Abstract
We evaluated a treatment strategy targeting both lymphoma cells (by rituximab) and the microenvironment (by thalidomide) in 16 patients with relapsed/refractory mantle cell lymphoma (MCL). Rituximab was administered at 375 mg/m2 for 4 weekly doses concomitantly with thalidomide (200 mg daily, with a dose increment to 400 mg on day 15), which was continued as maintenance therapy until progression/relapse. Thirteen patients (81%) experienced an objective response, with 5 complete responders (31%). Median progression-free survival (PFS) was 20.4 months (95% confidence interval [CI], 17.3-23.6 months), and estimated 3-year survival was 75%. In patients achieving a complete response, PFS after rituximab plus thalidomide was longer than PFS after the preceding chemotherapy. Severe adverse events included 2 thromboembolic events and 1 grade IV neutropenia associated with thalidomide. Our results suggest that rituximab plus thalidomide has marked antitumor activity in relapsed/refractory MCL and a low toxicity profile, which warrants further evaluation in MCL.
Introduction
Mantle cell lymphoma (MCL), which represents a distinct clinicopathologic entity among the non-Hodgkin lymphomas, is characterized by a low response rate to and short progression-free survival (PFS) after conventional chemotherapy.1,2 Administration of high-dose therapy with autologous stem cell rescue has also not resulted in long-term disease-free survival of patients with MCL,1,3,4 emphasizing the need for novel treatment strategies for this lymphoma entity. It has been recognized that stromal cells deliver important stimuli for growth and survival of normal and malignant B cells.5,6 Interactions between tumor and stromal cells can be modulated by agents like thalidomide and proteasome inhibitors. Activity of thalidomide has already been demonstrated in B-cell malignancies, in particular multiple myeloma7,8 and Waldenström macroglobulinemia.9 In a patient with heavily pretreated and rapidly progressive MCL, we have observed stabilization of the disease for a period of 6 months following administration of thalidomide (J.D., unpublished observation, September 2000). We, therefore, hypothesized that a treatment strategy targeting both lymphoma cells and the microenvironment could be active in MCL. We evaluated this treatment approach in patients with MCL pretreated with chemotherapy and combined thalidomide with rituximab, an antibody with documented efficacy in MCL.10
Study design
Patient characteristics
All 16 patients (Table 1) enrolled into this phase 2 protocol had already been treated by CHOP (cyclophosphamide, hydroxydaunorubicin, Oncovin, prednisone; n = 14) or a CHOP-like regimen (n = 2). Fifteen patients were at relapse (7 patients after 2 or more prior regimens), 1 patient was primary refractory to CHOP. Two patients had prior high-dose chemotherapy with autologous transplantation, and 1 patient underwent an allogeneic stem cell transplantation after reducedintensity conditioning. Three patients had prior rituximab (single-agent rituximab at relapse in 2 patients, and rituximab plus CHOP [R-CHOP] as induction treatment in 1 patient). Median time between initial diagnosis of MCL and initiation of study treatment was 21 months (range, 4-52 months). Each patient gave written informed consent prior to study inclusion.
Dose of thalidomide actually administered in patients with MCL
. | . | . | . | Thalidomide dose, mg . | . | |
|---|---|---|---|---|---|---|
| Patient . | Age, y* . | IPI . | Response . | Maximum . | During maintenance . | |
| 1 | 56 | Intermediate-low | PR | 400 | 200 (12 wk) → 100 (26 wk) → 50 (88 wk, continued during retreatment) | |
| 2 | 68 | Intermediate-high | PR | 400 | 400 (8 wk) → 300 (4 wk) → 200 (47 wk) → 100 (19 wk, continued during retreatment) | |
| 3 | 50 | Low | CR | 400 | 400 (70 wk) → 200 (8 wk, continued during retreatment) | |
| 4 | 70 | Intermediate-high | PR | 400 | 400 (8 wk) → 300 (8 wk; discontinued because of neutropenia) | |
| 5 | 74 | Intermediate-high | CR | 400 | 400 (8 wk) → 300 (15 wk) → 200 (32 wk) → 100 (36 wk) | |
| 6 | 65 | Intermediate-high | CRu | 200 | 200 (36 wk) → 100 (42 wk) → 50 (22 wk; discontinued because of neuropathy) | |
| 7 | 64 | Intermediate-high | PR | 200 | 200 (3 wk) → 100 (83 wk) | |
| 8 | 70 | Intermediate-low | SD | 400 | 400 (8 wk) → 200 (15 wk) | |
| 9 | 74 | Intermediate-low | CR | 200 | 200 (20 wk) → 100 (60 wk) → 50 (55+ wk) | |
| 10 | 71 | High | PR | 200 | 200 (36 wk) → 100 (20 wk) | |
| 11 | 62 | Intermediate-high | PR | 200 | 200 (32 wk) → 100 (44+ wk) | |
| 12 | 45 | Low | CR | 200 | 100 (8 wk) → 50 (44+ wk) | |
| 13 | 76 | High | NR | 200 | 100 (8 wk) | |
| 14 | 64 | Intermediate-low | PR | 200 | 200 (20 wk) | |
| 15 | 72 | Intermediate-low | NR | 200 | 200 (8 wk) | |
| 16 | 61 | High | PR | 200 | 200 (12+ wk) | |
. | . | . | . | Thalidomide dose, mg . | . | |
|---|---|---|---|---|---|---|
| Patient . | Age, y* . | IPI . | Response . | Maximum . | During maintenance . | |
| 1 | 56 | Intermediate-low | PR | 400 | 200 (12 wk) → 100 (26 wk) → 50 (88 wk, continued during retreatment) | |
| 2 | 68 | Intermediate-high | PR | 400 | 400 (8 wk) → 300 (4 wk) → 200 (47 wk) → 100 (19 wk, continued during retreatment) | |
| 3 | 50 | Low | CR | 400 | 400 (70 wk) → 200 (8 wk, continued during retreatment) | |
| 4 | 70 | Intermediate-high | PR | 400 | 400 (8 wk) → 300 (8 wk; discontinued because of neutropenia) | |
| 5 | 74 | Intermediate-high | CR | 400 | 400 (8 wk) → 300 (15 wk) → 200 (32 wk) → 100 (36 wk) | |
| 6 | 65 | Intermediate-high | CRu | 200 | 200 (36 wk) → 100 (42 wk) → 50 (22 wk; discontinued because of neuropathy) | |
| 7 | 64 | Intermediate-high | PR | 200 | 200 (3 wk) → 100 (83 wk) | |
| 8 | 70 | Intermediate-low | SD | 400 | 400 (8 wk) → 200 (15 wk) | |
| 9 | 74 | Intermediate-low | CR | 200 | 200 (20 wk) → 100 (60 wk) → 50 (55+ wk) | |
| 10 | 71 | High | PR | 200 | 200 (36 wk) → 100 (20 wk) | |
| 11 | 62 | Intermediate-high | PR | 200 | 200 (32 wk) → 100 (44+ wk) | |
| 12 | 45 | Low | CR | 200 | 100 (8 wk) → 50 (44+ wk) | |
| 13 | 76 | High | NR | 200 | 100 (8 wk) | |
| 14 | 64 | Intermediate-low | PR | 200 | 200 (20 wk) | |
| 15 | 72 | Intermediate-low | NR | 200 | 200 (8 wk) | |
| 16 | 61 | High | PR | 200 | 200 (12+ wk) | |
IPI indicates International Prognostic Index; SD, stable disease; and NR, no response.
Age at the time of initiation of study treatment.
Treatment regimen
Treatment with rituximab consisted of 4 weekly doses at 375 mg/m2, with standard premedication (diphenhydramine and paracetamol). Thalidomide was started on the evening of day 1 (scheduled daily dose, 200 mg during the first 2 weeks, then 400 mg) and continued as maintenance treatment after completion of rituximab until progression or relapse. The study protocol was approved by the institutional ethics committee.
Toxicity was assessed weekly during the first month of treatment and thereafter once a month during thalidomide-maintenance with use of standard National Cancer Institute common toxicity criteria. Response to treatment was assessed according to the International Workshop Response Criteria Guidelines,11 with restaging every 3 months. PFS and overall survival (OS) was estimated by the method of Kaplan and Meier.
Results and discussion
Response
Objective responses to rituximab plus thalidomide (R + T) were achieved in 13 of the 16 patients (overall response rate, 81%; 95% CI, 59.8%-102.7%), 1 patient experienced stable disease. Five patients (31%; 95% CI, 5.7%-56.8%) achieved a complete response (CR; 4 CR, 1 unconfirmed/uncertain complete remission [CRu]), including the patient with primary CHOP-resistance and 1 patient at relapse after autologous transplantation. Eight patients (50%; 95% CI, 22.5%-77.5%) had a partial response (PR); among them was the patient after allogeneic transplantation with reduced-intensity conditioning. Responses were observed both at nodal and extranodal manifestations of MCL (remission of gastrointestinal manifestations in 5 patients, disappearance of a lymphoma in the breast in 1 patient). In responding patients, shrinkage of peripheral lymph nodes and/or significant improvements of laboratory parameters (clearance of lymphoma cells in the peripheral blood in 3 patients, hematologic improvement, reduction of elevated serum levels of lactate dehydrogenase [LDH]) were noted during the first month of treatment.
Among the 13 responders, 8 patients experienced a relapse or progressive disease. In 3 of them, 1 course of rituximab (4 weekly infusions at 375 mg/m2) was again added to thalidomide as reinduction treatment. Second remissions were induced in 2 of them, and PFS was 18+ months (versus 20 months after the first administration of R + T) and 13 months (versus 30 months), respectively.
Progression-free and overall survival
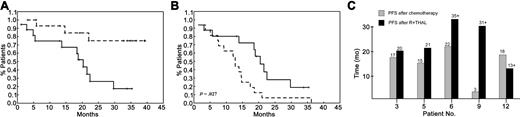
Median PFS of the 16 patients was 20.4 months (95% CI, 17.6-23.6 months; Figure 1), as opposed to 12.7 months after the line of chemotherapy preceding R + T. Improvement of PFS after R + T was particularly evident among the 5 patients achieving a CR (Figure 1C). Median OS has not yet been reached, with 13 patients alive at the time of analysis (February 22, 2004) and an estimated 3-year survival of 75%.
Progression-free survival. (A) Progression-free (median, 20.4 months; solid line) and overall survival (dashed line) of patients with relapsed/refractory MCL from the time of initiation of rituximab plus thalidomide. (B) Progression-free survival after rituximab plus thalidomide (median, 20.4 months; solid line) was significantly longer compared with progression-free survival after the previous line of chemotherapy (median, 12.7 months; dashed line) (P = .027). (C) Progression-free survival (PFS) of patients with MCL achieving a CR after rituximab plus thalidomide (▪) compared with PFS after the previous line of chemotherapy (▦).
Progression-free survival. (A) Progression-free (median, 20.4 months; solid line) and overall survival (dashed line) of patients with relapsed/refractory MCL from the time of initiation of rituximab plus thalidomide. (B) Progression-free survival after rituximab plus thalidomide (median, 20.4 months; solid line) was significantly longer compared with progression-free survival after the previous line of chemotherapy (median, 12.7 months; dashed line) (P = .027). (C) Progression-free survival (PFS) of patients with MCL achieving a CR after rituximab plus thalidomide (▪) compared with PFS after the previous line of chemotherapy (▦).
Toxicity
It was assumed that combination of R + T should not lead to added toxicity, which was confirmed during the course of this trial. All patients completed the scheduled 4 infusions of rituximab. Infusional reactions to rituximab, consisting of fever, rigors, and chills (grades I-II), developed in 7 patients (44%), mainly during the first application, but no other adverse events (grade II or greater) were encountered.
Fatigue, somnolence, and constipation were common, dose-dependent side effects of thalidomide; therefore, the planned dose of 400 mg daily could only be achieved in 6 patients, and it was necessary to adapt the maintenance dose of thalidomide on an individual basis (Table 1). A daily dose of 50 to 200 mg could be maintained with only limited toxicity, which was also reported by Damaj et al.12 Peripheral neuropathy (grades I-II) occurred in 7 patients (44%). Because of neuropathy, 1 patient discontinued thalidomide after 30 months. Grade IV neutropenia associated with thalidomide was observed in 1 patient, and his neutrophil counts recovered after discontinuation of thalidomide. As previously reported, there were 2 events of venous thromboembolism13 (1 deep-vein thrombosis 5 weeks after initiation of therapy, and one asymptomatic pulmonary embolism at routine computed tomography [CT] scanning during follow-up).
The efficacy of R + T is clearly beyond that of rituximab alone (35% remission rate of single-agent rituximab in patients with MCL, with virtually no CR and a median PFS of 12 months),10,14,15 and its activity compares favorably with results of other salvage strategies in MCL. For example, among 21 evaluable patients with relapsed MCL, the recently reported R-FCM (rituximab, fludarabine, cyclophosphamide, mitoxantrone) regimen induced remissions in 13 patients (62%), with a CR in 7 patients (33%).16 As recently published in 2 case reports,12,17 thalidomide has single-agent activity in relapsed MCL, and we can now assume that it may be additive to or synergistic with rituximab. Thalidomide is known to have pleiotropic effects,18 which mainly exert an indirect effect on tumor cells by modulating cytokine secretion19 and expression of adhesion molecules,20 and by enhancing the activity of natural killer (NK) cells and cytotoxic T lymphocytes.21,22 It is worth noting that rituximab can induce cytotoxic T-cell responses against lymphoma-associated antigens in vitro,23 an effect that could be further enhanced by the immunomodulatory activity of thalidomide. Finally, effects of thalidomide on the microenvironment also include inhibition of angiogenesis.24 Further studies are required to delineate the precise mechanism of action of thalidomide in MCL.
Despite the limited number of patients, our study provides evidence for promising antitumor activity and a low toxicity profile of R + T in patients with relapsed/chemotherapy refractory MCL. The observation of durable remissions warrants further evaluation in an attempt to improve the overall grim prognosis in MCL. We are, therefore, studying rituximab plus CHOP plus thalidomide as induction treatment, followed by thalidomide maintenance, in patients with previously untreated MCL.
Prepublished online as Blood First Edition Paper, May 27, 2004; DOI 10.1182/blood-2004-03-1091.
Supported in part by grants from CLEXO (Excellence Center of Clinical and Experimental Oncology at the University Hospital Vienna) and the Lymphoma Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr K. Zwingenberger (Gruenenthal GmbH, Aachen, Germany) for providing thalidomide for this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal