Abstract
Effective targeting of vectors to tumor cells that have metastasized to multiple different tissue sites remains a major challenge for gene therapy. Tumor-specific cytotoxic T lymphocytes (CTLs) have been shown in animal models and in humans to be able to cross tissue barriers and traffic to tumor cells. However, their capacity to eliminate malignancy has been limited by tumor immune evasion strategies. We now use a model of Epstein-Barr virus–mediated malignancy to show that human CTLs themselves may be modified to release therapeutic vectors following engagement of their antigen-specific receptors and that these vectors will effectively transduce and destroy tumor targets. We generated EBV-specific CTLs that were transgenic for the adenoviral E1 gene under the control of the cell activation-dependent CD40 ligand (CD40L) promoter. Following transduction with E1-deficient adenoviral vectors, these CTLs produced infectious virus when exposed to HLA-matched EBV-expressing targets, but not on exposure to major histocompatibility complex (MHC)–mismatched or otherwise irrelevant cells. This approach provides a means of delivering oncolytic/therapeutic vectors not only to locally accessible macroscopic tumors as is presently the case, but also to disseminated metastatic disease, while avoiding the risks associated with systemic administration of large doses of adenoviral vectors.
Introduction
Although gene transfer has shown promise for the therapy of cancer, a major constraint on its effectiveness has been the poor targeting of current vectors. While much has been accomplished in modifying the native target specificities of vectors, the fundamental problem remains that many human malignancies are metastatic. Consequently, they are distributed in many different tissues and tissue planes. Appropriate retargeting of a vector to allow it to traverse all such planes and effectively enter tumor cells regardless of the tissues in which they reside is a daunting task. By contrast, tumor antigen–specific cytotoxic T lymphocytes (CTLs) have been shown in a number of preclinical and clinical models to be highly effective at infiltrating tumor sites in a multiplicity of organs.1-5 However, while CTLs may eradicate some types of experimental and natural tumors, it has become evident that many malignancies exhibit a range of immune evasion mechanisms that diminish the effectiveness of attack.6-9 If it were possible to combine the trafficking and specific targeting of CTLs with tumor-localized delivery of a lethal viral vector, it might be possible to compensate for some of the deficiencies of each system alone.
When T cells engage their antigen-specific receptors, a complex series of activation events occurs, and a number of genes are expressed that are otherwise silent. Among these is the ligand for the CD40 molecule (CD40L or CD154), the expression of which can induce maturation of CD40+ dendritic cell and B lymphocytes. The CD40L promoter is tightly regulated by the AT hook transcription factor AKNA, which is expressed only transiently following antigen-mediated T-cell activation.10 We reasoned that if this promoter were used to drive the adenoviral E1 gene, expression would occur only after the T cell encountered its target.
If the T cell also contained an E1-deleted adenoviral vector, production should be similarly enhanced after antigen encounter. Because T and lymphoblastoid cell line (LCL) cells have low expression of the Coxsackie adenoviral receptor (CAR) required for uptake of conventional Ad5 vectors,11-14 we used a chimeric adenoviral vector in which the fiber protein of Ad5 is substituted by the fiber of Ad35.15,16 This Ad5F35 vector is CAR independent and transduces human T cells.15 To investigate the feasibility of this approach, we used the model of Epstein-Barr virus (EBV)–mediated B-cell immunoblastic lymphoma, in which the function of the specific CTLs and the antigens they target has been extensively characterized ex vivo and in vivo.4,17-20
We show that CTLs transgenic for E1 controlled by the CD40L promoter retain their normal functionality but will in addition produce infectious virus after exposure to EBV antigens on major histocompatibility complex (MHC)–matched target cells. The close proximity to the intended target cell is able to produce high levels of target cell transduction, even in cells such as EBV-infected B lymphocytes that are normally resistant to adenoviral transduction. By incorporating a gene such as herpes simplex virus thymidine kinase (HSV-Tk) into the CTL-delivered adenovector, it is possible to increase target cell killing beyond that produced by the CTL alone.
Materials and methods
Cell lines
The HEK293 (human embryonic kidney), A459 (human lung cancer), and cell lines were purchased from ATCC (American Type Culture Collection, Manassas, VA), as was PG-13, the amphotropic retrovirus packaging cell line that produces virus pseudotyped with the gibbon-ape leukemia virus (GALV). Epstein-Barr virus lymphoblastoid cell lines (EBV-LCLs) were obtained by B95-8–derived Epstein-Barr virus immortalization of B lymphocytes isolated from the blood of healthy donors.21
HEK293, A549, PG-13, and 293 T cells were maintained in Dulbecco modified Eagle medium (DMEM; BioWhittaker, Walkersville, MD), while EBV-LCLs were cultured in RPMI-1640 (BioWhitttaker). All cells were grown in their specific medium supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT), 100 unit/mL penicillin, and 100 μg/mL streptomycin (Life Technologies, Gaithersburg, MD) at 37° C, 5% CO2 in a humidified incubator.
Production of retroviral vectors
The CD40L promoter was extracted from the gfp.5′hcd4oL plasmid22 and subcloned into the retroviral vector SFG (provided by R. C. Mulligan, Cambridge, MA). The E1 gene (Ela, Elb) was polymerase chain reaction amplified and subcloned into the SGF-CD40Lpr, which was used to transfect Phoenix or 293T cells by calcium phosphate precipitation.
Fresh retroviral supernatants were collected from transiently transfected Phoenix-eco or 293T cells and used to infect the packaging cell lines PG13 (in the presence of polybrene [8 μg/mL] 4 times for 24 hours at 37° C). High titer retroviral supernatants (> 5 × 105/mL) were obtained from PG-13 cultures.
Production of adenovectors
We used an Ad5 or a chimeric adenovirus Ad5/F35 described previously.16 Both viruses have the identical E1/E3-deleted backbone structure and contain in their E3 region the gene of interest driven by the cytomegalovirus (CMV) promoter. In Ad5F35, the native Ad5 fiber gene is substituted by parts of the fiber from Ad35. All viruses were produced by calcium phosphate transfection of HEK293 cells. Ad5F35-TK and Ad5/F35-GFP viruses were prepared by expansion of a single plaque generated in transfected HEK293. The cells were harvested and pelleted when 90% or more of cells showed cytopathic effects. Virus was extracted by 3 consecutive freeze/thaw cycles and amplified by transduction of a larger culture of HEK293 cells. The viruses were purified on 2 series of cesium chloride gradient ultracentrifugation and desalted on an EconoPac 10DG exclusion column (Bio-Rad Laboratories, Hercules, CA). The infectious unit (IU) titer of the large-scale virus preparation was established by plaque assay using HEK293 cells. Preparations were routinely tested for replication-competent adenovirus (RCA) by plaquing on A549 cells, and all were less than 1 RCA/1010 vp. Viral titers were also quantitated by optical density 260, and the particle to plaque-forming unit ratios were 100:1 and 40:1 for Ad5F35-GFP and Ad5/F35-TK, respectively. Except where stated, cells were transduced at 37° C with 103 vp per cell in Opti-minimal essential medium (MEM) (BioWhittaker) medium. At 6 hours after transduction, the cells were washed in 4 mL phosphate-buffered saline (PBS) and resuspended in fresh medium supplemented with 10% FCS. Cells were analyzed for transgene expression and viability at 24 to 48 hours after transduction.
Generation of EBV-specific cytotoxic T lymphocytes (CTLs)
EBV-specific CTLs were prepared by stimulating PBMCs with the autologous EBV-transformed LCLs.21 PBMCs (2 × 106) were cocultured with 5 × 104 gamma-irradiated (40 Gy) autologous LCLs per well in a 24-well plate. Starting on day 10, the responder cells were restimulated weekly with irradiated (40 Gy) LCLs at a responder to stimulator ratio of 4:1. There were 2 weekly doses of recombinant human interleukin 2 (rhIL-2, 50 IU/mL) added from day 14.
Transduction of CTLs
By the retrovirus. At 24 hours after LCL stimulation, CTLs ready for transduction were transferred to a 24-well plate (Costar), precoated with OKT3 (1 μg/mL; Ortho Pharmaceuticals, Raritan, NJ) and anti-CD28 antibody (1 μg/mL; Pharmingen, San Diego, CA) at 1 × 106 cells per well, and incubated for 48 hours for optimal activation before transduction. Transductions were carried out in 24-well non–tissue-culture–treated plates (BD Bioscience, Franklin Lakes, NJ) coated with recombinant fibronectin fragment (FN CH-296, Retronectin; Takara Shuzo, Otsu, Japan) at a concentration of 4 μg/cm2. The stimulated CTL lines were resuspended at 1 × 106 cells/mL in complete medium supplemented with 45% EHAA (Clicks; GIBCO-BRL, Grand Island, NY) and rhIL-2 (100 IU/mL), and then incubated with equal volumes of freshly generated retrovirus viral supernatant for 36 hours at 37° C and 5% CO2. To increase the efficiency of transduction, 2 to 3 rounds of transduction were performed. The transduced CTLs (C-RE) were subsequently maintained in Yssel medium (Gemini Biological Products, Calabasas, CA) supplemented with 10% serum and stimulated with LCLs and IL-2 as described in “Generation of EBV-specific cytotoxic T lymphocytes (CTLs).”
By the Ad5F35 adenovirus. Except where stated, cells were transduced at 37° C with 103 vp per cell in Opti-MEM medium. At 6 hours after transduction, the cells were washed in 4 mL PBS and resuspended in fresh medium supplemented with 10% FCS. Cells were analyzed for transgene expression and viability at 24 to 48 hours after transduction.
Cytotoxicity assays
To compare the cytotoxic specificity of latent adenovector transduced and nontransduced CTLs, standard 51Cr release assays were performed at 72 to 96 hours after transduction. Dilutions of CTLs were coincubated in triplicate for 4 hours with 51Cr-labeled target cells (Amersham Pharmacia Biotech, Piscataway, NJ) in a total volume of 200 μL in a 96-well plate (Costar, Cambridge, MA) as previously described. The targets tested were autologous LCLs, HLA class I and II mismatched LCLs, and the EBV-negative HSB-2 cell line used to detect any lymphokine activated killer activity. Target cells incubated in RPMI 1620 alone or in 5% Triton X-100 (Sigma, St Louis, MO) were used to determine spontaneous and maximum 51Cr release, respectively. At the end of a 4-hour incubation period at 37° C and 5% CO2, supernatants were harvested, and 51Cr release was measured on a gamma counter (Tri-CARB 4640; Packard BioScience, Downers Grove, IL). The mean percentage of specific lysis of triplicate wells was calculated as follows: [(test counts spontaneous counts)/(maximum counts spontaneous counts)] × 100%.
Flow cytometry
CD19, CD20, CD3, and CD45 monoclonal antibodies (MAbs) were purchased from BD Bioscience (Mountain View, CA). Cells were washed and stained with the appropriate fluorescein isothiocyanate (FITC)–, R-phycoerythrin (PE)–, or peridinin chlorophyll protein (PercP)–conjugated antibodies for 20 minutes at 4° C in the dark in phosphate-buffered saline (PBS) supplemented with 0.1% bovine serum albumin (BSA). Control cells were stained with FITC, PE, or PercP-conjugated immunoglobulin G (IgG) control isotype; unstained cells were used as control for green fluorescent protein (GFP). After incubation, the cells were washed twice and resuspended in PBS. Dead cells were excluded from analysis by using propidium iodide (PI). GFP expression was measured using a standard filter setup for fluorescein (525 nm, bandpass filter). For intracellular immunofluorescence analysis of E1a expression in retrovirally transduced T cells, cells were incubated with serum to block nonspecific binding and fixed with 4% paraformaldehyde for 20 minutes at 4° C. The cells were washed and permeabilized with fresh saponin (0.5%) for 20 minutes at room temperature (RT), then washed in PBS 2% fetal calf serum (FCS; Hyclone) for 20 minutes at room temperature (RT). Cells were washed and incubated with the monoclonal antibody specific for adenovirus E1a (M58; Biomeda, Foster City, CA), diluted in PBS, 2% FCS, and 0.1% saponin for 30 minutes at RT, and washed 3 times before incubation with a secondary anti–mouse FITC-labeled antibody (Santa Cruz Biotechnology, Santa Cruz, CA). For the negative controls, cells were incubated with the second antibody alone for 30 minutes at RT, or with an isotype control and then the secondary FITC antibody. The cells were washed 3 times with the permeabilizing solution and resuspended in PBS and 0.2% FCS and analyzed immediately by flow cytometry.
Western blots
CTLs were transduced with the SFG retrovirus as described above; 4 × 106 of LCL-activated or nonactivated CTLs were lysed with 2 X sample buffer (125 μM Tris [tris(hydroxymethyl)aminomethane]–HCl [pH 6.8], 4% [wt/vol] sodium dodecyl sulfate, 20% glycerol, 0.05% [wt/vol] bromophenol blue, and β-mercaptoethanol [10% vol/vol]). After sonication, the lysates were centrifuged at 10 000g for 10 minutes. Protein concentrations were determined by commercial assay (Bio-Rad, Hercules, CA). A 293 cell lysate was used as the positive control for E1 detection. Proteins were separated on a Novex Tris-Glycine gel 4% to 20% (Invitrogen, Carlsbad, CA) under denaturing conditions and transferred to a supported nitrocellulose membrane (BioRad). Adenovirus E1 was detected using a monoclonal antibody specific for E1a (M58; Biomeda) and a Western Breeze chemiluminescent kit (Invitrogen). The secondary anti–mouse IgG antibody was conjugated with alkaline phosphatase. The signal was detected by incubation with CDP-star chemiluminescent substrate and developed on Hyperfilm (Amersham Pharmacia Biotech).
CTL proliferation
CTLs were plated at 1 × 106 cells per well, maintained in fresh medium (Yssel medium; Gemini Biological Products) supplemented with FCS 10%, and stimulated with EBV-LCLs + IL-2 as described for CTL cultures. The control cells were cultured in fresh medium + 10% FCS. The cell viability and count were determined by trypan blue exclusion on alternate days.
Infectious particle production by CTLs
CTLs (107 of each cell line) were transduced with Ad5F35-GFP (1000 vp/cell) for 6 hours and then washed and cultured in fresh medium. At 24 hours after transduction, cells were washed 5 times in PBS to remove free particles and resuspended in fresh medium. Duplicate supernatant samples (or lysates from freeze-thawed T cells) were used to transduce A549 cells in 200 μL Opti-MEM at 37° C. A459 cells were analyzed for GFP expression 24 hours after transduction. In some experiments, CTLs were cocultured with LCLs at an E/T ratio of 1:80; no IL-2 was added the day of the coculture, and the cells were stained with CD20 MAb and analyzed by fluorescence-activated cell sorter (FACS) 48 hours later.
Measurement of apoptosis
CTLs were cocultured with irradiated LCLs and analyzed for apoptosis using a BD Bioscience kit (BD Bioscience, Mountain View, CA). The cocultured cells were stained with CD3, 7AAD, and Annexin-V according to the manufacturer's instructions.
Measurement of cytokine release after exposure to adenoviral vectors
To detect the production of inflammatory cytokines by transduced CTLs, the cells were exposed to 5 × 103 vp/mL adenovirus (Ad5 or Ad5F35) and incubated in Opti-MEM in 48-well plates at 2 × 105 cells/well. Supernatants were harvested at 6, 12, 24, 48, 72, and 96 hours and analyzed using the Cytometric Bead Array (BD Bioscience human inflammation kit, BD Bioscience, Mountain View, CA). Transduced human peripheral blood mononuclear cells (PBMNCs) obtained from healthy donors were used as positive controls, while uninfected CTLs and PBMNCs were used to measure background cytokine release.
Statistical analysis
All data are presented as means ± SD of multiple replicate experiments. Intergroup differences were analyzed using paired t testing. P values of .05 and below were taken as significant.
Results
Preparation of retrovirally transduced advector producer T cells
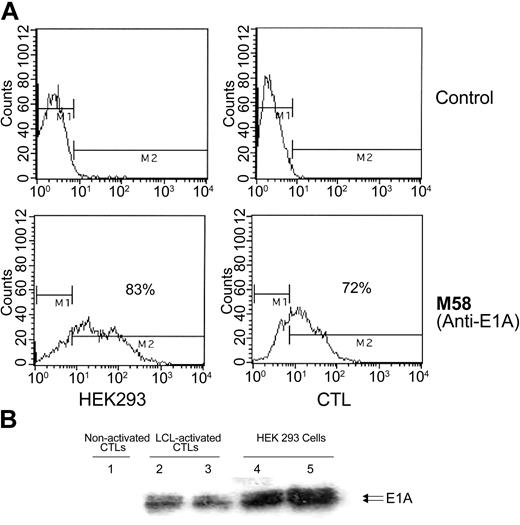
Figure 1 shows the schema for generating EBV-CTLs capable of producing adenovectors following T-cell receptor (TCR) engagement. We first transduced the CTLs with a Moloney-derived SFG retrovirus containing the adenovirus E1 gene driven by the CD40L promoter (C-RE cells). To determine the percentage of CTL transduction by the retrovirus, the cell lines were analyzed by intracellular immunofluorescence using a monoclonal antibody specific to E1a. 293 cells were used as a positive control and nontransduced CTLs, as negative control. Less than 5% of C-RE cells were E1 positive before target cell stimulation, but 32% to 70% of the C-RE expressed the E1a adenovirus protein within 24 hours of exposure to LCLs (Figure 2A). E1a protein production was confirmed by Western blot analysis of activated and nonactivated C-RE lysates. E1a protein production required CTL activation by EBV-LCLs (Figure 2B), and coculture of EBV-CTLs with an EBV-negative cell target failed to induce E1 protein (data not shown).
Generation of genetically modified EBV-specific cytotoxic T lymphocytes (CTLs). Peripheral blood mononuclear cells were used to generate both EBV-LCLs and EBV-specific CTLs. The CTLs were then transduced with an SFG retrovirus containing the human CD40L promoter driving the adenovirus E1 gene. These E1-transduced cells (C-RE) are then transduced with an Ad5F35 adenovector. Following activation by autologous EBV-LCLs, these CTLs (C-RE-AG) are activated and release infectious adenovectors. LTR indicates long terminal repeat.
Generation of genetically modified EBV-specific cytotoxic T lymphocytes (CTLs). Peripheral blood mononuclear cells were used to generate both EBV-LCLs and EBV-specific CTLs. The CTLs were then transduced with an SFG retrovirus containing the human CD40L promoter driving the adenovirus E1 gene. These E1-transduced cells (C-RE) are then transduced with an Ad5F35 adenovector. Following activation by autologous EBV-LCLs, these CTLs (C-RE-AG) are activated and release infectious adenovectors. LTR indicates long terminal repeat.
E1 protein is detected in modified CTLs after specific activation. (A) Immunofluorescence: C-RE (E1-transduced CTLs) and nontransduced CTLs were activated with autologous LCLs, fixed, permeabilized, and stained with an antibody specific for E1a or with an isotype control. 293 cells were used as a positive control for E1a production. Cells were analyzed by flow cytometry. (B) Western blot analysis: 4 × 106 activated E1-transduced CTLs (C-RE) were lysed and the proteins separated on a gel (lanes 2-3). M58 monoclonal antibody was used to detect the E1a protein. 293 cell extract was used as a positive control (lanes 4-5), and nonactivated CTL (nonactivated C-RE) extract was used as a negative control (lane 1). M1 indicates negative population; M2, positive population. Results are shown as the percentage of positive cells.
E1 protein is detected in modified CTLs after specific activation. (A) Immunofluorescence: C-RE (E1-transduced CTLs) and nontransduced CTLs were activated with autologous LCLs, fixed, permeabilized, and stained with an antibody specific for E1a or with an isotype control. 293 cells were used as a positive control for E1a production. Cells were analyzed by flow cytometry. (B) Western blot analysis: 4 × 106 activated E1-transduced CTLs (C-RE) were lysed and the proteins separated on a gel (lanes 2-3). M58 monoclonal antibody was used to detect the E1a protein. 293 cell extract was used as a positive control (lanes 4-5), and nonactivated CTL (nonactivated C-RE) extract was used as a negative control (lane 1). M1 indicates negative population; M2, positive population. Results are shown as the percentage of positive cells.
Function of E1-transduced EBV-CTLs
To ensure that E1-transduced EBV-CTLs (C-RE) continued to expand and respond to their targets, we tested their proliferative activity against LCLs. C-RE (transduced CTLs) and nontransduced CTLs were cultured in minimum media (Yssel 10% FCS) alone or with irradiated LCL cells and IL-2. The cell counts revealed that C-RE and nontransduced CTLs proliferated only when stimulated with the LCLs + IL-2 and that the proliferation rate was the same for both cultures (Figure 3A). The nonstimulated C-RE (transduced CTLs) rapidly died in culture, showing that the cells remain under normal growth control. The C-RE remained viable in culture for at least 6 weeks when the LCLs + IL-2 were added on a weekly basis. The cytotoxic cells (C-RE) also retained their MHC-restricted cytotoxicity against autologous LCLs. As shown in Figure 3B, the retrovirally transduced CTLs lysed autologous but not allogeneic EBV-LCLs. The proportions of CD4- or CD8-expressing CTLs were unchanged by transduction. FACS analysis confirmed that both transduced and nontransduced cells were predominantly CD8+ (97% versus 98%; Figure 3C). Expression of TCRαβ, CD56, and CD25 was also unchanged (data not shown).
E1-transduced CTL function and phenotype are unchanged. (A) Normal proliferation of modified CTLs (C-RE). The graphs illustrate the expansion rate of nontransduced and transduced CTL lines (C-RE) cultured in minimum media (Yssel + 10% FCS) alone or activated with irradiated LCLs + IL-2 (50 U/mL). The fold expansion is illustrated on the y-axis. ▪ indicates activated E1-CTLs (C-RE); ▴, nonactivated E1-CTLs (C-RE); and ♦, activated nontransduced CTLs. Cell count was evaluated by trypan blue exclusion. The rate of expansion of the stimulated transduced CTL line (C-RE) was not significantly different from the stimulated nontransduced CTL line. (B) Cytotoxic effector function is unaffected by the retrovirus transduction. EBV-specific CTLs were used in a cytotoxic assay before and after transduction with the E1 retroviral vector. We used allogeneic or autologous LCL lines as targets. The results are expressed as the percentage of lysis at multiple effector-target ratios. ♦ indicates allogeneic (Allo) LCLs and nontransduced (nt) CTLs; ▪, autologous (Auto) LCLs and nontransduced CTLs; ▴, allogeneic LCLs and E1-CTLs (C-RE); and ×, autologous LCLs and E1-CTLs (C-RE). (C) CD4 and CD8 expression of E1-transduced EBV-specific CTLs (C-RE) is unchanged. Nontransduced or transduced CTLs (C-RE) were stained with monoclonal antibodies specific for CD4 (PE labeled) and CD8 (FITC labeled). PE and FITC isotype antibodies were used for controls, and the cells were analyzed by flow cytometry. Error bars indicate mean ± SD. Upper left: percentage of CD4+ cells. Lower left: control. Lower right: percentage of CD8+ cells.
E1-transduced CTL function and phenotype are unchanged. (A) Normal proliferation of modified CTLs (C-RE). The graphs illustrate the expansion rate of nontransduced and transduced CTL lines (C-RE) cultured in minimum media (Yssel + 10% FCS) alone or activated with irradiated LCLs + IL-2 (50 U/mL). The fold expansion is illustrated on the y-axis. ▪ indicates activated E1-CTLs (C-RE); ▴, nonactivated E1-CTLs (C-RE); and ♦, activated nontransduced CTLs. Cell count was evaluated by trypan blue exclusion. The rate of expansion of the stimulated transduced CTL line (C-RE) was not significantly different from the stimulated nontransduced CTL line. (B) Cytotoxic effector function is unaffected by the retrovirus transduction. EBV-specific CTLs were used in a cytotoxic assay before and after transduction with the E1 retroviral vector. We used allogeneic or autologous LCL lines as targets. The results are expressed as the percentage of lysis at multiple effector-target ratios. ♦ indicates allogeneic (Allo) LCLs and nontransduced (nt) CTLs; ▪, autologous (Auto) LCLs and nontransduced CTLs; ▴, allogeneic LCLs and E1-CTLs (C-RE); and ×, autologous LCLs and E1-CTLs (C-RE). (C) CD4 and CD8 expression of E1-transduced EBV-specific CTLs (C-RE) is unchanged. Nontransduced or transduced CTLs (C-RE) were stained with monoclonal antibodies specific for CD4 (PE labeled) and CD8 (FITC labeled). PE and FITC isotype antibodies were used for controls, and the cells were analyzed by flow cytometry. Error bars indicate mean ± SD. Upper left: percentage of CD4+ cells. Lower left: control. Lower right: percentage of CD8+ cells.
Function of E1 and advector-transduced CTLs
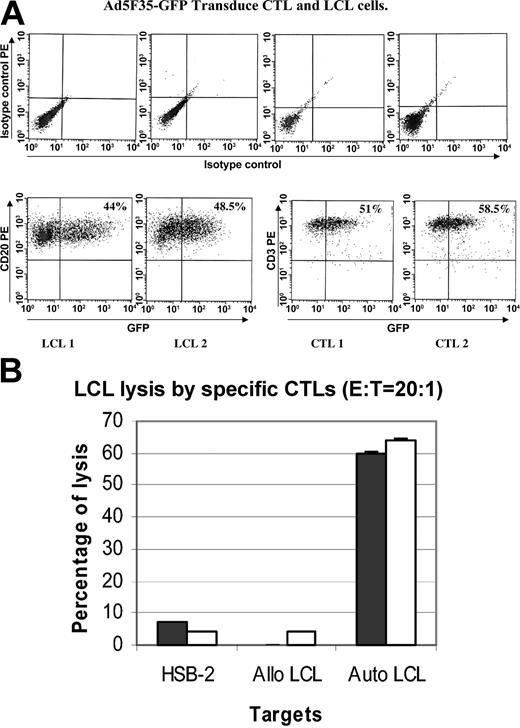
Next, the C-RE T cells were transduced with an E1-deleted adenoviral vector encoding enhanced GFP (eGFP; C-RE-AG cells). A mean of 45% of C-RE was transduced by Ad5F35 (1000 vp/mL) as measured by eGFP fluorescence (Figure 4A). Figure 4B shows that the cell population exposed to both retrovirus and Ad5F35-eGFP retains the ability to kill autologous but not allogeneic EBV-CTLs. There is no increase in killing of the EBV-negative HSB-2 target cell line.
Function of E1-CTLs following transduction with adenovectors. (A) There were 2 CTL and LCL cell lines transduced for 6 hours with Ad5/F35-GFP using 1000 vp per cell. At 24 to 48 hours after infection, the cells were stained with a PE-CD3 or PE-CD20 antibody and analyzed by FACS. The results are expressed as the percentage of cells expressing CD3 and GFP. Ad5F35 transduced 51% to 58.5% of the CTLs and 44% to 48.5% of the LCLs. (B) The killing activity of CTLs is not changed by double transduction. The cytotoxic activity of doubly transduced CTLs (C-RE-AG, □) was compared with nontransduced CTLs (▪). The graph shows the percentage of specific lysis at an effector to target ratio of 20:1 against autologous LCLs, allogeneic LCLs, and a complete HLA class I mismatch control target line (H-2B). Measurement at other ratios (40:1 and 10:1) was also unchanged. Results are shown as mean ± SD.
Function of E1-CTLs following transduction with adenovectors. (A) There were 2 CTL and LCL cell lines transduced for 6 hours with Ad5/F35-GFP using 1000 vp per cell. At 24 to 48 hours after infection, the cells were stained with a PE-CD3 or PE-CD20 antibody and analyzed by FACS. The results are expressed as the percentage of cells expressing CD3 and GFP. Ad5F35 transduced 51% to 58.5% of the CTLs and 44% to 48.5% of the LCLs. (B) The killing activity of CTLs is not changed by double transduction. The cytotoxic activity of doubly transduced CTLs (C-RE-AG, □) was compared with nontransduced CTLs (▪). The graph shows the percentage of specific lysis at an effector to target ratio of 20:1 against autologous LCLs, allogeneic LCLs, and a complete HLA class I mismatch control target line (H-2B). Measurement at other ratios (40:1 and 10:1) was also unchanged. Results are shown as mean ± SD.
Vector released from CTLs infects tumor targets
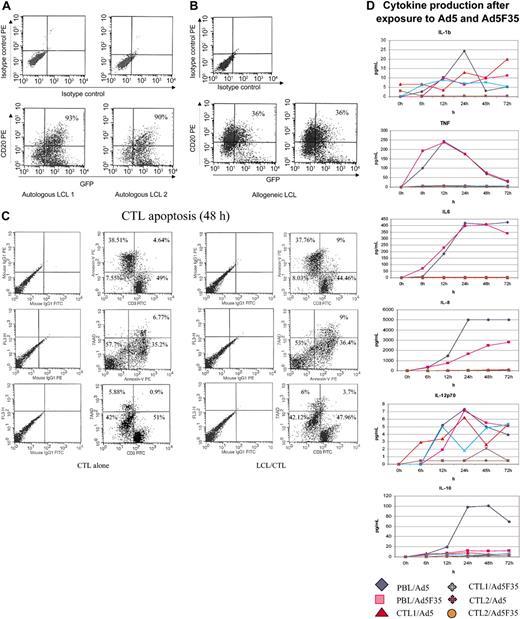
Exposure to specific antigen allowed the C-RE-AG cells to produce infectious vector that was able to infect the stimulating target cells—in this case EBV-LCLs. Figure 5 shows the percentage of autologous (Figure 5A) and allogeneic LCLs (Figure 5B) infected after exposure to C-RE-AG cells. The level of transduction is greater in MHC-identical (90%-93%) than in partially MHC-mismatched LCL target cells (31%-36%), indicating that MHC-restricted activation of CTLs through their antigen-specific T-cell receptor is required for optimal production of vector. CTLs infected with the adenoviral vector (C-RE-AG) alone produced no measurable adenoviral vectors (data not shown). The apoptosis rate of CTLs was unchanged by re-exposure to their target cells (Figure 5C). Transduced CTLs did not produce increased levels of inflammatory cytokines compared with nontransduced controls (Figure 5D). In contrast, exposure of monocyte-containing PBMNCs to the same adenoviral vectors induced substantial production of tumor necrosis factor, IL-8, and IL-6, and low levels of IL-10, IL-1β, and IL-12 (Figure 5D).
Adenovectors are produced by activated E1-CTLs and can transduce LCL targets. There were 2 separate EBV-CTL cell lines transduced with the E1 retrovirus and the Ad5F35-eGFP vector cocultured (at 80:1 LCL/CTL ratio) with autologous (A) or allogeneic (B) LCLs for 48 hours, and then the cells were stained with CD20 or isotype control antibodies. Results are expressed as the percentage of transduced target LCL cells (ie, double positive for GFP and CD20). (C) CTL apoptosis in the presence or absence of LCLs was evaluated using Annexin-V and 7AAD staining of CD3 cells. Cells were stained as recommended by the manufacturer and analyzed by FACS. Results are expressed as percentage in each quadrant. (D) Cytokine production was measured in the supernatant of CTL lines and PBMNCs exposed to 5 × 103 vp of Ad5 or Ad5F35 vector. Cells were transduced as described and supernatants harvested. The cytokines produced from nontransduced CTLs and from transduced PBMNCs were used as negative and positive controls, respectively.
Adenovectors are produced by activated E1-CTLs and can transduce LCL targets. There were 2 separate EBV-CTL cell lines transduced with the E1 retrovirus and the Ad5F35-eGFP vector cocultured (at 80:1 LCL/CTL ratio) with autologous (A) or allogeneic (B) LCLs for 48 hours, and then the cells were stained with CD20 or isotype control antibodies. Results are expressed as the percentage of transduced target LCL cells (ie, double positive for GFP and CD20). (C) CTL apoptosis in the presence or absence of LCLs was evaluated using Annexin-V and 7AAD staining of CD3 cells. Cells were stained as recommended by the manufacturer and analyzed by FACS. Results are expressed as percentage in each quadrant. (D) Cytokine production was measured in the supernatant of CTL lines and PBMNCs exposed to 5 × 103 vp of Ad5 or Ad5F35 vector. Cells were transduced as described and supernatants harvested. The cytokines produced from nontransduced CTLs and from transduced PBMNCs were used as negative and positive controls, respectively.
Time course of vector production from C-RE-AG cells
We next measured the time course of vector production by C-RE-AG cells after antigen exposure. Supernatants from these CTL cultures were recovered at multiple time points and the infectious adenovector particles titrated on A549 as indicator cells. Vector production declined after 2 to 3 days of stimulation, but could be reinduced by restimulating C-RE-AG cells with EBV-CTLs (Figure 6A). Similarly (Figure 6B), when the EBV-LCLs were removed from the CTL culture within 12 hours of the initial activation (Figure 6B, first arrow), Ad5F35 production dropped rapidly but could be reactivated upon restimulation (second arrow) of the CTLs with LCLs. The kinetics of virus production could be marginally modified by using CD3 and CD28 monoclonal antibodies as activating stimulus. These delayed the onset of vector production but increased its duration compared with the antigen-specific stimulus (Figure 6C). These data indicate that exposure to EBV-LCLs represents a potent and sustained signal for production of vector from C-RE-AG cells.
Kinetics of adenovector production by modified CTLs. (A-B) EBV-specific CTLs were sequentially transduced with both the retrovirus and the Ad5F35-eGFP. After washing, the cells were incubated with (♦) or without (▪) irradiated autologous LCLs for the entire culture period (A) or for 12 hours. (B) Supernatants were collected at different time points and used to transduce A459 cells. The latter were then analyzed by flow cytometry. Results are expressed as the percentage of eGFP-positive A549 cells after 24 hours of exposition to CTL supernatants. The arrows represent the times of stimulation with the irradiated LCLs. (C) The C-RE-AG (Ad5F35-eGFP–transduced CTLs, 40% efficiency) were activated with irradiated LCLs (▴) or CD3/CD28 MAb (▪). Nonactivated (NA) cells were used as negative control (♦). Error bars indicate mean ± SD.
Kinetics of adenovector production by modified CTLs. (A-B) EBV-specific CTLs were sequentially transduced with both the retrovirus and the Ad5F35-eGFP. After washing, the cells were incubated with (♦) or without (▪) irradiated autologous LCLs for the entire culture period (A) or for 12 hours. (B) Supernatants were collected at different time points and used to transduce A459 cells. The latter were then analyzed by flow cytometry. Results are expressed as the percentage of eGFP-positive A549 cells after 24 hours of exposition to CTL supernatants. The arrows represent the times of stimulation with the irradiated LCLs. (C) The C-RE-AG (Ad5F35-eGFP–transduced CTLs, 40% efficiency) were activated with irradiated LCLs (▴) or CD3/CD28 MAb (▪). Nonactivated (NA) cells were used as negative control (♦). Error bars indicate mean ± SD.
E1 transgenic CTLs can produce adenovectors with antitumor activity
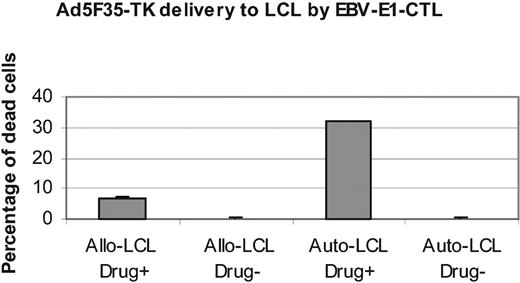
One potential use for CTL-targeted adenoviral vectors would be to add an oncolytic transgene in an adenovector to augment the tumor killing mediated by the T lymphocytes themselves. To determine if such an effect was indeed feasible, we generated an Ad5F35-TK vector and introduced it into E1 transgenic EBV-CTLs (C-RE-AT). These C-RE-AT cells were cocultured with autologous or allogeneic EBV-LCLs for 24 hours and the cells were then sorted by flow cytometry to eliminate LCLs that had been killed directly by the CTLs. The remaining LCLs were counted, and equal numbers of cells were then grown with or without ganciclovir, the prodrug target of the HSV-Tk transgene product. After 3 days, LCL viability demonstrated a substantial further reduction when autologous LCLs had been exposed to both C-RE-AT cells and ganciclovir compared with those exposed to C-RE-AT but cultured without ganciclovir (Figure 7). The survival of allogeneic EBV-LCLs exposed to C-RE-AT was little affected by the presence or absence of ganciclovir, indicating that they had been unable to induce significant vector release from the C-RE-AT producer cells (Figure 7 and also Figure 5).
Ad5F35-TK delivered by activated E1-CTLs killed LCLs. E1-CTLs (C-RE) were transduced (1000 vp per cell) with an Ad5F35-TK vector and cultured with autologous and allogeneic LCLs. At 24 hours after coculture, the CTLs (C-RE-AT) were removed by fluorescence-activated cell sorting and the LCLs counted. The same numbers of LCL cells were cultured with or without ganciclovir for 3 days. For each condition, 6 identical wells were used. The viability of the cells was then measured with an adenosine triphosphate (ATP) assay. Control cells were LCLs directly infected with Ad5F35-TK vector. Results are reported as the percentage of dead cells. Results are shown as mean ± SD.
Ad5F35-TK delivered by activated E1-CTLs killed LCLs. E1-CTLs (C-RE) were transduced (1000 vp per cell) with an Ad5F35-TK vector and cultured with autologous and allogeneic LCLs. At 24 hours after coculture, the CTLs (C-RE-AT) were removed by fluorescence-activated cell sorting and the LCLs counted. The same numbers of LCL cells were cultured with or without ganciclovir for 3 days. For each condition, 6 identical wells were used. The viability of the cells was then measured with an adenosine triphosphate (ATP) assay. Control cells were LCLs directly infected with Ad5F35-TK vector. Results are reported as the percentage of dead cells. Results are shown as mean ± SD.
Discussion
The availability of a chimeric adenoviral vector (Ad5F35) that can infect human T cells and of an activation-dependent promoter (CD40L) controlling a critical early adenoviral replicative gene has made it possible to generate cytotoxic T cells that produce adenoviral vectors when they encounter their target antigen. These antigen-specific T cells retain their specificity and function after exposure to Moloney retroviral vectors encoding the E1 antigen driven by a CD40L promoter and to E1-deleted Ad5F35 vectors encoding eGFP or a gene of interest. Upon antigen-induced activation, the T cells produce infectious virus that can enter proximate target cells. If the adenoviral vector contains a gene such as thymidine kinase, the approach can be used to produce a higher level of target cell killing than could be achieved by the CTL alone.
We developed this targeted delivery of adenoviral vectors by cytotoxic T cells in an effort to overcome some of the major limitations of adenoviral vectors and of T-cell therapies for cancer. While adenovectors can deliver highly toxic genes to a wide variety of malignant cell types, a lack of specific targeting makes systemic administration problematic, while the limited efficiency of transduction means that many tumor targets escape.23-27 Simply increasing the dose of adenovectors administered is not an option because induction of innate inflammatory and immune responses by large quantities of adenoviral proteins may prove lethal.28,29 One option is to genetically modify the structure of adenoviral vector proteins to remove their native specificities30,31 and introduce new target cell ligands.15,16,32,33 This approach is of great promise and will likely improve the efficiency and reduce the toxicity of adenoviral vectors.34-39 However, for cancer therapy in particular, retargeting may have a limited ability to allow adenoviral vectors to reach metastatic tumor cell targets that may be present in the multiplicity of different tissues. Reaching these metastatic targets will require a capacity not just for retargeting but also for traversing distinct tissue planes.40,41
T lymphocytes, by contrast, have excellent targeting ability, and their capacity to respond to a variety of different migration signals allows them to traverse many distinct tissues to reach their targets.42-44 While other cell types may also possess this property,45 they lack the receptors to make them specific for tumor targets. Moreover, once T cells are in place, they bring to bear a range of cytotoxic effector mechanisms, including perforins, granzymes, and cell surface death-inducing molecules that can be used to destroy tumor cells. Unfortunately, most tumors have developed means of evading these cytotoxic mechanisms either by release of cytokines such as IL-4 that favor recruitment of noncytotoxic effector cells or by production of inhibitory cytokines such as transforming growth factor β that rapidly inactivate lymphocytes and other immune system cells.42,46-48
We have used the capacity of a chimeric adenovirus vector 5 containing the shaft and knob domains of the fiber of the adenovirus serotype 35 to transduce human T cells, which are largely resistant to transduction by wild-type adenovirus type 5.11,15,16 Because the vector we used is E1 deleted, it is unable to replicate.49,50 By providing E1 in trans, under the regulation of a T-cell activation–dependent promoter (CD40 ligand), we ensure that viral replication occurs only when the cell engages its MHC-restricted antigen-specific receptor. Transduction with E1-encoding retrovirus and with Ad5/F35-GFP vector did not impair CTL proliferation or function, nor did it discernibly alter the CD4/CD8/CD56/CD25 phenotype of the cells or induce inflammatory cytokines. Infectious vector was released over a 24- to 72-hour period following cellular activation, kinetics that are consistent with the known pattern of expression of the CD40 ligand gene, the promoter of which is tightly regulated by the AT hook transcription factor AKNA.
The system we used to test the effects of released virus used EBV-specific CTLs and EBV-transformed B lymphoblastoid cell lines.51 Although this is primarily a viral antigen, rather than a tumor antigen–driven system, it models a genuine human malignancy, the immunoblastic lymphoma that occurs as part of posttransplantation lymphoproliferative disease.52 In this lymphoma, EBV antigen–expressing B lymphoblasts hyperproliferate. Our gene marking studies4,19,21 have shown that EBV-specific CTLs trafficto tumor sites and have activity against posttransplantation lymphoproliferative disease, and more recently against EBV-positive Hodgkin disease. The high level of ex vivo and in vivo activation we observe is likely due to the expression of a broad array of costimulator molecules on the EBV-infected target cells. These ensure that the target cells act as “semiprofessional” antigen-presenting cells and can therefore directly activate CTLs in tissues.
We show that stimulation of the CTLs transduced with both E1 retrovirus and E1-deficient Ad5F35 adenovectors (c-RE-Ad) could produce substantial quantities of infectious adenoviral vector when these cells were cultivated with MHC-identical EBV-positive target cells. Transduction of target cells was substantially lower when cultivation occurred with MHC-mismatched or EBV-negative targets, consistent with a lower degree of T-cell activation through an MHC-restricted receptor pathway. The adenovector produced was capable of infecting a high proportion of EBV-positive target cells. If the vector encoded a potentially oncolytic gene such as thymidine kinase, then it was possible to subsequently kill the target cells by exposure to ganciclovir. The substitution of C-RE-AT was capable of producing substantially greater tumor cell death than the use of cytotoxic T cells alone.
Will this approach work for more conventional tumor cell targets? The critical determinants of success are sufficient activation of T cells to induce CD40L promoter-driven expression of E1 and the susceptibility of the tumor target to the adenovectors produced by the T lymphocytes. The viral antigens expressed by EBV-LCLs induce high-affinity cytotoxic T lymphocytes, while most other tumor-specific antigens induce T cells expressing receptors with much lower affinity.53 These “weak” antigens would be anticipated to stimulate a lower level of T-cell activation than EBV proteins. Moreover, EBV-LCLs express a wide array of costimulator molecules that amplify T-cell responses to antigen-specific receptor engagement and that promote T-cell proliferation and survival.54 Most tumor cells lack such costimulator molecules and may be expected to induce lower levels of T-cell activation, and hence reduced E1 expression. But while these features might suggest that the application of our approach will be limited to the uncommon virally associated tumors of the immune system, it is also true that extremely low levels of E1 expression are permissive for adenoviral replication. Moreover, many primary human tumor cells are more susceptible than EBV-LCLs to adenovector transduction, so that even a more limited activation stimulus to T cells that produced a more limited adenovector burst size could still transduce an equivalent number of these more susceptible target cells.
There are 2 additional potential limitations of our proposed approach that should also be considered. The first is that C-RE-Ad cells may express sufficient adenoviral antigens even in the absence of antigen stimulation to render them susceptible to immune attack. Although we could not detect any adenoviral hexon proteins in unstimulated C-RE-Ad cells, we cannot formally exclude this possibility. Since Ad5F35 vectors infect murine T cells extremely poorly55 and cannot be shown to reproduce in them at all (even in the presence of abundant E1), it is not possible to accurately model this approach in general, or the possibility of stimulation of the human immune system in particular, in murine models. This possibility could be addressed only if the approach came to clinical studies. However, while it is true that transduced CTLs may become a target for an antiadenoviral vector response, so too may the tumor cells that have been infected. T cells release the bulk of their adenovirus within the first 48 to 72 hours of antigen exposure, and if the immune response peaks after this period, the major targets may well be the infected tumor cells (expressing processed adenoviral capsid antigens). There is also a concern that antigen loss variants would be selected among the tumor cells, so that production of therapeutic adenovectors would cease due to loss of T-cell antigen receptor stimulation.56,57 However, antigen loss variants are a concern for any immunotherapy approach, and in fact the delivery of both a T-cell–mediated and an adenovector-dependent cytotoxic signal may make it less likely that sufficient tumor cells would survive for there to be antigen loss variants among the remaining cells.
Finally, the system we have described is complex, requiring transduction with 2 different vectors, one of which has been associated with oncogenic events in human T cells.58 If, however, our approach is shown to be a general means of targeting adenovectors, it should prove possible to modify and simplify the transduction technology, for example by using physical techniques to simultaneously transduce the CTLs with appropriate vectors. Such a development would ease manufacturing and diminish patient risks.
These potential limitations notwithstanding, we have demonstrated that antigen-specific human CTLs can be modified to become adenovector producer cells on encountering their antigen-specific target. The function and specificity of these vector producer T cells appears unimpaired, and incorporation of an advector encoding a potentially oncolytic gene, such as TK, can increase target killing compared with CTLs alone. It will be of interest to discover whether targeted delivery of adenoviral vectors by cytotoxic T cells will have wider applicability in cancer therapy, above and beyond destruction of EBV-expressing target cells.
Prepublished online as Blood First Edition Paper, May 25, 2004; DOI 10.1182/blood-2003-11-3803.
Supported by The Methodist Foundation, the Laurie Strauss Leukemia Foundation, and the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Foundation Grant 1R03 DK067264-01.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Tatiana Goltsova and Frances Eustice for technical and editing help.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal