Abstract
Primary systemic amyloidosis (AL) is a fatal plasma cell disorder. Pilot data suggest survival is better in patients undergoing peripheral blood stem cell transplantation (PBSCT), but the selection process makes the apparent benefit suspect. We have reported that circulating cardiac biomarkers are the best predictors of survival outside of the transplantation setting. We now test whether cardiac troponins (cTnT and cTnI) and N-terminal pro-brain natriuretic peptide (NT-proBNP) are prognostic in transplant recipients. In 98 patients with AL undergoing PBSCT, serum cardiac biomarkers were measured (cTnT, 98 patients; cTnI, 65 patients; and NT-proBNP, 63 patients). Elevated levels of cTnT, cTnI, and NT-proBNP were present in 14%, 43%, and 48% of patients, respectively. At 20 months median follow-up, median survival has not been reached for patients with values below the thresholds; in patients with values above the thresholds, median survival is 26.1 months, 66.1 months, and 66.1 months, respectively. Our previously reported risk systems incorporating these markers were also prognostic, notably the cTnT/NT-proBNP staging. Using this system, 49%, 38%, and 13% of patients were in stage I, stage II, and stage III, respectively. Determining levels of circulating biomarkers may be the most powerful tool for staging patients with AL undergoing PBSCT.
Introduction
Primary systemic amyloidosis (AL) has been a uniformly fatal disease. With standard chemotherapy, median survival is 12 to 18 months.1-4 Over the past decade, we and others have introduced peripheral blood stem cell transplantation (PBSCT) as a therapeutic option for selected patients.5 Phase 2 and retrospective analysis suggest that overall survival is better in patients who receive this therapy.6-8 However, patients receiving this procedure are highly selected, bringing into question the interpretation of the apparent improved survival.9 A major drawback has been the lack of a prognostic staging system, making comparisons between different studies and treatment centers difficult to interpret.
We have recently reported that in patients receiving standard chemotherapy, circulating cardiac biomarkers are the most powerful independent predictors of survival.10 Cardiac troponins (cTnT, cTnI) are highly specific markers of myocardial injury.11 Elevations are common in patients with acute coronary syndromes and in those with acute congestive heart failure.12,13 Pro-brain natriuretic peptide is a 108–amino acid propeptide, made by myocytes in response to increased wall stress. It is produced predominantly in the left ventricle and when released, it is cleaved into 2 fragments, the active brain natriuretic peptide (amino acids 77-108) and a leader sequence known as N-terminal pro-brain natriuretic peptide (NT-proBNP; amino acids 1-76). NT-proBNP has been shown to be a sensitive indicator of cardiac abnormalities.14,15 Blood levels of NT-proBNP provide prognostic information in patients with AL treated with conventional chemotherapy.10,16 Moreover, cardiac troponins and NT-proBNP are independent predictors of survival in this population of patients, and we have constructed a powerful staging system for patients treated with conventional chemotherapy by combining these 2 blood tests.17 We have now used this staging system to determine its prognostic value in patients undergoing PBSCT.
Patients and methods
Patients
Serum cardiac troponin levels were measured in 99 patients with primary systemic amyloidosis who underwent transplantation between March 8, 1996, and June 30, 2003. All samples were obtained within 90 days prior to peripheral blood stem cell infusion. Serum cardiac troponin levels (troponin T [cTnT] and troponin I [cTnI]) were run from stored serum (–20°C) for 65 patients, and an additional 33 patients had cTnT levels measured prospectively as part of their routine evaluation. NT-proBNP levels were measured from stored serum in 63 of the same patients based on serum availability.
Other clinical and laboratory data were collected prospectively into the Amyloid Transplant Database. All patients consented to have their medical records reviewed according to institutional review board practices and Health Insurance Portability and Accountability Act (HIPAA) guidelines. The diagnosis of amyloidosis was predicated on biopsy proof of amyloid (congophilia with green birefringence), the presence of a plasma cell clone, and either kappa or lambda staining of the amyloid with immunohistochemical stains.
Stem cells were mobilized using either cyclophosphamide and granulocyte macrophage–colony-stimulating factor (GM-CSF) or granulocyte-CSF (G-CSF) alone. Prior to receiving their stem cells, patients received melphalan doses ranging from 100 mg/m2 to 200 mg/m2. Patients were given prophylactic antibiotics and supportive care as previously reported.6
Laboratory methods
Assays for cTnT and cTnI were performed with sensitive second- and third-generation assays with reagents provided by Roche Diagnostics (Indianapolis, IN) and DADE (Newark, DE).10,18 The troponin T assay has a limit of detection of less than 0.01 μg/L and coefficients of variability of 10% at 0.035 μg/L and 20% at 0.015 μg/L (Roche Diagnostics).18 The value of 0.035 μg/L is the lowest value that meets the European Society of Cardiology/American College of Cardiology (ESC/ACC) criteria for precision.19 The troponin I assay (DADE) has a limit of detection of 0.03 μg/L and a coefficient of variability of 10% at the upper limit of the normal range of 0.06 μg/L.19 NT-proBNP levels were measured with electrochemiluminescence sandwich immunoassay (ECLIA; Roche) on an Elecsys System 2010. The detection limit is 5 ng/L. Serum NT-proBNP levels are higher in women and increase with age. Upper reference limits (97.5 percentiles of healthy subjects) in men and women are, respectively, 87 ng/L and 150 ng/L in subjects younger than 50 years old; and 220 ng/L and 331.5 ng/L in individuals older than 50 years old (data from Roche from 712 healthy subjects). Precision with this assay is excellent but substantial biologic variability exists, especially at higher values.14 A small number of patients had only plasma samples, which can differ from serum samples by as much as 10%. Analyses done with correction for this effect and without taking it into account were identical.
Model systems
Two indices incorporating cardiac troponins as the only serum cardiac biomarker were used. We previously constructed a multivariate model in a cohort of patients who did not undergo transplantation, incorporating cTnT and resulting in an “cTnT Idealized Risk Score”:10 R = 3.19* √cTnT + 0.52* √urine M spike + 0.025* age + 0.34* ejection fraction, where cTnT and urine M spike are the actual values expressed in μg/L and g/24 hours, respectively; age is number of years when younger than 65, but 65 for those 65 years and older; ejection fraction is one when less than 55% and 0 when 55% or higher. The overall risk score yields values ranging between 0.77 and 5.2. We independently designed a similar “idealized” model for cTnI:10 R = 0.40* cTnI + 0.50* ejection fraction + 0.025* age + 0.34* septal thickness + 0.37* √urine M spike. The assignment of values for the variables are the same as for the cTnT model with the following exceptions: cTnI and septal thickness variables are each assigned a value of one if more than or equal to 0.1 μg/L or more than 15 mm, respectively, and 0 for less than 0.1 μg/L or less than or equal to 15 mm. The maximum value of this risk score is 3.18.10 The model including cTnT was more robust than the one with cTnI, but both are reported because of preferential assay usage at different medical facilities.
Because the “idealized” indices are complex and unwieldy for routine clinical practice, we developed 2 simplified staging systems for patients with AL who did not undergo transplantation, incorporating NT-proBNP and troponin levels.17 Rather than using transformed or normalized variables, threshold values were chosen (cTnT < 0.035 μg/L; cTnI < 0.1 μg/L and NT-proBNP < 332 ng/L). Patients are considered stage I (low risk) when both troponin and NT-proBNP are below the threshold, stage III (high risk) if both are equal to or above the threshold, and stage II (intermediate risk) if only one marker is below the threshold. The suffixes (-t or -i) refer to which troponin (cTnT or cTnI) assay was used in the staging system.
Statistical analysis
Demographic and baseline clinical and laboratory data between groups were compared using the rank-sum20 and Kruskal-Wallis tests. Fisher exact test was used to test differences in categoric variables.21 Survival was calculated from the time of transplantation and survival curves were constructed according to the Kaplan-Meier method,22 and the groups were compared using log-rank tests.23 We identified predictors of survival in univariate and multivariate Cox proportional hazards models and calculated the relative hazards and 95% confidence intervals using univariate and multivariate Cox proportional hazards regression models.24 Multivariate analyses were performed with the use of a stepwise forward regression model with an entry probability for each variable set at 0.05. All analyses were done using Statview Software (SAS, Cary, NC).
Results
Patient characteristics of 98 patients who underwent PBSCT are in Table 1. Median time from histologic diagnosis to transplantation was 4.2 months and median follow-up was 20 months. Twenty-one patients have died. Elevated (≥ 0.035 μg/L) cTnT values were found in 14% (14/98) of patients. A cTnI level greater than or equal to 0.1 μg/L was found in 44% of patients tested. The NT-proBNP level was greater than or equal to 332 ng/L in 48% of patients. The median cTnT and cTnI Idealized Risk Scores were each 1.8. Median overall survival for the entire transplantation group has not been reached, and 75% of patients are alive at 26 months.
Patient characteristics
Characteristic . | Median (range) . | Cut-off . | Percent . |
|---|---|---|---|
| Male | NA | NA | 54 |
| Age, y | 55 (35-71) | > 60 | 28 |
| Serum albumin, g/L | 29 (10-44) | < 20 | 25 |
| Diagnosis to transplantation, months | 4.2 (1.3-75) | ≥ 12 | 12 |
| Creatinine, μM/L [mg/dL] | 97 (53-247) | > 150 | 10 |
| [1.1 (.6-2.8)] | [>1.7] | ||
| Creatinine clearance, mL/s [mL/min] | 1.22 (0.33-2.10) [73 (20-126)] | 1.00 [< 60] | 31 |
| Alkaline phosphatase, IU/L (UNL 300) | 186 (85-2789) | > 1.5 UNL | 17 |
| β2-microglobulin, nM/L [μg/mL] | 201.4 [2.37] (85-858 [1-10.1]) | ≥ 229.5 [2.7] | 31 |
| Serum M spike, g/L | 10 (0-26) | ≥ 20 | 6 |
| Urine total protein, g/24 hours | 3.7 (0.02-26.2) | ≥ 3 | 57 |
| Urine M spike, g/24 hours | 0.17 (0-2.2) | ≥ 1 | 10 |
| Bone marrow plasma cells, % | 6 (.4-49) | > 30 | 5 |
| Bone marrow plasma cell labeling index, % | 0 (0-2.4) | > 1 | 7 |
| Interventricular septal thickness, mm | 12 (7-25) | > 15 | 15 |
| Left ventricular ejection fraction, % | 65 (28-84) | < 55 | 11 |
| Organ systems involved, n | 2 (1-3) | ≥ 3 | 19 |
| Troponin T, μg/L | 0.01 (0-1) | ≥ 0.03 | 14 |
| Troponin I, μg/L* | 0.07 (0-4.31) | ≥ 0.1 | 44 |
| NT-proBNP, ng/L† | 323 (0-35001) | ≥ 332 | 48 |
| Melphalan conditioning | |||
| Mel 200 mg/m2 | NA | NA | 54 |
| Mel 140 mg/m2/TBI | NA | NA | 12 |
| Mel 140 mg/m2 | NA | NA | 24 |
| Mel 100 mg/m2 | NA | NA | 9 |
Characteristic . | Median (range) . | Cut-off . | Percent . |
|---|---|---|---|
| Male | NA | NA | 54 |
| Age, y | 55 (35-71) | > 60 | 28 |
| Serum albumin, g/L | 29 (10-44) | < 20 | 25 |
| Diagnosis to transplantation, months | 4.2 (1.3-75) | ≥ 12 | 12 |
| Creatinine, μM/L [mg/dL] | 97 (53-247) | > 150 | 10 |
| [1.1 (.6-2.8)] | [>1.7] | ||
| Creatinine clearance, mL/s [mL/min] | 1.22 (0.33-2.10) [73 (20-126)] | 1.00 [< 60] | 31 |
| Alkaline phosphatase, IU/L (UNL 300) | 186 (85-2789) | > 1.5 UNL | 17 |
| β2-microglobulin, nM/L [μg/mL] | 201.4 [2.37] (85-858 [1-10.1]) | ≥ 229.5 [2.7] | 31 |
| Serum M spike, g/L | 10 (0-26) | ≥ 20 | 6 |
| Urine total protein, g/24 hours | 3.7 (0.02-26.2) | ≥ 3 | 57 |
| Urine M spike, g/24 hours | 0.17 (0-2.2) | ≥ 1 | 10 |
| Bone marrow plasma cells, % | 6 (.4-49) | > 30 | 5 |
| Bone marrow plasma cell labeling index, % | 0 (0-2.4) | > 1 | 7 |
| Interventricular septal thickness, mm | 12 (7-25) | > 15 | 15 |
| Left ventricular ejection fraction, % | 65 (28-84) | < 55 | 11 |
| Organ systems involved, n | 2 (1-3) | ≥ 3 | 19 |
| Troponin T, μg/L | 0.01 (0-1) | ≥ 0.03 | 14 |
| Troponin I, μg/L* | 0.07 (0-4.31) | ≥ 0.1 | 44 |
| NT-proBNP, ng/L† | 323 (0-35001) | ≥ 332 | 48 |
| Melphalan conditioning | |||
| Mel 200 mg/m2 | NA | NA | 54 |
| Mel 140 mg/m2/TBI | NA | NA | 12 |
| Mel 140 mg/m2 | NA | NA | 24 |
| Mel 100 mg/m2 | NA | NA | 9 |
n = 98 except where stated.
UNL indicates upper normal limit; NA, not applicable.
n = 65.
n = 63.
There were 10 patients (10%) who died within 3 months of undergoing transplantation, considered potential treatment-related mortality. On univariate logistic regression, only cTnI greater than or equal to 0.1 μg/L, β2-microglobulin greater than or equal to 229.5 nM/L (2.7 μg/mL), creatinine greater than or equal to 150 μM/L (1.7 mg/dL), and the numbers of organs involved were significant predictors of early death (Table 2). Pretransplantation cTnI values were measured in 7 of the 10 patients who died, and all but 1 (86%) had a cTnI greater than or equal to 0.1 μg/L. None of the other cardiac markers had predictive value for treatment-related mortality. Multivariate logistic regression was not performed given the limited number of events.
Probability of death by 3 months, using univariate logistic regression
Characteristic . | Odds ratio . | 95% CI . | P . |
|---|---|---|---|
| cTn1 greater than or equal to 0.1 μg/L | 9.8 | 1.1-87.1 | .04 |
| β2-microglobulin greater than or equal to 229.5 nM/L | 6.2 | 1.5-26.0 | .01 |
| Creatinine greater than or equal to 150 μM (1.7 mg/dL) | 5.0 | 1.0-23.5 | .04 |
| No. of organs involved | 3.6 | 1.4-9.2 | .008 |
| Albumin greater than or equal to 30 g/L | 0.1 | 0.02-1.0 | .06 |
| Alkaline phosphatase greater than or equal to 1.5 normal | 3.8 | 10.9-15.5 | .06 |
| cTnT greater than or equal to 0.035 μg/L | 3.0 | 0.7-13.3 | .1 |
| NT-proBNP greater than or equal to 332 ng/L | 3.1 | 0.5-17.3 | .2 |
| NT-proBNP greater than or equal to 1266 ng/L16 | 2.0 | 0.4-10.2 | .4 |
| LV EF greater than 50% | .3 | 0-1.7 | .2 |
| IVS greater than 15 mm | 1.4 | 0.3-7.6 | .7 |
| Age greater than 60 y | 1.10 | 0.3-4.5 | .9 |
Characteristic . | Odds ratio . | 95% CI . | P . |
|---|---|---|---|
| cTn1 greater than or equal to 0.1 μg/L | 9.8 | 1.1-87.1 | .04 |
| β2-microglobulin greater than or equal to 229.5 nM/L | 6.2 | 1.5-26.0 | .01 |
| Creatinine greater than or equal to 150 μM (1.7 mg/dL) | 5.0 | 1.0-23.5 | .04 |
| No. of organs involved | 3.6 | 1.4-9.2 | .008 |
| Albumin greater than or equal to 30 g/L | 0.1 | 0.02-1.0 | .06 |
| Alkaline phosphatase greater than or equal to 1.5 normal | 3.8 | 10.9-15.5 | .06 |
| cTnT greater than or equal to 0.035 μg/L | 3.0 | 0.7-13.3 | .1 |
| NT-proBNP greater than or equal to 332 ng/L | 3.1 | 0.5-17.3 | .2 |
| NT-proBNP greater than or equal to 1266 ng/L16 | 2.0 | 0.4-10.2 | .4 |
| LV EF greater than 50% | .3 | 0-1.7 | .2 |
| IVS greater than 15 mm | 1.4 | 0.3-7.6 | .7 |
| Age greater than 60 y | 1.10 | 0.3-4.5 | .9 |
LV EF indicates left ventricular ejection fraction; IVS, interventricular septum.
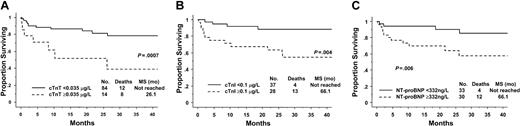
Median overall survival has not yet been reached for the cohort. On univariate analysis all 3 cardiac biomarkers were predictive for survival as single variables and as part of previously reported prognostic models or staging systems (see “Patients and methods” and Table 3). The Kaplan-Meier survival curves are shown in Figure 1. For the 14% of patients (14/98) with elevated cTnT levels, median survival was 26.1 months, whereas it had not been reached at 24 months for patients with normal levels: hazard ratio (HR) 4.4, 95% confidence interval (CI) 1.7-10.9 (Figure 1A). For the 43% of patients (28/65) with elevated cTnI levels, the median survival was 66 months, whereas it had not be reached at 24 months in the patients with normal values: HR 4.9, 95% CI 1.6-15.1 (Figure 1B). The patients with abnormal NT-proBNP also had survival rates inferior to those of their healthy counterparts, median survival 66 months versus not reached, HR 3.8 95% CI 1.2-12.0 (Figure 1C).
Predictors for overall survival
Predictor . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Univariate | ||
| Troponin T greater than or equal to 0.035 μg/L | 4.4 (1.7-10.9) | .002 |
| Troponin I greater than or equal to 0.1 μg/L | 4.9 (1.6-15.1) | .006 |
| NT-proBNP greater than or equal to 332 ng/L | 3.8 (1.2-12) | .02 |
| cTnT Idealized Risk Score* | 2.2 (1.4-3.3) | .0003 |
| cTnI Idealized Risk Score† | 2.8 (.8-9.4) | .09 |
| cTnT/NT-proBNP stage‡ | 3.1 (1.5-6.2) | .001 |
| cTnI/NT-proBNP stage‡ | 3.8 (1.7-8.4) | .001 |
| β2-microglobulin greater than 2.7 U/L | 3.8 (1.5-9.5) | .004 |
| Organ systems involved, no. | 3.5 (1.9-6.5) | < .0001 |
| Serum creatinine | 2.3 (1.0-5.5) | .05 |
| Melphalan intensity, high dose | 0.5 (0.2-1.1) | .08 |
| Left ventricular EF less than or equal to 50% | 1.4 (0.3-6) | .7 |
| IVS greater than or equal to 15 mm | 1.8 (0.6-5.0) | .3 |
| Age greater than 60 y | 1.0 (0.4-2.5) | .99 |
| Female gender | 1.2 (0.5-2.9) | .7 |
| Time to transplantation | 0.8 (0.8-1.0) | .06 |
| Serum M spike | 1.0 (0.5-1.9) | .99 |
| Bone marrow plasmacytosis less than 30% | 1.1 (0.1-8.4) | .9 |
| Bone marrow plasma cell labeling index | 1.9 (0.8-4.2) | .1 |
| Multivariate model 1 | ||
| cTnT/NT-proBNP stage | 2.3 (1.1-4.7) | .02 |
| β2-microglobulin, U/L | 2.4 (1.5-3.8) | .0005 |
| Multivariate model 2 | ||
| cTnI/NT-proBNP stage | 3.2 (1.4-7.4) | .007 |
| β2 microglobulin, U/L | 2.4 (1.4-3.9) | .0006 |
Predictor . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Univariate | ||
| Troponin T greater than or equal to 0.035 μg/L | 4.4 (1.7-10.9) | .002 |
| Troponin I greater than or equal to 0.1 μg/L | 4.9 (1.6-15.1) | .006 |
| NT-proBNP greater than or equal to 332 ng/L | 3.8 (1.2-12) | .02 |
| cTnT Idealized Risk Score* | 2.2 (1.4-3.3) | .0003 |
| cTnI Idealized Risk Score† | 2.8 (.8-9.4) | .09 |
| cTnT/NT-proBNP stage‡ | 3.1 (1.5-6.2) | .001 |
| cTnI/NT-proBNP stage‡ | 3.8 (1.7-8.4) | .001 |
| β2-microglobulin greater than 2.7 U/L | 3.8 (1.5-9.5) | .004 |
| Organ systems involved, no. | 3.5 (1.9-6.5) | < .0001 |
| Serum creatinine | 2.3 (1.0-5.5) | .05 |
| Melphalan intensity, high dose | 0.5 (0.2-1.1) | .08 |
| Left ventricular EF less than or equal to 50% | 1.4 (0.3-6) | .7 |
| IVS greater than or equal to 15 mm | 1.8 (0.6-5.0) | .3 |
| Age greater than 60 y | 1.0 (0.4-2.5) | .99 |
| Female gender | 1.2 (0.5-2.9) | .7 |
| Time to transplantation | 0.8 (0.8-1.0) | .06 |
| Serum M spike | 1.0 (0.5-1.9) | .99 |
| Bone marrow plasmacytosis less than 30% | 1.1 (0.1-8.4) | .9 |
| Bone marrow plasma cell labeling index | 1.9 (0.8-4.2) | .1 |
| Multivariate model 1 | ||
| cTnT/NT-proBNP stage | 2.3 (1.1-4.7) | .02 |
| β2-microglobulin, U/L | 2.4 (1.5-3.8) | .0005 |
| Multivariate model 2 | ||
| cTnI/NT-proBNP stage | 3.2 (1.4-7.4) | .007 |
| β2 microglobulin, U/L | 2.4 (1.4-3.9) | .0006 |
EF indicates ejection fraction; IVS, interventricular septum.
See “Patients and methods” for equation. If risk levels 3 through 5 are collapsed into one level: HR 3.8, 95% CI 1.5-9.6, P = .004.
See “Patients and methods” for equation. If levels 2 and 3 are collapsed into one level: HR 6.6, 95% CI .9-49.6, P = .07.
Stage I is when troponin (either cTnT or cTnI) and NT-proBNP are both below threshold. Stage II is when troponin (either cTnT or cTnI) or NT-proBNP is greater than or equal to threshold value. Stage III is when troponin (either cTnT or cTnI) and NT-proBNP are both greater than or equal to threshold value. Threshold values for cTnT, cTnI, and NT-proBNP are < 0.035 μg/L, < 0.1 μg/L, and < 332 ng/L. Suffix (-t or -i) used depending on whether the troponin measured is cTnT or cTnI.
Survival according to circulating cardiac biomarkers. (A) Troponin T (cTnT). (B) Troponin I (cTnI). (C) N-terminal pro-brain natriuretic peptide (NT-proBNP).
Survival according to circulating cardiac biomarkers. (A) Troponin T (cTnT). (B) Troponin I (cTnI). (C) N-terminal pro-brain natriuretic peptide (NT-proBNP).
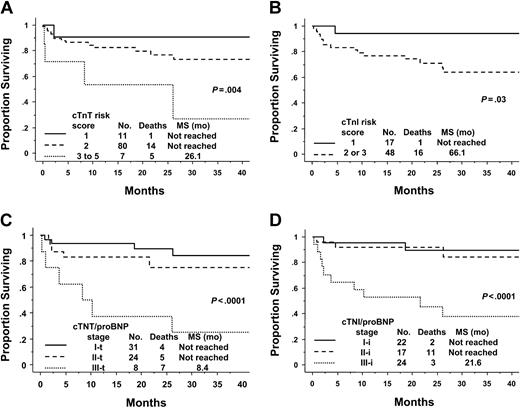
Previously, we have described the value of models incorporating these circulating cardiac biomarkers in nontransplanted amyloid.10 These models are also of value in the PBSCT population (Table 3 and Figure 2). The cTnT Idealized Risk Score, incorporating cTnT, age, ejection fraction (EF), and urine M spike, predicted for survival. This model demonstrates that the transplantation cohort is a relatively low-risk population. In the transplantation cohort, 11% of patients had a score of one, while the majority (82%) of patients had a score of 2 and 7% had a score of 3 to 5 (Figure 2A). This is in stark contrast to the risk scores of 242 unselected conventionally treated patients in whom the respective percentages are 11, 52, and 3817 (P < .0001); Table 4. The cTnI Idealized Risk Score, also constructed in amyloid patients undergoing conventional low-dose therapies, had slightly less prognostic value in the PBSCT cohort. There was excellent separation of the survival curves between patients with a score of one and more than one (P = .03; Figure 2B). Once again, higher risk groups had to be collapsed to account for the small numbers of the highest risk patients in the transplantation cohort.
Survival according to risk scores or stage. (A) The cTnT Idealized Risk Score. Risk scores 3 through 5 were merged due to low numbers of patients in higher risk groups. Score 3, n = 6; score 4, n = 0; and score 5, n = 1. See “Patients and methods” for calculation. (B) The cTnI Idealized Risk Score. Risk scores 2 and 3 were merged because there were only 3 patients in the score 3 category. See “Patients and methods” for calculation. (C) cTnT/NT-proBNP Staging System. Stage I-t is when both are below threshold. Stage II-t is when either is greater than or equal to threshold value. Stage III-t is when both are greater than or equal to threshold value. Threshold values for cTnT and NT-proBNP are less than 0.035 μg/L and less than 332 ng/L, respectively. (D) cTnI/NT-proBNP Staging System. Stage I-i is when both are below threshold. Stage II-i is when either is greater than or equal to threshold value. Stage III-i is when both are greater than or equal to threshold value. Threshold values for cTnI and NT-proBNP are less than 0.1 μg/L and less than 332 ng/L, respectively.
Survival according to risk scores or stage. (A) The cTnT Idealized Risk Score. Risk scores 3 through 5 were merged due to low numbers of patients in higher risk groups. Score 3, n = 6; score 4, n = 0; and score 5, n = 1. See “Patients and methods” for calculation. (B) The cTnI Idealized Risk Score. Risk scores 2 and 3 were merged because there were only 3 patients in the score 3 category. See “Patients and methods” for calculation. (C) cTnT/NT-proBNP Staging System. Stage I-t is when both are below threshold. Stage II-t is when either is greater than or equal to threshold value. Stage III-t is when both are greater than or equal to threshold value. Threshold values for cTnT and NT-proBNP are less than 0.035 μg/L and less than 332 ng/L, respectively. (D) cTnI/NT-proBNP Staging System. Stage I-i is when both are below threshold. Stage II-i is when either is greater than or equal to threshold value. Stage III-i is when both are greater than or equal to threshold value. Threshold values for cTnI and NT-proBNP are less than 0.1 μg/L and less than 332 ng/L, respectively.
Prognostic markers in patients who underwent transplantation and in those who did not
. | SCT N = 98 . | . | No SCT17 N = 242 . | . | ||
|---|---|---|---|---|---|---|
| Characteristic and cut-off . | % . | Median survival, mos. . | % . | Median survival, mos. . | ||
| cTnT | ||||||
| Less than 0.035 μg/L | 86 | NR | 56 | 17 | ||
| Greater than or equal to 0.035 μg/L | 14 | 26.1 | 44 | 3.7 | ||
| cTnI* | ||||||
| Less than 0.10 μg/L | 57 | NR | 46 | 16.4 | ||
| Greater than or equal to 0.10 μg/L | 43 | 66.1 | 54 | 5.9 | ||
| NT-proBNP† | ||||||
| Less than 332 ng/L | 52 | NR | 40 | 20.0 | ||
| Greater than or equal to 332 ng/L | 48 | 66.1 | 60 | 5.8 | ||
| Ejection fraction | ||||||
| Less than 50% | 92 | NR | 68 | 14.0 | ||
| Greater than or equal to 50% | 8 | NR | 32 | 3.9 | ||
| Septal thickness | ||||||
| Less than or equal to 15 mm | 76 | NR | 67 | 12.3 | ||
| Greater than 15 mm | 24 | NR | 33 | 4.3 | ||
| cTnT Idealized Risk Score‡ | ||||||
| 1 | 11 | NR | 11 | 23.4 | ||
| 2 | 82 | NR | 52 | 16.4 | ||
| 3-5 | 7 | 26.1 | 38 | 2.9 | ||
| cTnI Idealized Risk Score*‡ | ||||||
| 1 | 23 | NR | 11 | 17.0 | ||
| 2 | 72 | NR | 52 | 11.1 | ||
| 3 | 5 | 66.1 | 32 | 2.2 | ||
| cTnT/NT-proBNP stage†§ | ||||||
| I-t | 49 | NR | 33 | 26.4 | ||
| II-t | 38 | NR | 30 | 10.5 | ||
| III-t | 13 | 8.4 | 37 | 3.5 | ||
| cTnI/NT-proBNP stage†§ | ||||||
| I-i | 35 | NR | 24 | 27.2 | ||
| II-i | 38 | NR | 38 | 11.1 | ||
| III-i | 27 | 21.6 | 38 | 4.1 | ||
. | SCT N = 98 . | . | No SCT17 N = 242 . | . | ||
|---|---|---|---|---|---|---|
| Characteristic and cut-off . | % . | Median survival, mos. . | % . | Median survival, mos. . | ||
| cTnT | ||||||
| Less than 0.035 μg/L | 86 | NR | 56 | 17 | ||
| Greater than or equal to 0.035 μg/L | 14 | 26.1 | 44 | 3.7 | ||
| cTnI* | ||||||
| Less than 0.10 μg/L | 57 | NR | 46 | 16.4 | ||
| Greater than or equal to 0.10 μg/L | 43 | 66.1 | 54 | 5.9 | ||
| NT-proBNP† | ||||||
| Less than 332 ng/L | 52 | NR | 40 | 20.0 | ||
| Greater than or equal to 332 ng/L | 48 | 66.1 | 60 | 5.8 | ||
| Ejection fraction | ||||||
| Less than 50% | 92 | NR | 68 | 14.0 | ||
| Greater than or equal to 50% | 8 | NR | 32 | 3.9 | ||
| Septal thickness | ||||||
| Less than or equal to 15 mm | 76 | NR | 67 | 12.3 | ||
| Greater than 15 mm | 24 | NR | 33 | 4.3 | ||
| cTnT Idealized Risk Score‡ | ||||||
| 1 | 11 | NR | 11 | 23.4 | ||
| 2 | 82 | NR | 52 | 16.4 | ||
| 3-5 | 7 | 26.1 | 38 | 2.9 | ||
| cTnI Idealized Risk Score*‡ | ||||||
| 1 | 23 | NR | 11 | 17.0 | ||
| 2 | 72 | NR | 52 | 11.1 | ||
| 3 | 5 | 66.1 | 32 | 2.2 | ||
| cTnT/NT-proBNP stage†§ | ||||||
| I-t | 49 | NR | 33 | 26.4 | ||
| II-t | 38 | NR | 30 | 10.5 | ||
| III-t | 13 | 8.4 | 37 | 3.5 | ||
| cTnI/NT-proBNP stage†§ | ||||||
| I-i | 35 | NR | 24 | 27.2 | ||
| II-i | 38 | NR | 38 | 11.1 | ||
| III-i | 27 | 21.6 | 38 | 4.1 | ||
NR indicates not reached.
For TnI, only 65 transplantation patients had levels measured.
For NT-proBNP, 63 transplantation patients had levels measured
See “Patients and methods” for calculations.
See Table 3 for definitions.
The staging systems incorporating troponins and NT-proBNP were also applied to this PBSCT cohort. These systems, although less complex than the “idealized” scores, are as powerful (if not more so) in this setting. For the cTnT/NT-proBNP staging system (-t), the groups split as follows: stage I-t, 49%; stage II-t, 38%; and stage III-t, 13%. Most patients are low to intermediate risk. Only in the stage III-t group has median survival been reached, at 8.4 months (Figure 2C). Of the stage II-t patients, 22 of 24 are upstaged from stage I-t due to elevated NT-proBNP, rather than cTnT. The findings for the cTnI/NT-proBNP staging system (-i) are similar, except patients are classified as slightly higher risk using this system: stage I-i, 34%; stage II-i, 28%; and stage III-i, 38% (Figure 2D). Of the 24 stage II-i patients, 11 are so classified because of elevated NT-proBNP. The stage I-i and stage II-i patients appear to have comparable outcomes; longer follow-up will be required to fully understand the distinction between these 2 stages in the transplantation setting. If one compares the 2 troponin/NT-proBNP staging systems, their correlation is very good at 0.76, P less than .0001. Sixty-five percent have the same relative stage. Of the 22 patients with discordant staging, the cTnI/NT-proBNP system upstages 20 patients relative to the cTnT/NT-proBNP system (11 from stage I-t to stage II-i and 9 from stage II-t to stage III-i).
Other factors predictive for survival on univariate analysis include number of organs involved, serum β2-microglobulin, and serum creatinine (Table 3). Age, sex, time to transplantation, melphalan dose, serum or urine M spike size, percent bone marrow plasmacytosis, bone marrow plasma cell labeling index, and left ventricular (LV) ejection fraction do not predict for survival.
Table 3 illustrates 2 multivariate models using the compound troponin/NT-proBNP staging system: one utilizing cTnT and the other cTnI. On multivariate analysis, only 2 variables could be entered due to the paucity of events.
Finally, in Table 4, the cTnT, cTnI, and NT-proBNP values and scores are compared with the values previously reported in a cohort of 242 patients who did not undergo transplantation.17 Not surprisingly, the patients who did not undergo transplantation have significantly higher serum troponin levels and risk scores, demonstrating the selection that enters into offering patients PBSCT.
Discussion
Our data illustrate several important issues. The first is that serum levels of cardiac troponins and NT-proBNP are valuable predictors of overall survival in patients with AL undergoing PBSCT, as they are in patients not undergoing this intensive procedure.10,16 The second is that elevations of cTnI, but not cTnT and/or NT-proBNP, are predictive for mortality within 90 days of transplantation. The third is that patients undergoing PBSCT are lower than average risk because they are highly selected to undergo the rigorous procedure.
Our troponin/NT-proBNP staging system builds on our previous work with troponins10 and the work of Palladini et al16 who used NT-proBNP. Palladini et al selected an NT-proBNP threshold of 152 pM (1266 ng/L) based on their receiver operator characteristic analysis derived from a clinical diagnosis of cardiac involvement. Their methodology empirically restricted the utility of the biomarker to that of previously described cardiac characteristics, that is, congestive heart failure symptoms as defined by New York Heart Association (NYHA) class II or higher, unexplained low voltage on the electrocardiogram, or echocardiographic findings consistent with amyloidosis. In contrast, we have selected 332 ng/L (approximately the fiftieth percentile for our amyloid patients undergoing transplantation) as a threshold for NT-proBNP. An additional 19% of our patients were classified as having an inferior prognosis using this latter cut-off. Our threshold predicted for overall survival and may therefore be more meaningful. Although our data cannot be used to unequivocally define cardiac involvement, they are suggestive. In a nonamyloid setting, levels of NT-proBNP have been shown to correlate with left ventricular dilatation, remodeling, dysfunction, congestive heart failure, and death among patients presenting with acute myocardial infarction, and there is a strong association between levels of NT-proBNP and mortality even in the absence of cardiac troponin release.25
Moreover, the present data emphasize the importance of staging patients for the purpose of making meaningful comparisons between studies from different treatment centers and for stratification within randomized studies.9 Although multiple prognostic factors have been identified, until now no staging system existed for patients with primary systemic amyloidosis.8,9,16,26-33 While “numbers of organs involved” is a valuable descriptor that has been used as a form of classification, there is subjectivity and no uniform agreement in designating organ involvement.6,8,34,35 In an effort to rectify this problem, we recently modeled 2 different systems to stage patients with AL. The first system is a complicated but very powerful troponin-based Idealized Risk Score with separate equations for cTnT and cTnI to serve investigators who have only one or the other test available.10 The second system is a simpler troponin/NT-proBNP based staging system.17 Both are now tested in the transplantation population and are demonstrated to be relevant.
Despite the relatively small numbers of patients (63 to 98) and the relatively short follow-up (median 20 months), using our models the highest risk patients are clearly distinguished from the lower risk patients. Using the “idealized” models, there is good separation between the lowest-risk and intermediate-risk patients. Separation of the stage I and stage II patients is less dramatic in troponin/NT-proBNP staging systems, but with longer follow-up and more events, the stage I and stage II curves may diverge as they do in the nontransplantation cohort.
Although troponin and NT-proBNP levels are known to be elevated in patients with end-stage renal disease, it has been repeatedly demonstrated that these markers are still predictive of survival in these patients.15,36-39 There are very few data documenting the renal elimination of cTnT, NT-proBNP, or cTnI although this is often speculated to be the case when the renal impairment is severe. Renal function thus could play a role but it could also be that increases in patients with end-stage renal disease reflect concomitant comorbidities rather than changes in clearance. The interaction, however, is complex. Renal impairment also may be a marker of the cumulative extent of multifactorial endothelial and vascular damage occurring throughout the affected individual,25 and a component of the elevation of these markers observed in dialysis patients may be a reflection of this principle. In our series, only 10% of patients had significant renal impairment (serum creatinine above 150.28 μM/L [1.7 mg/dL]). There was no correlation between the serum creatinine, urinary total protein, or urinary M spike with any of the cardiac biomarkers (data not shown). The contrasts between the patients who did not undergo transplantation and the patients who did are striking though not surprising. Whereas 44% of the former group and 14% of the latter have elevated cTnT levels, the difference in the percentages of patients with elevated NT-proBNP is less notable. In this sense troponin levels appear less sensitive but more specific than NT-proBNP levels in the context of amyloid patients. While serum troponins detect even minor amounts of cardiac injury when it occurs, NT-proBNP detects cumulative effects that may represent cardiac or noncardiac conditions such as volume overload or right ventricular stress due to pulmonary disease. The relevance of our findings, however, lies in the observation that these markers are prognostic.
It is also important to note disparity in risk scores and stages between the transplantation and nontransplantation groups. Using our risk stratification approach based on troponin, the transplantation group was much lower risk. Instead of approximately one third of patients falling into each stage of the cTnT/NT-proBNP system, about half were stage I-t and only one eighth were stage III-t. The cTnT Idealized Risk Scores were significantly different across the groups as well. Only a minority of amyloid patients are transplantation candidates because a high proportion are too sick to withstand high-dose chemotherapy.5,9
Why are serum levels of sensitive cardiac biomarkers the most powerful predictors of survival? To date, our studies have not been designed to directly answer this important question. Perhaps the answer lies in the fact that the heart is involved with AL in at least 40% of patients and that the cause of death in most patients is cardiac1,2,31 —either cardiomyopathy that progresses to congestive heart failure or sudden death due to ventricular fibrillation or asystole.26,40 The mechanism of troponin and NT-proBNP release is unknown in these patients, but one can hypothesize that either the free light chains or the amyloid fibrils directly or indirectly cause myocyte damage resulting in cardiac troponin and NT-proBNP release which can be measured in the blood stream as a routine assay. More study is required to elucidate the mechanisms whereby patients with the highest levels of cardiac biomarkers have the shortest survivals and their utility as serial measurements in this cohort of patients. Palladini et al16 have demonstrated in a small set of patients that reductions in NT-proBNP occur in a majority of patients who enjoy a hematologic response. Palladini et al have demonstrated in a small set of patients that reductions in NT-proBNP occur in a majority of patients who enjoy a hematologic response.16 We therefore conclude that with simple measurements of serum levels of cardiac troponins and NT-proBNP, one can predict patient outcome whether the patient has received standard chemotherapy10,17 or stem cell transplantation.
Prepublished online as Blood First Edition Paper, March 25, 2004; DOI 10.1182/blood-2004-01-0390.
Supported in part by CA 62 242 (R.A.K), CA 91 561 (A.D) from the National Cancer Institute and the Robert A. Kyle Hematologic Malignancies Fund.
Presented in part in abstract form at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 8, 2003.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal