Abstract
Hereditary hemochromatosis (HH) is an iron-overload disorder caused by a C282Y mutation in the HFE gene. In HH, plasma nontransferrin-bound iron (NTBI) levels are increased and NTBI is bound mainly by citrate. The aim of this study was to examine the importance of NTBI in the pathogenesis of hepatic iron loading in Hfe knockout mice. Plasma NTBI levels were increased 2.5-fold in Hfe knockout mice compared with control mice. Total ferric citrate uptake by hepatocytes isolated from Hfe knockout mice (34.1 ± 2.8 pmol Fe/mg protein/min) increased by 2-fold compared with control mice (17.8 ± 2.7 pmol Fe/mg protein/min; P < .001; mean ± SEM; n = 7). Ferrous ion chelators, bathophenanthroline disulfonate, and 2′,2-bipyridine inhibited ferric citrate uptake by hepatocytes from both mouse types. Divalent metal ions inhibited ferric citrate uptake by hepatocytes, as did diferric transferrin. Divalent metal transporter 1 (DMT1) mRNA and protein expression was increased approximately 2-fold by hepatocytes from Hfe knockout mice. We conclude that NTBI uptake by hepatocytes from Hfe knockout mice contributed to hepatic iron loading. Ferric ion was reduced to ferrous ion and taken up by hepatocytes by a pathway shared with diferric transferrin. Inhibition of uptake by divalent metals and up-regulation of DMT1 expression suggested that NTBI uptake was mediated by DMT1.
Introduction
Hereditary hemochromatosis (HH) is a common autosomal recessive disorder of iron (Fe) metabolism. This disease affects up to 1 in 200 individuals of North European descent.1 Most HH patients are homozygous for a C282Y missense mutation in the HFE gene that encodes for a major histocompatibility complex class I–like protein.2 HH is characterized by an increase in dietary Fe absorption, with a chronic rise in body Fe stores, leading to hepatic Fe overload, followed by the progressive loading of other parenchymal organs such as the heart, pancreas, and joints. The abnormally high levels of Fe in the liver can lead to hepatic fibrosis and cirrhosis, increasing the risk of hepatocellular carcinoma.3,4
Hepatocytes of the liver are the main site of deposition of Fe in HH and hence play an important role in maintaining Fe homeostasis. It is well established that hepatocytes and hepatoma cells take up Fe from transferrin (Tf)5 and nontransferrin-bound iron (NTBI).6-8 The normal range of Tf saturation in plasma is usually 20% to 50%, but in Fe-overload diseases like HH, Tf saturation is elevated and can reach 100%. Under these conditions, a greater proportion of Fe in the plasma occurs as NTBI. It has been reported that NTBI can coexist with diferric Tf in the serum of patients with Fe overload.9 NTBI in the plasma is directed to hepatocytes in the liver in vivo,10 where it is rapidly cleared. The contribution of plasma NTBI to the toxicity associated with Fe overload is uncertain. Serum NTBI is found mainly in the form of ferric citrate.9,11,12 The uptake of ferric (Fe(III))–citrate involves the dissociation of Fe from citrate at the cell surface before being transported into the cell by an Fe carrier–mediated step.8,13
Recently, Zhou and colleagues14 produced an Hfe knockout (KO) mouse model of HH. These Hfe KO mice display similar characteristics of Fe overload observed in patients with HH. These animals have elevated serum Tf saturation and increased hepatic Fe levels by 4 weeks of age. Iron deposition occurs mainly in the hepatocytes, with little or no Fe loading in the reticuloendothelial cells of the liver and the spleen.15 The Hfe KO mouse is a good animal model of HH and it provides the opportunity to investigate the mechanisms underlying an increased deposition of Fe in the liver in HH.
In previous studies, we and others have shown that the Fe absorbed by the duodenum in vivo is increased in Hfe KO mice compared with control mice.16,17 In addition, we found that the amount of absorbed Fe deposited in the liver was increased. These results indicated that Fe uptake by the liver from the Hfe KO mice is up-regulated. As NTBI levels are elevated in the serum of HH patients,18,19 even when Tf is not completely saturated with Fe, we hypothesize that NTBI uptake by hepatocytes is up-regulated in HH.
In this study, we characterized the uptake of NTBI in the form of ferric citrate by hepatocytes from Hfe KO and control C57BL/6J mice. Results showed that the rate of NTBI uptake by hepatocytes from Hfe KO mice was increased more than 2-fold compared with control cells, demonstrating that NTBI contributes to Fe loading of the liver in HH.
Materials and methods
Animals
The Hfe KO mouse, a mouse model of HH, was kindly provided by Professor W. Sly (St Louis University, St Louis, MO). In these mice, the Hfe gene has been disrupted and is a null allele.14 Control C57BL/6J and Hfe KO mice were bred at the Animal Resource Centre (Murdoch, Australia). The mice had access to a standard rodent chow and water ad libitum.
Plasma NTBI
NTBI concentration in the plasma from Hfe KO and control mice was measured using a method similar to that described previously.18,19 Briefly, 100 μL Tris-carbanatocobaltate(III) trihydrate solution was added to 450 μL plasma and incubated at 37° C for 1 hour. Nitriloacetic acid (Sigma Chemical, St Louis, MO) was then added to give a final concentration of 80 mM. This mixture was left to stand for 30 minutes at room temperature. The samples were ultrafiltered using Centricon-30 (Millipore, North Ryde, Australia) at 3000g for 1 hour and assayed for NTBI using a colorimetric method.
Serum Fe and Tf saturation
Serum iron concentration and Tf saturation in the Hfe KO and control mice were determined using a colorimetric assay according to the manufacturer's instructions (Sigma Chemical).
Ferritin levels
The amount of ferritin protein in the hepatocytes from both types of mice was measured using an immunoturbidometric method according to the manufacturer's instructions (Roche Diagnostics, Mannheim, Germany).
Isolation and culture of mouse hepatocytes
Mouse hepatocytes were isolated from livers of 11-week-old female Hfe KO and control C57BL/6J mice using a modified method of Knight et al20 based on the method first described by Seglen.21 The liver was initially perfused at a rate of 10 mL/min with a perfusion buffer (137 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 0.65 mM MgSO4, and 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]) containing 0.5 mM EGTA (ethyleneglycoltetraacetic acid; Sigma Chemical) for 5 minutes and then with a second buffer at a rate of 12 mL/min (67 mM NaCl, 6.7 mM KCl, 4.8 mM CaCl2, 100 mM HEPES, and 65 mM NaOH) containing 0.01% collagenase Type I (Worthington Biochem, Freehold, NJ) for 5 minutes. The hepatocytes were dispersed in Leibowitz (L15) medium (Invitrogen, Melbourne, Australia) and 0.04% bovine serum albumin (Sigma Chemical), filtered through gauze, and centrifuged at 200g. The isolated hepatocytes were cultured in Dulbecco modified Eagle medium (DMEM; Invitrogen) containing 2 mM l-glutamine (Invitrogen), 1 mM sodium pyruvate (Invitrogen), 2.5 μg/mL amphotericin B (ThermoTrace, Sydney, Australia), 100 U/mL and 100 μg/mL of penicillin/streptomycin sulfate (Invitrogen), 10 nM dexamethasone (Sigma Chemical), and 10 nM insulin (Sigma Chemical) supplemented with 10% (vol/vol) fetal bovine serum (Invitrogen). The hepatocytes were maintained in a humidified atmosphere of 5% CO2 for 24 hours before use. This procedure was approved by the Animal Ethics Committee of The University of Western Australia.
NTBI uptake studies
Ferric (Fe(III)) citrate solution was labeled with 59FeCl3 (Perkin Elmer, Melbourne, Australia) as previously described.6 Hepatocytes from both Hfe KO and control mice were washed 3 times with Hanks buffer (HBSS) at 37° C and the medium was replaced with incubation medium (DMEM + 2% bovine serum albumin [pH 7.4] containing 59Fe-citrate [0-25 μM Fe; ratio of 1 Fe to 100 citrate]). The cells were incubated for 0 to 120 minutes at 37° C and then washed 5 times with HBSS at 4° C. The hepatocytes were incubated with 1 mg/mL Pronase (Boehringer Mannheim, Mannheim, Germany) in HBSS for 30 minutes at 4° C to release membrane Fe.8 The cell suspension was then centrifuged at 14 000g for 1 minute to separate into the supernatant and cell pellet fractions that will be referred to as membrane Fe and internalized Fe, respectively. The radioactivity in both fractions was measured in a LKB Wallac 1282 CompuGamma gamma-counter (Wallac, Turku, Finland). An aliquot of the cell suspension was then taken for protein estimation using the bicinchoninic acid (BCA) protein method (Pierce, Rockford, IL). All data were calculated as nmol Fe/mg protein to correct for variation in cell density. The amount of protein/hepatocyte for Hfe KO mice (921 ± 29 pg protein/cell; mean ± SEM; n = 5) and control (864 ± 29 pg protein/cell; mean ± SEM; n = 11) was similar (P = .25).
In other experiments, the effects of a 100-fold excess of various divalent metals (iron [Fe], manganese [Mn], cobalt [Co], nickel [Ni], copper [Cu], and zinc [Zn]) on 59Fe(III)–citrate (ratio of 1 μM Fe to 100 μM citrate) uptake by the Hfe KO and control hepatocytes were investigated. The cells were incubated in the presence or absence of the metals for 1 hour at 37° C. Also, the effects of specific ferrous ion chelators (0-1000 μM), bathophenanthroline disulfonate (BPS; Sigma Chemical) and 2′,2-bipyridine (BP; BDH AnalaR, Kilsyth, Australia), and diferric Tf (ratio of 0-10 μM Tf to 0-20 μM Fe) on 59Fe-citrate (ratio of 1 μMFeto100 μM citrate) by hepatocytes from both types of mice were investigated. In these experiments, the hepatocytes were incubated with up to 20-fold molar excess of diferric Tf for 2 hours or with the chelators for 1 hour at 37° C. The effect of extracellular pH (range 5.5-8, buffered with 20 mM HEPES-Tris) on 59Fe-citrate (ratio of 10 μM Fe to 1000 μM citrate) uptake by hepatocytes from Hfe KO and control mice was also examined.
RNA expression
Total RNA was isolated from cultured hepatocytes using RNA-Bee (Tel-Test, Friendswood, TX) and treated with DNAse I (Ambion, Austin, TX). Reverse transcription was performed on 1 μg of total RNA, 0.5 μg oligoDT15 (Promega, Sydney, Australia), and 1 mM each of deoxynucleotide triphosphate (Promega) and 12 units avian myeloblastosis virus (AMV) reverse transcriptase (Promega) according to the manufacturer's instructions. Mouse DMT1 and β-actin mRNA (endogenous control) transcripts were quantified by real-time polymerase chain reaction (PCR) using a Rotorgene (Corbett Research, Mortlake, Australia). The PCR reaction mixture contained cDNA converted from 50 ng RNA, 0.25 μM each of the forward and reverse primers (Geneworks, Thebarton, Australia), 0.2 units Platinum Taq (Invitrogen), 0.5 × SYBR Green (Molecular Probes, Sydney, Australia), and 2 mM MgCl2. The cycling parameters were as follows: an initial denaturation step at 95° C for 300 seconds followed by 40 cycles of denaturation at 95° C for 15 seconds, annealing at 55° C (DMT1 and β-actin) for 20 seconds and extension at 72° C for 20 seconds, and a final extension step at 72° C for 30 seconds. Plasmids containing DMT1 and β-actin cDNA were made by PCR amplification using their primer sets (DMT1 [GenBank accession no. NM_008732] forward primer 5′-TCTATCGCCATCATCCCCACCC-3′ and reverse primer 5′-TCCACAGTCCAGGAAAGACAGACCC-3′; and β-actin [GenBank accession no. NM_007393] forward primer 5′-CTGGCACCACACCTTCTA-3′ and reverse primer 5′-GTCACCCACACTGTGCCC-3′) and mouse duodenum cDNA as a template. The PCR products were then inserted into pGEM-T Easy vector (Promega) and cloned. Serial dilutions of known copy numbers of the plasmids were used to generate standard curves to quantify gene expression by control and Hfe KO mouse hepatocytes. DMT1 mRNA expression was then normalized against β-actin mRNA expression.
DMT1 Western blotting
Hepatocytes from both types of mice were lysed in 150 mM NaCl, 25 mM Tris-HCl (pH 7.5), and 0.5% Triton X-100 (BDH AnalaR) containing the protein inhibitors phenylmethylsulfonyl fluoride (PMSF; 0.5 mM; Sigma Chemical), leupeptin (10 μg/mL; Sigma Chemical), and aprotinin (2 μg/mL; Boehringer Mannheim), the reducing agent dithiothreitol (DTT; 1 mM; Sigma Chemical), and EDTA (ethylenediaminetetraacetic acid; 5 mM; BDH AnalaR). Sodium dodecyl sulfate–polyacrylamide gel electrophoresis was performed according to the method of Laemmli.22 Protein samples were heated at 90° C for 10 minutes in Laemmli buffer and separated by 7% acrylamide gel electrophoresis. The proteins were then electroblotted onto a polyvinylidene fluoride membrane (Millipore). For detection of DMT1 protein, the blot was incubated with rabbit anti-DMT1, which recognizes both the iron responsive element (IRE) and non-IRE isoforms.23 After incubation with the DMT1 antibody (1:1500 dilution), the blot was incubated with goat antirabbit immunoglobulin G–horseradish peroxidase (IgG-HRP) antibody (1:2000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). DMT1(total) protein expression was then detected using enhanced chemiluminescence (ECL; Amersham Biosciences, Castle Hill, Australia) chemiluminescence kit. Western blot analysis was quantified using QuantityOne software (Bio-Rad, Regents Park, Australia).
Statistical analysis
Results are expressed as mean ± SD of triplicate measurements within each experiment, unless otherwise stated. Each experiment was performed at least 2 to 7 times. Student unpaired t test was used to determine differences between groups and statistical significance was obtained for P values less than .05.
Results
Iron parameters
In the Hfe KO mice, plasma NTBI concentration was approximately 2.5-fold higher compared with control C57BL/6J mice (Table 1). Serum Fe levels and Tf saturation were also significantly higher in the Hfe KO mice. Hepatocytes from Hfe KO mice contained 5.5-fold more ferritin protein than hepatocytes from control mice (Table 1).
Iron parameters in Hfe KO and control mice
. | Mouse types . | . | |
|---|---|---|---|
. | Control . | Hfe KO . | |
| Plasma NTBI, μM; n = 5 | 1.5 ± 0.1 | 3.7 ± 0.1 | |
| Serum iron, μM; n = 3 | 23 ± 1 | 31 ± 2 | |
| Serum transferrin saturation, %; n = 3 | 40 ± 1 | 77 ± 5 | |
| Hepatocyte ferritin, ng/μg protein; n = 30 | 1.0 ± 0.1 | 5.5 ± 0.4 | |
. | Mouse types . | . | |
|---|---|---|---|
. | Control . | Hfe KO . | |
| Plasma NTBI, μM; n = 5 | 1.5 ± 0.1 | 3.7 ± 0.1 | |
| Serum iron, μM; n = 3 | 23 ± 1 | 31 ± 2 | |
| Serum transferrin saturation, %; n = 3 | 40 ± 1 | 77 ± 5 | |
| Hepatocyte ferritin, ng/μg protein; n = 30 | 1.0 ± 0.1 | 5.5 ± 0.4 | |
All values are mean ± SEM. P < .05 Hfe KO versus control mice.
Kinetics of NTBI uptake from 59Fe-citrate
Hepatocytes isolated from Hfe KO and control mice were cultured for 24 hours prior to incubation with 59Fe-citrate (ratio of 10 μMFe to 1000 μM citrate) for 0 to 2 hours at 37° C. Total (internalized and membrane) Fe uptake by hepatocytes from both types of mice increased over time (Figure 1). The rate of Fe internalization was greater than the rate of Fe accumulation on the membrane of hepatocytes from both types of mice. The rate of Fe internalization by the cells increased by over 2-fold by hepatocytes from the Hfe KO compared with control mice. (28.0 ± 2.4 pmol Fe/mg protein/min vs 13.0 ± 2.2 pmol Fe/mg protein/min; mean ± SEM, n = 7; P < .001). However, no statistical difference was observed in the rates of membrane uptake by hepatocytes from both Hfe KO and control mice (7.13 ± 1.11 vs 4.79 ± 0.69 pmol Fe/mg protein/min; mean ± SEM; n = 7; P = .10).
Kinetics of NTBI uptake from ferric citrate by hepatocytes. Hepatocytes from Hfe KO (filled symbols) and control (open symbols) mice were incubated for 0 to 90 minutes with 59Fe-citrate (ratio of 10 μM Fe to 1000 μM citrate) at 37°C. (A) Internalized (•, ○), (B) membrane (▪, □), and (C) total (▴, ▵) uptake are shown. Results are expressed as mean ± SD (n = 3). This graph is a typical representation from 7 independent experiments performed.
Kinetics of NTBI uptake from ferric citrate by hepatocytes. Hepatocytes from Hfe KO (filled symbols) and control (open symbols) mice were incubated for 0 to 90 minutes with 59Fe-citrate (ratio of 10 μM Fe to 1000 μM citrate) at 37°C. (A) Internalized (•, ○), (B) membrane (▪, □), and (C) total (▴, ▵) uptake are shown. Results are expressed as mean ± SD (n = 3). This graph is a typical representation from 7 independent experiments performed.
Effects of iron concentration on NTBI uptake
As the extracellular concentration of 59Fe-citrate was increased, the uptake of Fe by hepatocytes from both types of mice reached saturation (Figure 2). The maximal velocity of uptake (Vmax) and the affinity constant (Km) for iron uptake were estimated using the software program GraphPad Prism (GraphPad Software, San Diego, CA) based on the Michaelis-Menten equation. The Vmax was greater in the hepatocytes from the Hfe KO mice (4823 pmol Fe/mg protein/2 h) compared with the control mice (2842 pmol Fe/mg protein/2 h; Figure 2), whereas Km values were 6.4 μM and 6.8 μM for the Hfe KO and control mice, respectively. When this experiment was repeated (n = 4), there was a significant increase in Vmax values by 70% by the hepatocytes from Hfe KO mice compared with the control mice (P < .0001) but there was no statistically significant difference in their Km values (P = .13).
The effects of increasing ferric citrate concentration on Fe uptake. Hepatocytes from Hfe KO (filled symbols) and control (open symbols) mice were incubated with 0 to 20 μM 59Fe-citrate (ratio of 1 Fe to 100 citrate) for 2 hours at 37° C. Internalized (•, ○), membrane (▪, □), and total (▴, ▵) uptakes are shown. Results are expressed as mean ± SD (n = 3). This graph is a typical representation from 4 independent experiments performed.
The effects of increasing ferric citrate concentration on Fe uptake. Hepatocytes from Hfe KO (filled symbols) and control (open symbols) mice were incubated with 0 to 20 μM 59Fe-citrate (ratio of 1 Fe to 100 citrate) for 2 hours at 37° C. Internalized (•, ○), membrane (▪, □), and total (▴, ▵) uptakes are shown. Results are expressed as mean ± SD (n = 3). This graph is a typical representation from 4 independent experiments performed.
Effects of iron chelators on NTBI uptake
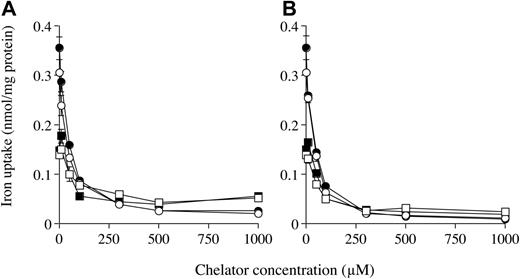
The hepatocytes from Hfe KO and control mice were incubated with 59Fe-citrate in the presence of the specific ferrous (Fe2+) ion chelators BPS and BP up to 1000-fold excess. These chelators inhibited intracellular and membrane Fe uptake by hepatocytes from both Hfe KO and control mice in a concentration-dependent manner with Fe uptake decreasing as the concentration of the chelators was increased (Figure 3). At 1 mM, both BPS and BP almost abolished intracellular Fe uptake, reducing uptake to approximately 5% of the control values in the absence of chelators. At the same concentration, membrane Fe uptake was reduced by BPS and BP to 40% and 15% of the control values, respectively. The effects of the chelators BP and BPS on intracellular and membrane uptake by the hepatocytes from both mouse types were similar.
The effects of specific ferrous ion chelators on NTBI uptake. Hepatocytes from Hfe KO (filled symbols) and control (open symbols) mice were incubated with 59Fe-citrate (ratio of 1 μM Fe to 100 μM citrate) in the presence of 0 to 1000 μM BPS (A) and BP (B) for 1 hour at 37° C. Internalized (•, ○) and membrane (▪, □) uptakes are shown. Results are expressed as mean ± SD (n = 3). This graph is a typical representation from 2 independent experiments performed.
The effects of specific ferrous ion chelators on NTBI uptake. Hepatocytes from Hfe KO (filled symbols) and control (open symbols) mice were incubated with 59Fe-citrate (ratio of 1 μM Fe to 100 μM citrate) in the presence of 0 to 1000 μM BPS (A) and BP (B) for 1 hour at 37° C. Internalized (•, ○) and membrane (▪, □) uptakes are shown. Results are expressed as mean ± SD (n = 3). This graph is a typical representation from 2 independent experiments performed.
Effects of divalent metals on NTBI uptake
59Fe-citrate uptake was inhibited by most of the divalent metals presented to the hepatocyte in culture in a 100-fold excess. Divalent metals manganese (Mn), iron (Fe), zinc (Zn), and cobalt (Co) significantly diminished intracellular Fe and membrane Fe uptake from citrate by 40% to 80% of control values in the absence of divalent metals, whereas divalent metals such as copper (Cu) only decreased uptake by about 20% and nickel (Ni) had no effect. Similar effects of the divalent metals on Fe uptake were observed for hepatocytes from both types of mice (Figure 4).
The effects of divalent metals on internalization and membrane ferric citrate uptake. (A) Internalized. (B) Membrane. Hepatocytes from Hfe KO mice (▪) and control (□) were incubated with 59Fe-citrate (ratio of 1 μM Fe to 100 μM citrate) in the absence and presence of 100 μM divalent metals for 1 hour at 37° C. Results are expressed as mean ± SD (n = 3). *P < .001; #P < .05. This graph is a typical representation from 3 independent experiments performed.
The effects of divalent metals on internalization and membrane ferric citrate uptake. (A) Internalized. (B) Membrane. Hepatocytes from Hfe KO mice (▪) and control (□) were incubated with 59Fe-citrate (ratio of 1 μM Fe to 100 μM citrate) in the absence and presence of 100 μM divalent metals for 1 hour at 37° C. Results are expressed as mean ± SD (n = 3). *P < .001; #P < .05. This graph is a typical representation from 3 independent experiments performed.
Effects of pH on NTBI uptake
59Fe-citrate uptake by hepatocytes from both Hfe KO and control mice was pH dependent (data not shown). Iron uptake from citrate increased as the pH of the incubation solution was raised from pH 5.5 to about 7 to 7.5 and then declined slightly as the pH was further increased to 8.
Effects of diferric Tf on NTBI uptake
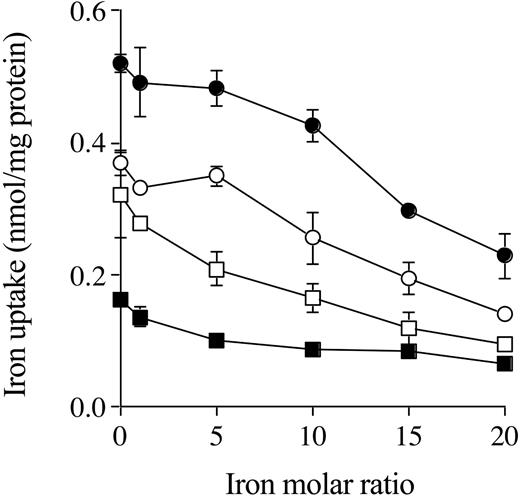
When the hepatocytes from Hfe KO and control mice were incubated with 59Fe-citrate in the presence of increasing concentrations of diferric Tf, intracellular and membrane Fe uptake decreased as diferric Tf concentration increased. In the presence of 20-fold molar ratio of diferric Tf, internalized Fe uptake from citrate was reduced by about 50% to 60%, as was membrane Fe uptake by hepatocytes from both Hfe KO and control mice (Figure 5).
The effects of increasing concentrations of diferric transferrin on NTBI uptake. Hepatocytes from Hfe KO (filled symbols) and control (open symbols) mice were incubated with 59Fe-citrate (ratio of 1 μM Fe to 100 μM citrate) in the presence of Fe 0 to 20 molar ratio for diferric transferrin to Fe-citrate for 2 hours at 37° C. Internalized (•, ○) and membrane (▪, □) uptake are shown. Results are expressed as mean ± SD (n = 3). This graph is a typical representation from 2 independent experiments performed.
The effects of increasing concentrations of diferric transferrin on NTBI uptake. Hepatocytes from Hfe KO (filled symbols) and control (open symbols) mice were incubated with 59Fe-citrate (ratio of 1 μM Fe to 100 μM citrate) in the presence of Fe 0 to 20 molar ratio for diferric transferrin to Fe-citrate for 2 hours at 37° C. Internalized (•, ○) and membrane (▪, □) uptake are shown. Results are expressed as mean ± SD (n = 3). This graph is a typical representation from 2 independent experiments performed.
Expression of DMT1 by hepatocytes
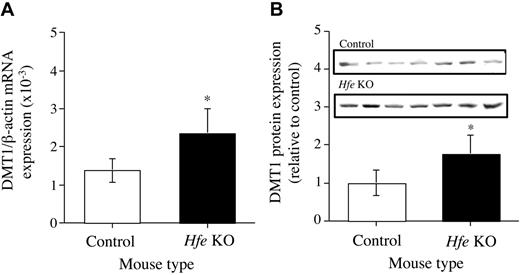
The expression of DMT1 mRNA by hepatocytes from both Hfe KO and C57BL/6J mice was determined by real-time PCR. Hepatocytes from the Hfe KO mice were shown to express approximately 2-fold more DMT1 than hepatocytes from the control mice (Figure 6A). DMT1(IRE) mRNA expression was up-regulated by 50% by hepatocytes from the Hfe KO mice compared with control mice. DMT1(non-IRE) mRNA was not detected in the hepatocytes from either type of mice (data not shown). Expression of DMT1 protein by the hepatocytes was detected by Western blot analysis. There was an up-regulation of DMT1 protein expression by about 2-fold in the hepatocytes from the Hfe KO mice compared with hepatocytes from control mice (Figure 6B).
Expression of DMT1 mRNA and protein. (A) mRNA. (B) Protein. Messenger RNA and protein expression of DMT1 in the hepatocytes from Hfe KO (▪) and control (□) mice were quantified by real-time PCR and Western blotting, respectively, and performed as described in the “Materials and methods.” Results are expressed as mean ± SD (n = 6-8) for real-time PCR and mean ± SD (n = 7) for Western blotting. *P < .01.
Expression of DMT1 mRNA and protein. (A) mRNA. (B) Protein. Messenger RNA and protein expression of DMT1 in the hepatocytes from Hfe KO (▪) and control (□) mice were quantified by real-time PCR and Western blotting, respectively, and performed as described in the “Materials and methods.” Results are expressed as mean ± SD (n = 6-8) for real-time PCR and mean ± SD (n = 7) for Western blotting. *P < .01.
Discussion
In this study, the mechanisms of Fe loading of hepatocytes from the Hfe KO mice were investigated. We demonstrated for the first time that plasma NTBI levels were significantly increased in the Hfe KO mice and that NTBI uptake by hepatocytes from the Hfe KO mice was significantly increased compared with control mice. NTBI as ferric citrate was reduced from +3 to +2 redox state at the cell surface of the hepatocytes before being internalized. The inhibition of ferric citrate uptake by divalent metals such as Mn, Zn, and Co is consistent with a common transporter mediating the uptake of these metals. Iron uptake from citrate was inhibited by Tf-Fe, suggesting that both forms of Fe share a common uptake pathway. DMT1 mRNA and protein expression were up-regulated in hepatocytes from the Hfe KO mice. These results suggest that NTBI uptake contributed to hepatic Fe loading in the Hfe KO mice and that DMT1 may mediate NTBI uptake by hepatocytes.
In patients with HH, serum Tf saturation is increased and NTBI concentration is elevated, with levels in the range of 3 to 5 μM,12,18,19 although higher levels of up to 22 μM have been reported.24,25 Indeed, we found an increase in plasma NTBI concentration in the Hfe KO mice compared with control mice. The elevated serum Fe levels and Tf saturation in the Hfe KO mice, as well as the higher concentration of ferritin protein in hepatocytes from the Hfe KO mice (Table 1), are consistent with the degree of Fe overload observed in HH patients.26 The increase in ferritin levels in hepatocytes from Hfe KO mice relative to hepatocytes from control mice was similar to that reported previously for nonheme iron levels in whole liver from these animals.14,17 This indicates there was no loss of Fe from the hepatocytes in vitro.
Hepatocytes from the Hfe KO mice took up Fe from citrate at twice the rate of control hepatocytes (Figure 1). Iron uptake from citrate by both Hfe KO and control hepatocytes was saturable, indicating that the uptake is mediated by an Fe transporter (Figure 2).7,8 The higher maximal rate of uptake by the Hfe KO hepatocytes compared with the control cells implies that there was an upregulation of the number of Fe transporters on the cell surface of the hepatocytes. Although the rate of NTBI uptake by the hepatocytes from Hfe KO mice increased by 2-fold in vitro, the increased concentration of NTBI in their circulation compared with control mice suggests that hepatocytes from Hfe KO mice have the capacity to take up more Fe from NTBI in vivo. It has been reported that approximately 60% to 75% of NTBI in the serum is cleared by rat liver in the first pass, possibly more in vivo.10,27 If so, the plasma NTBI levels reported in HH patients and in the Hfe KO mice may be an underestimation of the amount of NTBI that is present in the portal blood and cleared by the liver in Fe-overload diseases such as HH. These results indicate that NTBI is important in the pathogenesis of hepatic Fe loading in HH.
Specific ferrous ion chelators, the membrane-impermeable BPS and membrane-permeable BP, inhibited both internalized and membrane Fe uptake by hepatocytes from Hfe KO and control mice (Figure 3), indicating that ferric iron was reduced to the ferrous form at the cell surface before being taken up by the hepatocytes. Similarly, in other studies using hepatoma cells, it was shown that NTBI uptake was inhibited by the ferrous chelators.8,28 Also the membrane-impermeable ferricyanide competitively inhibited NTBI uptake by both hepatoma and erythroleukemic cells, indicating uptake was dependent on the reduction of Fe3+ to Fe2+ at the cell surface.8,29 These observations provide strong evidence for the presence of a ferrireductase at the cell surface that is coupled to NTBI transport in both hepatocytes and hepatoma cells. NTBI uptake by Hfe KO and control hepatocytes was also pH dependent, confirming the previous findings of others using wild-type rat hepatocytes.6,7
We found that divalent metals such as Fe, Mn, Zn, and, to a lesser extent, Co and Cu inhibited ferric citrate uptake by hepatocytes from both Hfe KO and control mice. This suggests that uptake of these metals by hepatocytes from both types of mice was mediated by the same carrier. The varying degree of inhibition of ferric citrate uptake by the different divalent metals is likely to be due to the affinity that the carrier has for the different metals. Similar results have been reported by other investigators that show NTBI uptake was inhibited by the divalent metals Zn, Co, and Ni in isolated rat hepatocytes6 ; Zn, Co, and Cd in hepatoma cells28 ; and Zn, Mn, and Co in perfused rat liver.27
In the present study, internalized and membrane Fe uptake from citrate by hepatocytes from both Hfe KO and control mice were inhibited in the presence of increasing concentrations of Tf-Fe. This implies that the uptake of Fe from both citrate and Tf shares a common uptake mechanism. Similar results have been reported in rat hepatocytes.7,30 We also showed the converse where Tf-Fe uptake by hepatoma cells was reduced by the presence of excess ferric citrate.31 In the latter study, we demonstrated that the inhibition of ferric citrate uptake by Tf-Fe was not due to the transfer or exchange of Fe between citrate and Tf. When radiolabeled diferric Tf was incubated with unlabeled ferric citrate for up to 24 hours at 37° C and Tf-bound and citrate-bound Fe were separated using a 30-kDa molecular weight cutoff membrane, the rate of exchange of 59Fe between Tf and citrate was less than 1.5%/hour, which was insufficient to account for the observed reduction in Fe-citrate uptake.31
It is well established that hepatocytes and hepatoma cells accumulate Tf-Fe by several pathways including high-affinity Tf receptor 1–mediated endocytic process and low-affinity Tf receptor 1–independent processes, which may involve Tf receptor 2.32 The mechanisms responsible for the low-affinity processes are unclear but evidence suggests there may be 2 pathways, one involving the endocytosis of Tf-Fe and the other, the release of Fe from transferrin at the cell surface.33-35 Using hepatoma cells, we have shown previously that NTBI had no effect on the uptake of Tf-Fe by a high-affinity transferrin receptor-1–mediated process. However, NTBI competitively inhibited the uptake of Tf-Fe released at the surface by the low-affinity process, suggesting a common Fe carrier mediates the uptake of both NTBI and Tf-Fe at the cell surface. A similar mechanism is likely to be present in hepatocytes.31
In 1997, Gunshin et al36 cloned a duodenal NTBI transporter called divalent metal transporter 1 (DMT1). Electrophysiologic studies performed on Xenopus oocytes expressing DMT1 showed that it transported not only Fe but also other divalent metals with affinity decreasing in the order of Zn, Mn, Co, Cd, Cu, Ni, and Pb. DMT1 is widely expressed in many other tissues including the liver.23,36 DMT1 has also been identified as the endosomal Fe carrier. Tf receptor 1–mediated uptake of Tf-Fe involves the internalization of Tf-Fe into an endosome where the Fe is released from Tf and transported by DMT1 across the endosomal membrane to the cell cytosol.37 Immunofluorescence and immunohistochemical studies have localized DMT1 to both recycling endosomes and plasma membranes,23,38-40 suggesting DMT1 cycles between the endosomal membrane and the plasma membrane where it may possibly mediate Fe uptake across both the endosome and cell membrane.
There is evidence to suggest that DMT1 may be a putative NTBI transporter in hepatocytes. In this study we found both DMT1 mRNA and protein expression were increased in the hepatocytes from the Hfe KO mice compared with cells from control animals, and this increase parallels the increase in NTBI uptake observed in the Hfe KO mice. Also the inhibitory effects of the ferrous chelators and other divalent metals on NTBI uptake suggest DMT1 may be involved. As Tf-Fe impairs the uptake of NTBI across the hepatocyte cell surface, it suggests that one Fe transporter, possibly DMT1 cycling between the endosome and the cell membrane, may be responsible for the uptake of Fe from both sources. Hence, further study to elucidate the site of NTBI and Tf-Fe interaction is warranted. Nevertheless, others failed to see an increase in liver DMT1 mRNA expression from Hfe KO mice.15,41 However, in both of these latter studies, only DMT1 mRNA and not protein expression was measured in whole liver rather than in isolated hepatocytes.
Iron loading of wild-type rat hepatocytes has been shown in at least some previous studies to lead to increases in hepatocellular DMT1 expression23,42 and/or uptake of NTBI.42,43 Thus, the increases in NTBI uptake and DMT1 expression observed in the Hfe KO mice in this study might reflect Fe loading rather than the loss of HFE per se. The studies presented in this current body of work cannot determine whether the increases in DMT1 expression and NTBI uptake by the Hfe KO mouse hepatocytes are the primary cause of Fe loading or secondary to Fe loading by another mechanism.
Hepcidin is synthesized by hepatocytes and has been postulated to be a key player in the regulation of iron metabolism. Mice that lack hepcidin develop Fe overload,44 whereas those with high expression develop severe Fe deficiency anemia,45 indicating an inverse relationship between dietary Fe absorption and hepatic hepcidin expression. Recent studies showed that hepcidin expression is inappropriately low in Hfe KO mice and HH patients despite Fe loading46,47 and this may be a key to the defective regulation of Fe metabolism in HH. Whether hepcidin is important in the up-regulation of NTBI transport or DMT1 expression by the hepatocytes from Hfe KO mice is yet to be determined.
In conclusion, this study provides evidence supporting our hypothesis that NTBI uptake by the liver is up-regulated in the Hfe KO mice and contributes to increased Fe deposition in the liver. These results demonstrate that Hfe KO mouse hepatocytes have increased NTBI uptake even when hepatic Fe stores are high. These findings provide new and important information on the mechanisms of hepatic Fe loading in HH.
Prepublished online as Blood First Edition Paper, May 20, 2004; DOI 10.1182/blood-2003-11-3872.
Supported by a grant from the National Health and Medical Research Council of Australia (D.T., J.K.O., and P.J.L.). A.C.G.C. is a recipient of the Dora Lush Biomedical Scholarship from the National Health and Medical Research Council of Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank S. Drake, C. Herbison, C. Smith, and T. De-Jong for skillful technical assistance and the Department of Biochemistry at Fremantle Hospital for performing the ferritin measurements.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal