Abstract
Supporting roles of stromal cells in preferential colonization of myeloma cells in bone marrow and development of associated osteoclastic osteolysis through cell-cell interactions have been indicated. Here we examined the effects of a monoclonal antibody to α4 integrin (anti-α4 Ab) that disrupts myeloma cell-stromal cell interactions mediated via α4β1 integrin and vascular cell adhesion molecule-1 (VCAM-1) on myeloma cell growth in bone marrow and accompanying osteolysis. The anti-α4 Ab decreased VCAM-1-stimulated 5TGM1/luc cell growth in culture. The 5TGM1 murine myeloma cells stably transfected with the firefly luciferase (5TGM1/luc) were inoculated from tail vein in bg/xid/nd mice. Preventative administration of the anti-α4 Ab suppressed the elevation of serum IgG2b levels, decreased 5TGM1/luc tumor burden with increased apoptosis in bone and spleen, reduced bone destruction with diminished number of osteoclasts, and prolonged survival of 5TGM1/luc-bearing mice. In contrast, therapeutic administration of the antibody failed to show these effects. However, therapeutic administration of the antibody combined with melphalan significantly suppressed serum IgG2b levels and tumor burden in bone. Our results suggest that the interactions with stromal cells via α4β1/VCAM-1 are critical to the development of myeloma and associated osteolysis and that disruption of these interactions using anti-α4 Ab is a potential therapeutic approach for myeloma. (Blood. 2004;104:2149-2154)
Introduction
Multiple myeloma is a B-cell malignancy characterized by the preferential accumulation of plasma cells that secrete monoclonal immunoglobulins in bone marrow and accompanying osteoclastic bone destruction with severe pain.1,2 It has been demonstrated that the marrow stromal cells play supportive roles in the colonization of myeloma cells in bone marrow by an establishment of cell-cell interactions through expressing cell adhesion molecules (CAMs) that recognize corresponding CAMs expressed on myeloma cells.3,4 Both myeloma cells and marrow stromal cells are shown to express various types of CAMs.5 In this context, it is notable that myeloma cells express α4β1 integrin (also known as VLA4)6-8 and that marrow stromal cells constitutively express VCAM-1 that is a ligand for α4β1 integrin.9,10 Previous in vitro studies have reported that the interactions with stromal cells via α4β1 integrin/VCAM-1 promote myeloma cell colonization in bone marrow.11 Consistent with these results, our in vitro data have shown that the contact of the 5TGM1 mouse myeloma cells with marrow stromal cells via α4β1 integrin/VCAM interactions enhances the production of osteoclastogenic factors and that disruption of this cell-cell contact using a neutralizing monoclonal antibody to α4 integrin (anti-α4 Ab)12 reduced the production of these factors.13 The 5TGM1 myeloma was derived from the 5T murine myeloma that spontaneously arose in C57BL/KaLwRij mice14 and reproducibly developed myeloma disease associated with monoclonal gammopathy and osteolytic lesions following intravenous inoculation in these mice or immunodeficient mice.15 We have previously demonstrated that ibandronate, which is one of the bisphosphonates that are widely used specific inhibitors of osteoclasts,16 suppresses osteolytic bone diseases associated with 5TGM1 myeloma.17 These results collectively led us to test the anti-α4 Ab in 5TGM1 myeloma model in vivo.
Here we newly established 5TGM1 cells stably transfected with the firefly luciferase cDNA (5TGM1/luc cells), which allowed us to determine tumor burden in a quantitative manner. We found that 5TGM1/luc cells caused myeloma disease and osteolysis in a manner identical to the parental 5TGM1 cells. Using this model, we examined the effects of the anti-α4 Ab on the capacity of these cells to develop myeloma disease and cause osteolysis. Our results demonstrate that administration of the anti-α4 Ab in a preventative manner decreases 5TGM1/luc tumor burden in bone and spleen with increased apoptotic 5TGM1/luc cells and suppresses osteoclastic bone destruction, leading to an inhibition of the development of 5TGM1/luc myeloma and improvement of survival of 5TGM1 myeloma-bearing mice. On the other hand, therapeutic administration of the antibody failed to suppress the progression of the disease. However, combination of the antibody with melphalan significantly inhibited myeloma growth in bone and reduced serum IgG2b levels.
Materials and methods
Cell culture
5TGM1 myeloma cells were derived from murine myeloma 5T33.17 These cells were grown in Iscove modified Dulbecco media (IMDM, Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS, Summit, Fort Collins, CO) and 100 U/mL penicillin-streptomycin (Life Technologies). The firefly luciferase cDNA (pGL3, Promega, Madison, WI) was cotransfected with neomycin-resistant gene into 5TGM1 cells using Effectane (Qiagen, Valencia, CA) according to the manufacturer's protocol. Positive clones were selected in the presence of G418 (1 mg/mL), and 5TGM1 cell clones stably expressing the firefly luciferase cDNA (5TGM1/luc cells) were established.
5TGM1/luc cell growth on rsVCAM-1-coated plates
Recombinant soluble form of VCAM-1 (rsVCAM-1)18 and the neutralizing monoclonal antibody against murine α4 integrin (PS/2)19 were gifts of Biogen (Cambridge, MA). A stock solution (1 mg/mL) was diluted with the binding buffer (15 mM sodium bicarbonate/35 mM sodium carbonate, pH 9.2) to make a final concentration of 10 μg/mL. One hundred-forty microliters of this solution in 48-well plates was incubated overnight at 4°C, and plates were blocked with 1% bovine serum albumin in phosphate buffered saline (PBS) for 1 hour at room temperature and washed once with IMDM containing 1% FBS. 5TGM1/luc cells (105/0.5 mL/well) were incubated with anti-α4 Ab or rat IgG (Sigma, St Louis, MO) (1 μg/mL) in IMDM containing 1% FBS for 48 hours. Cell growth was quantified by luciferase activity, measured according to the manufacturer's instructions.
Inoculation of 5TGM1/luc cells
5TGM1/luc cells were suspended in PBS at a density of 5 × 106 cells/mL. One million cells in 200 μL were inoculated into 6- to 8-week-old female bg/nd/xid mice (National Cancer Institute, Frederick, MD) via tail vein.
Administration of anti-α4 integrin antibody and melphalan
Anti-α4 Ab was purified endotoxin-free as described.20 It was diluted in PBS at a concentration of 2 mg/mL and given either 4 days (preventative administration) or 14 days (therapeutic administration) after the cell inoculation. Administration of the antibody was carried out as described20 with some modifications. In the preventative experiments, the antibody was given at 200 μg/mouse, intraperitoneally, daily, from day 4 to 6 and thereafter 80 μg/mouse, intraperitoneally, twice per week, until the end of the experiments (Figure 1). In the therapeutic experiments, the antibody was given at 200 μg/mouse, intraperitoneally, daily, from day 14 to 16 and thereafter 80 μg/mouse, intraperitoneally, twice per week until the end of the experiments combined with or without melphalan (100 μg, intraperitoneally, once a week, Sigma) (Figure 1). Rat IgG served as a control. Each group had 8 to 10 mice, and these experiments were conducted twice (n = 8 to 10 × 2 = 16 to 20 for each group). All the animal studies were performed according to the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) (protocol number 00143-34-03-C) of the University of Texas Health Science Center at San Antonio.
Experimental protocol. One million cells per mouse were inoculated through tail vein in 6- to 8-week-old female bg/nd/xid mice under anesthesia. Anti-α4 Ab and melphalan were given intraperitoneally.
Experimental protocol. One million cells per mouse were inoculated through tail vein in 6- to 8-week-old female bg/nd/xid mice under anesthesia. Anti-α4 Ab and melphalan were given intraperitoneally.
Measurement of serum IgG2b levels
Blood samples were collected by retro-orbital puncture under isoflulene-inhaled anesthesia. IgG2b concentration in sera was measured using a specific 2-site enzyme-linked immunosorbent assay (ELISA).15,17 Briefly, the capture antibody (rat anti-mouse IgG2b; Zymed, San Francisco, CA) was coated onto plates overnight at 4°C at a concentration of 2 μg/mL, and the plates were blocked with 5% bovine serum albumin for 3 hours at room temperature. After incubation with mouse IgG2b standard (Cappel, West Chester, PA) or serum samples for 1 hour at 37°C, plates were washed and incubated with the detection antibody at a dilution of 1:5000 (peroxidase-conjugated rat anti-mouse IgG; Biodesign International, Kennebunk, ME) for 1 hour at 37°C. Then o-phenylenediamine dihydrochloride tablets (Sigma) were used as reaction substrate according to the manufacturer's instructions. The absorbance at 450 nm was read on an enzyme immunoassay (EIA) plate reader.
Measurement of luciferase activity
At the time of death, left femurs and tibiae and spleen were harvested and homogenized in 0.5 mL cell culture lysis reagent (Promega). Luciferase activity was measured in 20 μL of the lysates using luciferase assay system (Promega) according to the manufacturer's instructions.
Histology and histomorphometry
Mice were killed on day 27, and their right hindlimbs were harvested and subjected to histological examinations. Right femurs and tibiae were fixed in 10% formalin for 48 hours, decalcified in 14% EDTA (ethylenediaminetetraacetic acid) for 14 days, and embedded in paraffin. Sections were stained with hematoxylin and eosin (H-E). Histomorphometric analysis was done by using osteomeasure histomorphometry system (Osteometrics, Atlanta, GA) as described previously.21
Determination of apoptosis
Apoptosis was determined by manually counting apoptotic 5TGM1/luc myeloma cells that exhibited characteristic morphology including cell shrinkage, nuclear pyknosis, and chromatin condensation under a microscope as described.22 Five different fields on H-E-stained histological sections of bone were randomly examined at a magnification of × 200.
Statistical analysis
Student t test or ANOVA followed by Tukey-Kramer test were used. For statistical analysis of survival data, Wilcoxon test was used. Data were accepted as significantly different with a probability of 0.05 or less.
Results
Effects of anti-α4 Ab on 5TGM1/luc cell growth in culture
We have reported that anti-α4 Ab inhibited the production of bone-resorbing activity in 5TGM1 cells that were cultured on VCAM-1-coated plates.13 Prior to in vivo experiments, we examined the effects of the antibody on 5TGM1 cell growth in culture. As shown in Figure 2, the growth of 5TGM1/luc cells was increased on VCAM-1-coated plates compared with noncoated plates. This increase in 5TGM1 cell growth was reduced in the presence of the anti-α4 Ab.
Effects of anti-α4 Ab on 5TGM1/luc cell growth in culture. 5TGM1/luc cells were cultured on noncoated wells or rsVCAM-1-coated wells for 48 hours. Anti-α4 Ab blocked the stimulatory effect of VCAM-1 on the growth of 5TGM1/luc. Data are means ± SEM (n = 4).
Effects of anti-α4 Ab on 5TGM1/luc cell growth in culture. 5TGM1/luc cells were cultured on noncoated wells or rsVCAM-1-coated wells for 48 hours. Anti-α4 Ab blocked the stimulatory effect of VCAM-1 on the growth of 5TGM1/luc. Data are means ± SEM (n = 4).
Effects of preventative administration of anti-α4 Ab on serum IgG2b levels
Measurement of luciferase activity in bone marrow and histological examination verified that 5TGM1/luc cells colonized in bone marrow 4 to 7 days after the inoculation (data not shown). Accordingly, we started preventative administration of the antibody 4 days after 5TGM1/luc cell inoculation (Figure 1). Serum IgG2b, a widely used systemic indicator for myeloma tumor burden,15,17 began to elevate from 3 weeks in 5TGM1/luc-bearing mice (Figure 3). Animals treated with the anti-α4 Ab showed reduced serum IgG2b levels compared with untreated and control rat IgG-treated animals. There was no significant difference between untreated and control rat IgG-treated group. These results indicate that the anti-α4 Ab decreases 5TGM1/luc myeloma tumor burden in mice.
Effects of preventative administration of the anti-α4 Ab on serum IgG2b levels in 5TGM1/luc myeloma-bearing mice. Monoclonal IgG2b was measured by sandwich ELISA as described in “Materials and methods.” Data are mean ± SEM of 2 separate experiments (n = 10 × 2 = 20). *Significantly different from untreated group. **Significantly different from control IgG group.
Effects of preventative administration of the anti-α4 Ab on serum IgG2b levels in 5TGM1/luc myeloma-bearing mice. Monoclonal IgG2b was measured by sandwich ELISA as described in “Materials and methods.” Data are mean ± SEM of 2 separate experiments (n = 10 × 2 = 20). *Significantly different from untreated group. **Significantly different from control IgG group.
Effects of preventative administration of anti-α4 Ab on 5TGM1/luc myeloma development
Since myeloma cells preferentially disseminate in bone marrow, we next determined the 5TGM1/luc tumor burden in the hindlimb by assessing for luciferase activity. The 5TGM1/luc tumor burden in bone was significantly decreased following the antibody treatment (Figure 4A). Consistent with these results, histological examinations revealed significantly reduced 5TGM1/luc tumor area in bone marrow in antibody-treated mice compared with untreated mice (Figure 5). As a unique feature of 5TGM1/luc myeloma model, these cells also disseminated in spleen. The anti-α4 Ab markedly diminished 5TGM1/luc myeloma development in spleen as well (Figure 4B). Control IgG had no effects (data not shown).
Effects of preventative administration of the anti-α4 Ab on tumor burden in hindlimbs and in spleen. Mice were killed at day 23. Luciferase activity was measured as described in “Materials and methods.” Data are mean ± SEM (n = 5). *Significantly different from untreated group.
Effects of preventative administration of the anti-α4 Ab on tumor burden in hindlimbs and in spleen. Mice were killed at day 23. Luciferase activity was measured as described in “Materials and methods.” Data are mean ± SEM (n = 5). *Significantly different from untreated group.
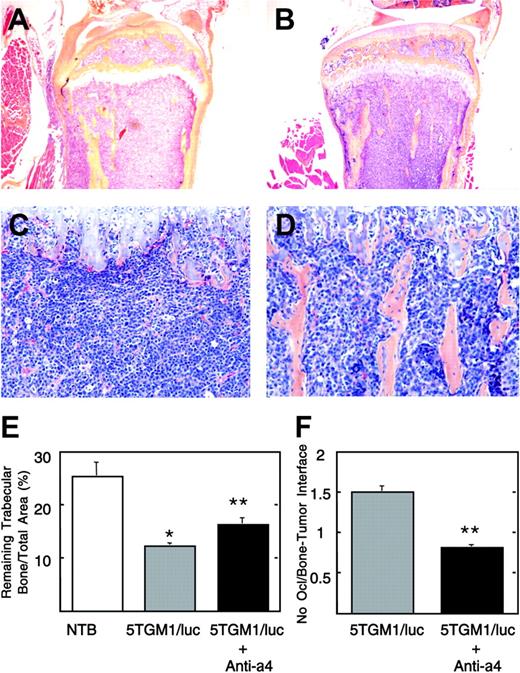
Histological view of tibia of untreated and anti-α4 Ab-treated 5TGM1/luc-bearing mice. Note that the bone marrow cavity is occupied by 5TGM1/luc myeloma cells, and no trabecular bones are seen in the untreated group (A, C). On the other hand, normal marrow elements and trabecular bones are still observed in the anti-α4 Ab-treated group (B, D). Slides were examined using an Olympus BX-40F4 microscope equipped with a film camera (Olympus, Melville, NY). Panels A and B: lower magnification (H-E, × 40); panels C and D: higher magnification (H-E, × 200). Histomorphometric analysis of remaining trabecular bone/total area (E) and osteoclast number at the interface between myeloma and bone (F). Data are mean ± SEM (n = 5). *Significantly different from non-tumor-bearing (NTB) mice. **Significantly different from untreated 5TGM1/luc-bearing mice.
Histological view of tibia of untreated and anti-α4 Ab-treated 5TGM1/luc-bearing mice. Note that the bone marrow cavity is occupied by 5TGM1/luc myeloma cells, and no trabecular bones are seen in the untreated group (A, C). On the other hand, normal marrow elements and trabecular bones are still observed in the anti-α4 Ab-treated group (B, D). Slides were examined using an Olympus BX-40F4 microscope equipped with a film camera (Olympus, Melville, NY). Panels A and B: lower magnification (H-E, × 40); panels C and D: higher magnification (H-E, × 200). Histomorphometric analysis of remaining trabecular bone/total area (E) and osteoclast number at the interface between myeloma and bone (F). Data are mean ± SEM (n = 5). *Significantly different from non-tumor-bearing (NTB) mice. **Significantly different from untreated 5TGM1/luc-bearing mice.
Effects of preventative administration of anti-α4 Ab on osteoclastic osteolysis
We subsequently examined the effects of the antibody on osteoclastic osteolysis associated with 5TGM1/luc myeloma cell colonization in bone. A representative histology revealed that bone marrow cavity was completely occupied by 5TGM1/luc cells, and trabecular bones were destroyed in untreated mice (Figure 5A, C). On the other hand, considerable parts of marrow elements and trabecular bones remained intact in antibody-treated mice (Figure 5B, D). In agreement with these histological pictures, histomorphometric analyses showed that there was a significant reduction in the remaining trabecular bone mass due to osteoclastic bone resorption in 5TGM1/luc-bearing mice (Figure 5E) compared with non-tumor-bearing (NTB) mice. The antibody significantly increased the remaining trabecular bone mass with decreased osteoclast number at the interface between myeloma and bone (Figure 5F). Control IgG had no effects (data not shown).
Effects of preventative administration of anti-α4 Ab on apoptosis of 5TGM1/luc myeloma cells in bone
Since previous studies showed that interactions with stromal cells increased survival of myeloma cells in bone marrow,23,24 we next examined the effects of the antibody on apoptosis of 5TGM1/luc myeloma cells in bone by histology. The antibody significantly increased apoptosis in 5TGM1/luc myeloma cells in bone (Figure 6). Control IgG had no effect (data not shown).
Effects of preventative administration of the anti-α4 Ab on apoptosis of 5TGM1/luc myeloma cells in bone. Apoptotic 5TGM1/luc myeloma cells with characteristic morphology including cell shrinkage, nuclear pyknosis, and chromatin condensation were counted on H-E-stained histological sections as described in “Materials and methods.” Data are mean ± SEM (n = 5). *Significantly different from untreated group.
Effects of preventative administration of the anti-α4 Ab on apoptosis of 5TGM1/luc myeloma cells in bone. Apoptotic 5TGM1/luc myeloma cells with characteristic morphology including cell shrinkage, nuclear pyknosis, and chromatin condensation were counted on H-E-stained histological sections as described in “Materials and methods.” Data are mean ± SEM (n = 5). *Significantly different from untreated group.
Effects of preventative administration of anti-α4 Ab on survival of 5TGM1/luc-bearing mice
Finally, we examined the effects of the antibody on survival of 5TGM1/luc myeloma-bearing mice. Mice bearing 5TGM1/luc myeloma began to die 4 weeks after the cell inoculation (Figure 7). 5TGM1/luc myeloma-bearing mice that were treated with the anti-α4 Ab showed increased survival rate compared with untreated 5TGM1/luc myeloma-bearing mice. Control IgG showed no effects on survival.
Effects of preventative administration of the anti-α4 Ab on survival of 5TGM1/luc myeloma-bearing mice. Survival was assessed by Wilcoxon test. Data are mean ± SEM of 2 separate experiments (n = 10 × 2 = 20). *Significantly different from untreated group. **Significantly different from control IgG.
Effects of preventative administration of the anti-α4 Ab on survival of 5TGM1/luc myeloma-bearing mice. Survival was assessed by Wilcoxon test. Data are mean ± SEM of 2 separate experiments (n = 10 × 2 = 20). *Significantly different from untreated group. **Significantly different from control IgG.
Effects of therapeutic administration of anti-α4 Ab in combination with or without melphalan on 5TGM1/luc myeloma
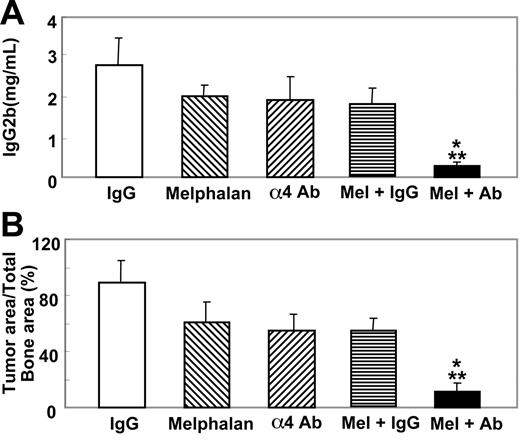
Subsequently, we examined the effects of therapeutic administration of the antibody combined with or without an anticancer agent. In these experiments the antibody was given 14 days after the cell inoculation together with or without melphalan (100 g/mouse, once a week, intraperitoneally) as described in Figure 1. Melphalan has been one of the most widely used agents for the treatment of patients with multiple myeloma.25 In a preliminary experiment, we examined the effects of melphalan at doses of 50, 100, and 200 μg/mouse on serum IgG2b levels in 5TGM1/luc-bearing mice and found that 200 μg/mouse melphalan significantly suppressed serum IgG2b levels, while 100 μg/mouse and 50 μg/mouse melphalan showed marginal and no effects, respectively (data not shown). Accordingly, we decided to use a suboptimal dose of melphalan (100 μg/mouse) in combination with the antibody. The antibody or melphalan alone administered according to this protocol showed no suppressive effects on serum IgG2b levels (Figure 8A) and the 5TGM1/luc tumor burden in bone as determined by histomorphometry (Figure 8B) in 5TGM1/luc myeloma-bearing mice. However, a combination of the antibody and melphalan markedly reduced serum IgG2b levels and the tumor burden of 5TGM1/luc myeloma in bone (Figure 8).
Effects of therapeutic administration of the anti-α4 Ab combined with melphalan on serum IgG2b levels and 5TGM1/luc tumor burden in bone. Anti-α4 Ab and melphalan were given as described in Figure 1. Data are mean ± SEM of 2 separate experiments (n = 8 × 2 = 16). *Significantly different from control IgG. **Significantly different from anti-α4 Ab or melphalan alone.
Effects of therapeutic administration of the anti-α4 Ab combined with melphalan on serum IgG2b levels and 5TGM1/luc tumor burden in bone. Anti-α4 Ab and melphalan were given as described in Figure 1. Data are mean ± SEM of 2 separate experiments (n = 8 × 2 = 16). *Significantly different from control IgG. **Significantly different from anti-α4 Ab or melphalan alone.
Discussion
We have previously reported that the direct contact of 5TGM1 myeloma cells with marrow stromal cells via α4β1/VCAM-1 interactions enhances the production of osteoclastogenic factors in 5TGM1 cells and that disruption of this cell-cell interaction by an anti-α4 integrin Ab significantly suppressed osteoclastogenic factor production.13 To determine the significance of these in vitro observations in the pathogenesis of myeloma and associated osteolytic bone disease, we examined the effects of the anti-α4Ab on the 5TGM1 myeloma behavior in vivo. Our results show that the preventative administration of the anti-α4 Ab significantly reduced the 5TGM1/luc myeloma tumor burden in bone marrow and spleen and inhibited osteoclastic osteolysis. Consequently, the antibody reduced serum IgG2b levels and improved survival rate of the 5TGM1/luc myeloma-bearing mice. These effects of the antibody are at least in part due to inhibition of 5TGM1/luc cell growth that is promoted through the contact with stromal cells expressing VCAM-1. Our results suggest that the α4β1 integrin/VCAM-1 interactions are critical to the development of the 5TGM1/luc myeloma colonization and accompanying osteolysis. More importantly from therapeutic viewpoints, the results that the anti-α4 Ab suppressed myeloma disease and associated bone destruction and prolonged survival suggest that interference with cell-cell interactions mediated via VCAM-1 and α4β1 integrin using agents such as anti-α4 Ab may be a potential therapeutic intervention for the treatment of myeloma. The importance of the α4β1 integrin/VCAM-1 interactions in the pathogenesis of various diseases other than myeloma has been also demonstrated. A recent study has reported that a recombinant anti-α4 monoclonal antibody (natalizumab) exhibits beneficial effects on multiple sclerosis and Crohn disease in which the interactions between microvessel wall and T lymphocytes mediated via VCAM-1 and α4β1 integrin were critical to the pathophysiology of these diseases.26
Failure of the antibody to reduce serum IgG2b levels and the 5TGM1/luc tumor burden in bone marrow following therapeutic administration suggests that once myeloma is well established, contact of myeloma cells with stromal cells may no longer be important for myeloma progression. The reason for this is not known at the moment. It is possible that myeloma cells acquire the capacity to grow independent from stromal cell support that is mediated through the VCAM-1 and α4β1 integrin interactions as a consequence of reciprocal decrease in the number of stromal cells due to the progressive colonization of myeloma cells in bone marrow. Thus, coadministration of anticancer agents is likely required for the suppression of the progression of established myeloma in bone. Indeed, our results demonstrate that therapeutic administration of the anti-α4 Ab combined with melphalan significantly decreased serum IgG2b levels and the 5TGM1/luc tumor burden in bone. Furthermore, it should be noted that a suboptimal dose of melphalan showed inhibitory effects on 5TGM1/luc myeloma when combined with the anti-α4 Ab. These results suggest that the anti-α4 Ab can be a mechanism-based adjuvantic agent that allows us to lower the dose of anticancer agents such as melphalan, thereby reducing the adverse effects of anticancer agents in the treatment of multiple myeloma.
In accordance with the suppression of 5TGM1/luc myeloma development in bone marrow, our results also show that the anti-α4 Ab increases apoptosis in 5TGM1/luc myeloma cells in bone. Consistent with our results, an anti-α4 antibody is shown to cause apoptosis in B cells by disrupting the interactions of B cells with follicular dendritic cells.27 It is, therefore, likely that the interactions between myeloma cells and marrow stromal cells mediated via α4β1 integrin and VCAM-1 also contribute to cell survival of B-cell lineages including myeloma.
The intracellular mechanism by which the ligand-receptor interactions between VCAM-1 and α4β1 integrin alter the growth in bone marrow, osteoclastogenic factor production and apoptosis of 5TGM1 myeloma cells is unknown. Integrins do not have intrinsic enzymatic activity that causes an activation of cytoplasmic signaling pathways. However, ligand-integrin interactions generate outside-in signals and activate nonreceptor protein tyrosine kinases including focal adhesion kinase (FAK), which subsequently propagates mitogen-activated protein (MAP) kinase pathways.28,29 FAK is shown to suppress p53-mediated apoptosis and promote cell survival,30 and MAP kinase pathways are known to mediate cell proliferation.31 Whether these signaling molecules are involved in the regulation of 5TGM1/luc myeloma cell behaviors upon contact with marrow stromal cells needs to be elucidated.
The α4β1 integrin, which is a major integrin expressed in myeloma cells,6 is also shown to play a role in distant metastasis of other malignant cells.32 Okahara et al12 reported that an anti-α4 antibody inhibited pulmonary metastasis of melanoma cells. Matsuura et al33 have shown that an introduction of α4β1 integrin into CHO cells renders them to preferentially metastasize to bone and that an anti-VCAM-1 antibody selectively inhibits bone metastases. From these results, the authors suggest that the interactions between α4β1 integrin expressed in tumor and VCAM-1 expressed in marrow stromal cells play a specific role for bone metastases. Our results suggest that the interactions are critical to the development of not only solid tumors but also myeloma cells in bone.
In conclusion, our results show that the anti-α4 integrin antibody has beneficial effects in animals bearing 5TGM1/luc myeloma. Disruption of the interactions between α4β1 integrin and VCAM-1 using anti-α4 integrin antibody may be a unique and specific therapeutic approach for multiple myeloma and associated osteolytic bone disease.
Prepublished online as Blood First Edition Paper, May 11, 2004; DOI 10.1182/blood-2004-01-0236.
Supported by grants from the National Institutes of Health (grants PO1-CA40035, RO1-AR28149, and RO1-DK45229).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Dr Roy R. Lobb for helpful discussion and generous gifts of rsVCAM-1 and anti-α4 integrin antibody.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal