy are at risk for long-term complications from their treatment, including therapy-related myelodysplastic syndrome (tMDS) and acute myeloid leukemia (tAML). Although a causal link has not yet been proven, these neoplasms are thought to be a direct consequence of mutational events caused by cytotoxic therapy and to be independent of the primary disease. Careful clinicopathologic and cytogenetic analyses of individual cases by many investigators have defined distinctive subtypes of this disease.1
Large epidemiologic surveys have defined high-risk patient populations. Patients with Hodgkin lymphoma were the first large cohort of cancer patients who experienced prolonged survival; hundreds of cases of therapy-related leukemia have now been reported within this group. More recently, treatment has extended the survival of patients with other cancers and they too have become at risk. In a recent report on 306 patients with therapy-related myeloid leukemia studied at the University of Chicago, 171 had lymphoma or myeloma as their primary disease, and 117 had solid tumors.2 Of note, 18 had received cytotoxic therapy for nonmalignant disorders, such as autoimmune diseases, or for immunosuppression after renal allografts.
Both ionizing radiation and many, but not all, chemotherapy drugs alter cellular DNA. If not repaired, this damage is most often lethal to cells. This, of course, is the desired consequence if the target cell were a tumor cell. Occasionally, however, nonlethal and heritable mutations occur in single somatic cells. Such an alteration in DNA might involve a single base change, deletion or inactivation of a growth suppressor gene, or changes in the expression of certain critical oncogenes or growth factor receptor genes. Alkylating agents, in particular, and radiation both possess strong mutagenic activity in vitro plus carcinogenic potency in vivo.FIG1
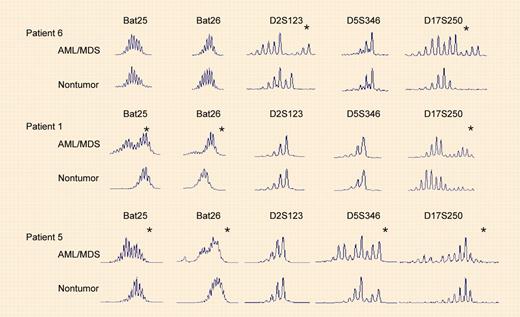
Microsatellite instability in AML/MDS from recipients of organ transplants. See the complete figure in the article beginning on page 822.
Microsatellite instability in AML/MDS from recipients of organ transplants. See the complete figure in the article beginning on page 822.
It has not yet been possible to determine whether the development of tMDS/tAML is a stochastic event, occurring by chance, or whether certain individuals are at higher risk, perhaps due to a DNA-repair deficiency or a heritable predisposition, such as altered drug metabolism. The identification of such an underlying pre-existing condition would help the screening and counseling of patients at the time of treatment for their primary disease.
Offman and colleagues now add important new data that may illuminate one mechanism for the etiology of therapy-related leukemia. Analyzing data for 180 000 recipients of heart, lung, or kidney transplants from 300 transplant centers, the authors report a significantly increased risk for developing tMDS/tAML among these recipients. A latency of 3 to 4 years was observed, and the risk was proportional to the dose of the thiopurine azathioprine received. Additionally, they confirm that thiopurines select for mismatch repair (MMR)–deficient cells in culture. Finally, they report that all 7 tAML patients examined demonstrated microsatellite instability, diagnostic for defective MMR.
Lymphoproliferative disorders that occur within a few months after transplantation are most likely due to immunosuppression from agents such as cyclosporine. In contrast, the prolonged use of thiopurines after solid organ transplants may favor the outgrowth of MMR-deficient cells, perhaps even within hematopoietic stem cells. Such a clone would have an intrinsic mutator phenotype, accumulating mutations due to uncorrected replication errors as well as increased sensitivity to DNA-damaging agents, both iatrogenic and environmental. Greater insight into the molecular pathogenesis of therapy-related leukemia would allow treatment regimens to be changed to prevent this complication plus provide an important model for the origin of similar leukemias that arise de novo.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal