Abstract
Since increased fetal hemoglobin diminishes the severity of β-thalassemia and sickle cell anemia, a strategy using autologous, stem cell–targeted gene transfer of a γ-globin gene may be therapeutically useful. We previously found that a γ-globin lentiviral vector utilizing the β-globin promoter and elements from the β-globin locus control region (LCR) totaling 1.7 kb could correct murine β-thalassemia. However, therapeutic consistency was compromised by chromosomal position effects on vector expression. In contrast, we show here that the majority of animals that received transplants of β-thalassemic stem cells transduced with a new vector containing 3.2 kb of LCR sequences expressed high levels of fetal hemoglobin (17%-33%), with an average vector copy number of 1.3. This led to a mean 26 g/L (2.6 g/dL) increase in hemoglobin concentration and enhanced amelioration of other hematologic parameters. Analysis of clonal erythroid cells of secondary spleen colonies from mice that underwent transplantation demonstrated an increased resistance of the larger LCR vector to stable and variegating position effects. This trend was also observed for vector insertion sites located inside genes, where vector expression was often compromised, in contrast to intergenic sites, where higher levels of expression were observed. These data emphasize the importance of overcoming detrimental position effects for consistent therapeutic globin vector expression.
Introduction
Significant progress in developing gene therapy approaches to both β-thalassemia and sickle cell disease has recently been made using murine models.1-5 Important concepts have emerged from these studies, some of which highlight the challenges to be overcome in order to translate preclinical progress into successful human clinical trials. Lentiviral vectors have a unique ability to efficiently transfer globin expression cassettes comprised of complex configurations of regulatory/enhancer sequences from the endogenous β-globin locus control region (LCR). This attribute improves globin expression due to the inclusion of the sequences from the LCR without detrimentally affecting vector stability or titer. Despite this advance, therapeutic correction of the mouse hemoglobinopathy models has only been obtained when most or all of the diseased hematopoietic stem cells (HSCs) are genetically modified with the vector. In contrast, using the same murine models of β-thalassemia and sickle cell disease, bone marrow chimera studies indicate that chimerism levels from 30% to 50% with normal HSCs result in nearly complete hematologic and pathologic correction.6,7
Although the reasons for the discrepancies between the gene therapy and chimera studies may be several, it is likely that a major factor underlying these contrasting results is the variable globin expression obtained from unique proviral integration sites.2,3 Previously, using a γ-globin lentiviral vector, we showed that an average vector copy number of 0.8 in peripheral blood leukocytes (PBLs) resulted in only slight hematologic improvement in murine β-thalassemia intermedia but that significant disease correction occurred with a copy number of 2.1 to 2.4. Analysis of clonal erythroblasts in secondary spleen colony-forming cells (CFU-Ss) demonstrated that many integration events resulted in poor vector expression.3 Likewise, using a similar murine model of β-thalassemia, Imren et al4 found that an average β-globin vector copy number of one led to detectable β-globin expression in only 30% of repopulated erythroid cells and resulted in no hematologic improvement. Indeed, phenotypic correction required an average vector copy number of 3. Pawliuk et al2 reported that correction of a murine model of sickle cell disease was also obtained with an average vector copy number of 3. Finally, Rivella and colleagues8 observed variable degrees of hematologic improvement of a model of β-thalassemia major in animals with bone marrow vector copy numbers ranging from 0.9 to 2.4. Together, these data suggest that phenotypic improvement required that most HSCs be genetically modified with one or more vector copies in order to ensure the engraftment of a subset of clones containing vector integration sites favorable to therapeutic levels of globin expression.
Achieving a level of genetically modified human HSCs similar to that in the murine studies is not currently likely based on data from recent clinical trials and nonhuman primate studies. In addition, since multiple vector copies per stem cell are not likely to be desirable based on safety concerns,9 vector modifications that will result in more consistent globin expression from single-copy vector insertions are highly desirable. Toward this goal, we report here the development of a second-generation γ-globin lentiviral vector having more extensive LCR-derived regulatory sequences relative to our previous vector. This vector directed high levels of chimeric mα2hγ2 hemoglobin molecules (termed HbF) in the majority of mice, resulting in an enhanced therapeutic effect in the context of a reduced average vector copy number. This improved performance resulted from an increased resistance of the vector to chromosomal position effects as demonstrated by the high frequency of globin-expressing cells in clonal CFU-S erythroid populations.
Materials and methods
Plasmid constructions
pCL10.1 d432βΔγm was derived from pCL10.1 d432βΔγ by replacing the 2860-bp NsiI-SbfI fragment with the same fragment from p72d432βΔγm. p72d432βΔγm, which contains a point mutation within the 3′ untranslated region (UTR) of the Aγ-globin sequences, was generated from p72d432βΔγ10 by using oligonucleotides 5′-GGCTTTTTTCTGCAAGCAATACAA-3′ and 5′-TTGCTTGCAGAAAAAAGCCTATCC-3′, and the QuickChange XL site-directed mutagenesis kit (Stratagene Cloning Systems, La Jolla, CA) to introduce an A-to-T change at nucleotide 8581 (GenBank accession no. M91036). This altered a canonical polyadenylation signal hexamer, “AATAAA,” which occurred in reverse orientation of the γ-globin expression cassette.
To construct a γ-globin vector with extended LCR sequences and a larger β-globin promoter, the shuttle plasmid p72mLARβΔγV1 was made by ligating a 3896-bp XhoI-BlnI LCR-promoter fragment from L1-432mLAR-bEGFP-1L11 (kindly provided by R. Hardison, Penn State University, University Park, PA) with the 3755-bp BlnI-XhoI fragment from p72d432βΔγm. In p72mLARβΔγV1, 4 polyadenylation signal hexamers identified in reverse orientation (2 within HS4 and 2 within HS2) were mutated to “AATATA,” “AATTAA,” “AATATT,” and “TATAAA,” as above. pCL20c mLARβΔγV1, with the largest LCR configuration (HS4: 1254 bp; HS3: 851 bp; HS2: 1407 bp) and promoter (373 bp), was made by inserting the globin expression cassette (5266-bp XhoI [klenow fill-in]–EcoRV fragment from p72mLARβΔγV1) into the pCL20c backbone12 (5647-bp BstZ17I-HpaI fragment from pCL20c MSCV-GFP) in reverse orientation. pCL20c mLARβΔγV3 was made by removing a 40-bp fragment from the 5′ end of the β-globin promoter of pCL20c mLARβΔγV1 (details available upon request). pCL20c mLARβΔγV4 was made by replacing the 1670-bp XbaI fragment (which cuts between HS2 and the β-globin promoter, and between the γ-globin downstream sequence and HIV-1 RRE) of pCL20c mLARβΔγV1 with the 1411-bp XbaI fragment from pCL10.1 d432βΔγm. pCL20c mLARβΔγV5 was made by removing the 349-bp XbaI fragment (which cuts between HS2 and β-globin promoter, and within HS2) from pCL20c mLARβΔγV4, leaving a 1094-bp HS2 sequence.
Lentiviral vector preparation
VSV-G pseudotyped lentiviral vector particles were prepared using a 4 plasmid system by transient transfection of 293T cells as previously described.3 In brief, 293T cells were transfected with a mixture of plasmid DNA consisting of 6 μg pCAGkGP1R (Gag/Pol), 2 μg pCAG4-RTR2 (Rev/Tat), 2 μg pCAG-VSVG (VSV-G envelope),13 and 10 μg of gene transfer vector plasmid per 10-cm dish using the calcium phosphate precipitation technique. Viral particles were concentrated by ultracentrifugation. Titers of globin vector preparations were determined by genome transfer to HeLa cells, as previously described.3
Northern blot analysis of viral vector genomes produced in transiently transfected 293T cells
After harvesting the conditioned media containing the lentiviral vector particles, total RNA was extracted from the transfected 293T cells. RNA (5 μg) was electrophoresed using a 1% agarose gel containing 2.2 M formaldehyde and transferred onto a Hybond-N membrane. A radiolabeled HIV-1 RRE element DNA probe (777-bp MunI-XbaI fragment from pCL10.1 d432βΔγm) was hybridized with the blot and the resulting hybridizing bands were visualized using a Molecular Dynamic Storm 860 Phosphorimager (Sunnyvale, CA) and its accompanying software.
RACE (3′ rapid amplification of cDNA ends) analysis to identify truncated mLARβΔγV1 globin vector genomes resulting from premature polyadenylation
One half of a microgram of the total RNA from transiently transfected, 293T viral producer cells was reverse transcribed using primer Adapter-dT (5′-GGCCACGCGTCGACTAGTACTTTTTTTTTTTTTTTTT-3′) and the First Strand cDNA Synthesis Kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer's specifications. Polymerase chain reaction (PCR) was then used to amplify the 3′ ends of the cDNAs representing the polyadenylated RNA species by using the primers Adapter-primer (5′-GGCCACGCGTCGACTAGTACTT-3′) and GSP1 (5′-CCGCTCTAGAACTAGTGGAT-3′), or GSP2 (5′-TCCTCCAGCATCTTCCACAT-3′) and the Expand High Fidelity PCR System (Roche Applied Science) according to the manufacturer's instructions. Amplified PCR products were visualized in 0.8% agarose gel with ethidium bromide staining. The bands were subcloned and sequenced using the pCR2.1-TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA).
Transduction and transplantation of murine BM cells
Bone marrow (BM) cells from β-thalassemic or congenic, normal HW80 mice (B6.C-TyrcH1b Hbbd/By; Jackson Laboratory, Bar Harbor, ME) were harvested 48 hours after treatment with 150 mg/kg 5-fluorouracil (Pharmacia, Kalamazoo, MI). Cells were cultured at a concentration of 2 × 106/mL in Dulbecco modified Eagle medium (DMEM) containing 20% fetal bovine serum (FBS; Hyclone, Logan, UT) and 20 ng/mL murine interleukin 3 (IL-3), 50 ng/mL murine IL-6, and 50 ng/mL murine stem cell factor (SCF; R&D Systems, Minneapolis, MN). After 48 hours, cells were washed and resuspended at a concentration of 1 × 107/mL to 2 × 107/mL in XVIVO 10 containing the above concentrations of serum/cytokines, polybrene at 6 μg/mL, and concentrated vector at approximately 1 × 109/mL to 3 × 109/mL. This mixture was placed into a RetroNectin (TAKARA Shuzo, Otsu, Japan)–coated (20 μg/cm2) 6-well plate and incubated at 37° C in a humidified incubator with 5% CO2. After 6 hours, cells were collected, washed with phosphate-buffered saline (PBS), and resuspended in PBS containing 2% FCS. Lethally irradiated (1050 cGy) C57Bl/6J mice (Jackson Laboratories) underwent transplantation with 1 × 106 to 2 × 106 cells by tail vein injection and were subsequently analyzed about 5 months after transplantation.
CFU-S assay
BM cells (0.5-1.0 × 105) from primary mice that underwent transplantation were transplanted into normal C57Bl/6J mice pretreated with 900 cGy irradiation. Mice were killed 13 days after transplantation, and discrete splenic colonies were dissected and single-cell suspensions prepared. A portion of the cells was used to prepare genomic DNA, while the rest of the cells were fixed, permeabilized, and stained with the TER119-PE antibody, which recognizes erythroid cells, and a fluorescein isothiocyanate (FITC)–labeled antibody against human γ-globin. In all cases, brightly positive TER119 cells were gated on to determine the percentage of HbF-expressing cells using a FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Hematologic analysis
Complete blood and reticulocyte counts were determined using an automated blood cell analyzer and FACS analysis as previously described.3 Hb cellulose acetate gel electrophoresis and quantitation of Hb bands was performed as previously described.6 FACS analysis of red cells for expression of human γ-globin was performed as previously described.3
DNA analysis
Spleen colony DNA samples were digested with BsrGI or HincII, both of which cut once within the provirus, in order to determine the pattern and number of vector integrations, thereby enabling each colony to be uniquely identified. BglII, which cuts at the ends of the provirus and liberates a near unit-length provirus, was used to verify the presence of unrearranged lentiviral vector. A radiolabeled HIV-1 RRE element DNA probe (as in “Northern blot analysis of viral vector genomes produced in transiently transfected 283T cells”) was hybridized with the blot and the resulting hybridizing bands were visualized as above.
To determine the average vector copy in the peripheral blood leukocytes (PBLs) of transplant recipients, semiquantitative duplex PCR using primers to amplify mouse β-major and vector sequences (5′ mouse β-major primer 5′-CCTATCCTCTGCCTCTGCTA-3′ and 3′ primer 5′-CTTCTGGAAGGCAGCCTGTG-3′; 5′ γ-globin vector primer 5′-AGCAACCTCAAACAGACACC-3′ and 3′ primer 5′-GGCCACTCCAGTCACCATCTT-3′) was performed on the PBL genomic DNA of mice that received transplants of β-thalassemic BM transduced with the γ-globin vectors as previously described.3
Identification of globin vector insertion sites using ligation-mediated–polymerase chain reaction (LM-PCR)
Genomic DNA (100 ng) from 13-day mouse spleen colonies and a double-stranded, asymmetric DNA linker containing a single HinfI restriction site were digested with HinfI (New England Bio Labs, Beverly, MA). The restricted genomic and linker DNAs were ligated using T4 ligase (Promega, Madison, WI) and this material served as a template for the first step of a 2-step nested PCR. The primers for the first PCR reaction were: outside LTR 5′-AACAGACGGGCACACACTAC-3′, outside linker 5′-GCACTCGTGATCGACTGATA-3′. PCR reactions were performed using HotStarTaq (Qiagen, Valencia, CA) according to the manufacturer's specification using a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA). Cycling parameters were: 1 cycle of 15 minutes at 94° C followed by 30 cycles of 1 minute at 94° C, 1 minute of 60° C, and 1 minute of 72° C, with a final extension cycle of 5 minutes at 72° C.
Nested PCR was then performed using 1 μL of the first PCR reaction as a template using the following primers: LTR Nest3 5′-CTAACCAGAGAGACCCAGTA-3′, and linker Nest3 5′-GCACGCGTCGCATCGTACTA-3′. Amplified DNA was cloned using the pCR2.1-TOPO TA Cloning Kit (Invitrogen). Plasmid clones containing inserts were sequenced and analyzed for the presence of the expected LTR DNA-genomic DNA-linker DNA fragments. If these criteria were met, the intervening genomic sequence was blasted using the National Center for Biotechnology Information (NCBI) mouse genomic database.
PCR detection of specific vector–genomic DNA junctions
Vector insertion site data for the single vector copy clones designated “A” and “S” were used to develop PCR primers to specifically allow amplification of the unique vector–genomic DNA junctions in these 2 clones. For d432βΔγm clone “A,” the presence of the unique 3′ vector-genomic DNA junction was determined by PCR using a primer, 5′-GAGCTCTCTGGCTAACTAGG-3′, complementary to sequences within the R region of the U3-deleted vector 3′ LTR and a primer, 5′-CTGCACATAGGAACCAGAAC-3′, complementary to downstream genomic sequences. For the mLARβΔγV5 clone “S,” the presence of the unique 5′ vector–genomic DNA junction was determined by PCR using a primer, 5′-AACAGACGGGCACACACTAC-3′, complementary to sequences within the U5 region of the U3-deleted vector 5′ LTR and a primer, 5′-GCTTCCTCCTCTTCCAACTC-3′, complementary to upstream genomic sequences.
Statistical analyses
The differences in the mean values of the variables of interest between the 2 vectors were assessed using a 2-sample t test for unequal variances (Behrens-Fisher test).14 The Behrens-Fisher statistic was used since in most cases the observed variance estimates for the 2 groups being compared were very different. Furthermore, for some of the comparisons, one-sided tests were performed as indicated in cases where an improvement in the variable with the new vector was predicted and tested. For all other cases, 2-sided tests were performed as indicated.
Results
Development of a γ-globin vector with extended β-globin LCR regulatory sequences
Previously we demonstrated the therapeutic efficacy in murine β-thalassemia of a γ-globin lentiviral vector (d432βΔγm) containing 1.7 kb of regulatory sequences from hypersensitive site (HS) elements HS4, HS3, and HS2 of the β-globin LCR.3 However, high-level γ-globin expression and major phenotypic improvement occurred in only 25% of mice due to detrimental chromosomal position effects in many cases. In order to improve the consistency of high-level expression, a second-generation γ-globin vector, mLARβΔγV1, was constructed containing 3.5 kb of LCR sequences (Figure 1A) and an extended β-globin promoter (+50 to –373).11 This larger LCR configuration and β-globin promoter size was chosen since sequences flanking the HS cores have significant interspecies conservation and are known to provide synergistic enhancement of expression, including expression at “nonpermissive” chromosomal locations in erythroid cell lines.11,15,16 Since we previously observed that canonical polyadenylation signal sequences (“AATAAA”)17 in the globin cassette can compromise vector titer through the generation of truncated, viral vector genomic RNA at the expense of full-length viral genomes,3,10 canonical hexamer polyadenylation signal sequences at 5 noncoding sites were mutated in mLARβΔγV1 (Figure 1A, vertical solid arrowheads; see “Materials and methods”). Northern blot analysis was then used to assess the level of full-length, viral vector genomic RNA produced in 293T cells transfected with mLARβΔγV1 and the lentiviral packaging plasmids. Surprisingly, the predominant viral vector genomic RNA species was truncated, with only a small amount of genomic species of the predicted correct size present (Figure 1B). The mLARβΔγV1 vector titer was almost 2 logs lower than the MSCV-GFP or small LCR γ-globin (d432βΔγm) lentiviral vectors, both of which led to the production of viral vector genomic RNA of the correct predicted size. As expected, the level of full-length viral vector RNA correlated with the unconcentrated titer of the vectors (Table 1).
Specific DNA sequences cause premature polyadenylation of globin vector genomic RNA and compromise vector titer. (A) Schematic representations of γ-globin lentiviral vectors. Shown at top is the SJ-1 self-inactivating (SIN) lentiviral vector backbone which contains both the central polypurine tract (cppt) and the rev-responsive element (RRE).13 The genomic γ-globin sequences, as previously described,3 are indicated by the hatched design, the β-globin promoter sequences are indicated by the solid horizontal arrow, and the HS elements, with the size and restriction sites that define the fragments from the β-globin LCR, are represented as horizontal open rectangles. Vertical solid arrowheads indicate the sites of mutagenesis that eliminated canonical “AATAAA” polyadenylation signal sequences. (B) Northern blot analysis, using a radiolabeled RRE probe, of RNA from 293T cells transfected with the indicated vectors.Arrows indicate the predicted size of the full-length vector genomic RNAspecies. Shown on the left are the migration positions of a set of RNA markers of the indicated sizes. (C) 3′ RACE analysis to map premature polyadenylation cleavage sites in mLARβΔγV1 vector genomic RNA transcripts. RNA from 293T cells transfected with the mLARβΔγV1 vector was subjected to 3′ RACE analysis as described in “Materials and methods.” The amplified reaction products, derived using either the GSP1 or GSP2 primers, were fractionated using agarose gel electrophoresis and visualized by ethidium bromide staining (right). The products of each reaction were also subsequently cloned and sequenced. At left, the vertical solid arrows indicate the major sites of aberrant cleavage of vector RNA as determined from sequencing data.
Specific DNA sequences cause premature polyadenylation of globin vector genomic RNA and compromise vector titer. (A) Schematic representations of γ-globin lentiviral vectors. Shown at top is the SJ-1 self-inactivating (SIN) lentiviral vector backbone which contains both the central polypurine tract (cppt) and the rev-responsive element (RRE).13 The genomic γ-globin sequences, as previously described,3 are indicated by the hatched design, the β-globin promoter sequences are indicated by the solid horizontal arrow, and the HS elements, with the size and restriction sites that define the fragments from the β-globin LCR, are represented as horizontal open rectangles. Vertical solid arrowheads indicate the sites of mutagenesis that eliminated canonical “AATAAA” polyadenylation signal sequences. (B) Northern blot analysis, using a radiolabeled RRE probe, of RNA from 293T cells transfected with the indicated vectors.Arrows indicate the predicted size of the full-length vector genomic RNAspecies. Shown on the left are the migration positions of a set of RNA markers of the indicated sizes. (C) 3′ RACE analysis to map premature polyadenylation cleavage sites in mLARβΔγV1 vector genomic RNA transcripts. RNA from 293T cells transfected with the mLARβΔγV1 vector was subjected to 3′ RACE analysis as described in “Materials and methods.” The amplified reaction products, derived using either the GSP1 or GSP2 primers, were fractionated using agarose gel electrophoresis and visualized by ethidium bromide staining (right). The products of each reaction were also subsequently cloned and sequenced. At left, the vertical solid arrows indicate the major sites of aberrant cleavage of vector RNA as determined from sequencing data.
Higher ratios of full-length genomic to truncated subgenomic viral RNA correlate with higher globin vector titers
Transfer vector . | HeLa titer, TU/mL, mean ± SEM . | Full-length genomic RNA . | Truncated subgenomic RNA . |
|---|---|---|---|
| MSCV-GFP | 4.4 ± 0.7 × 107 | +++ | ± |
| d432βΔγm | 1.6 ± 0.5 × 107 | +++++ | ± |
| mLARβΔγV1 | 3.8 ± 1.8 × 105 | + | ++++ |
| mLARβΔγV3 | 5.5 × 105 | ++ | +++ |
| mLARβΔγV4 | 7.6 ± 5.5 × 105 | +++ | + |
| mLARβΔγV5 | 1.8 ± 0.5 × 106 | +++ | + |
Transfer vector . | HeLa titer, TU/mL, mean ± SEM . | Full-length genomic RNA . | Truncated subgenomic RNA . |
|---|---|---|---|
| MSCV-GFP | 4.4 ± 0.7 × 107 | +++ | ± |
| d432βΔγm | 1.6 ± 0.5 × 107 | +++++ | ± |
| mLARβΔγV1 | 3.8 ± 1.8 × 105 | + | ++++ |
| mLARβΔγV3 | 5.5 × 105 | ++ | +++ |
| mLARβΔγV4 | 7.6 ± 5.5 × 105 | +++ | + |
| mLARβΔγV5 | 1.8 ± 0.5 × 106 | +++ | + |
Unconcentrated globin vector titers were determined by genome transfer to HeLa cells as described in “Materials and methods.” The relative amounts of the indicated RNA species were visually estimated with levels ranging from minimal (±) to high (+++++).
The size of the truncated mLARβΔγV1 viral vector RNA species suggested the possibility that premature polyadenylation was occurring in a region near the HS2/β-globin promoter junction (Figure 1A). Therefore, primers (GSP1 and GSP2) were designed for 3′ RACE analysis to identify possible polyadenylation cleavage sites within this region (Figure 1C). The 3′ RACE products were cloned and sequenced. These data showed that predominant cleavage sites were occurring within the distal β-globin promoter (Figure 1C), despite the lack of either canonical (“AATAAA”), alternative (“ATTAAA”), or variant polyadenylation signals17 within 20 nucleotides of the cleavage sites (data not shown). Additional cleavage sites were also identified within the proximal HS2 element and in this case a variant “AATATA” polyadenylation signal was present 15 bp to 20 bp upstream of the cleavage site (Figure 1C). Although deletion of the cleavage sites and an additional 17 bp upstream of the major cleavage site in the distal β-globin promoter resulted in a slight increase in full-length viral vector mRNA (mLARβΔγV3), major rescue of the full-length species required truncation of the β-globin promoter back to the size of that used in d432βΔγm (mLARβΔγV4, Figure 1A-B). A 349-bp deletion (mLARβΔγV5) was then used to eliminate the variant polyadenylation signal and cleavage sites present within the proximal HS2 element (Figure 1A,C). The majority of viral vector genomic transcripts produced in cells transfected with mLARβΔγV5 were of the predicted full-length size (Figure 1B), resulting in about a one-log improvement in vector titer over mLARβΔγV1 (Table 1). We chose to focus on studying the in vivo performance of this vector.
mLARβΔγV5 directs improved γ-globin expression and consistent phenotypic correction of murine β-thalassemia intermedia
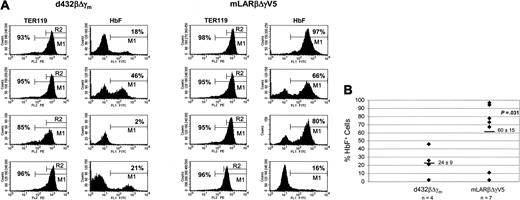
Lethally irradiated C57Bl/6 mice underwent transplantation with mock-transduced, d432βΔγm-transduced, or mLARβΔγV5-transduced β-thalassemic (HW80 background) BM cells. In addition, a control cohort of mice underwent transplantation with mock-transduced normal, HW80 BM cells. PB from the mice that underwent transplantation was analyzed for hematologic parameters, red cell γ-globin expression, HbF levels, and PBL vector copy number 5 months after transplantation. Complete hematologic reconstitution with the donor graft occurred in all recipient mice as verified by the absence of the endogenous C57Bl/6 “single” Hb phenotype on Hb electrophoresis analysis (Figure 2A and data not shown). Only 2 of the 5 mice that underwent transplantation with d432βΔγm-transduced cells showed HbF levels above 10%, whereas 5 of 6 mice that underwent transplantation with mLARβΔγV5-transduced cells had HbF levels above 15% (Figure 2A). On average, mice that underwent transplantation with mLARβΔγV5-transduced cells had almost 3-fold more HbF (22% versus 8%, P < .02) than mice receiving d432βΔγm-transduced cells (Table 2). In addition, mLARβΔγV5 mice had a much higher proportion of F cells than d432βΔγm mice (71% versus 32%, P < .004; Table 2). Importantly, the improved expression observed with mLARβΔγV5 was achieved with a lower average copy number (1.3 versus 2.0) and led to an output of 17% F per vector copy, which was 4-fold higher than that achieved with d432βΔγm (Figure 2A).
Consistent, therapeutic, γ-globin expression of the mLARβΔγV5 lentiviral vector in β-thalassemic mice. (A) Cellulose acetate Hb electrophoresis gels were used to separate the different Hb species in red cell lysates from mice that underwent transplantation with β-thalassemic BM cells transduced with the indicated vectors. This β-thalassemic mouse strain has the “diffuse” Hb pattern characterized by an uppermost
Consistent, therapeutic, γ-globin expression of the mLARβΔγV5 lentiviral vector in β-thalassemic mice. (A) Cellulose acetate Hb electrophoresis gels were used to separate the different Hb species in red cell lysates from mice that underwent transplantation with β-thalassemic BM cells transduced with the indicated vectors. This β-thalassemic mouse strain has the “diffuse” Hb pattern characterized by an uppermost
Improved expression and efficacy of the mLARβΔγV5 vector in murine β-thalassemia
Transplant group . | n . | % F* . | % F cells† . | Vector copy‡ . | Hb, g/L . | % Reticulocytes . | Spleen weight, mg . |
|---|---|---|---|---|---|---|---|
| Mock | 5 | NA | NA | NA | 86 ± 3 | 22 ± 3.4 | 205 ± 21 |
| d432βΔγm | 5 | 8.4 ± 2 | 31 ± 5 | 2.0 ± 0.3 | 103 ± 3§ | 17.3 ± 1.0∥ | 159 ± 7¶ |
| mLARβΔγV5 | 6 | 22 ± 4♯ | 71 ± 10♯ | 1.3 ± 0.3** | 112 ± 3§†† | 6.4 ± 0.7§♯ | 115 ± 8§♯ |
| Normal control | 5 | NA | NA | NA | 122 ± 02§ | 4.0 ± 0.3§ | ND |
Transplant group . | n . | % F* . | % F cells† . | Vector copy‡ . | Hb, g/L . | % Reticulocytes . | Spleen weight, mg . |
|---|---|---|---|---|---|---|---|
| Mock | 5 | NA | NA | NA | 86 ± 3 | 22 ± 3.4 | 205 ± 21 |
| d432βΔγm | 5 | 8.4 ± 2 | 31 ± 5 | 2.0 ± 0.3 | 103 ± 3§ | 17.3 ± 1.0∥ | 159 ± 7¶ |
| mLARβΔγV5 | 6 | 22 ± 4♯ | 71 ± 10♯ | 1.3 ± 0.3** | 112 ± 3§†† | 6.4 ± 0.7§♯ | 115 ± 8§♯ |
| Normal control | 5 | NA | NA | NA | 122 ± 02§ | 4.0 ± 0.3§ | ND |
Values represent the mean and SEM.
Hb indicates hemoglobin concentration; NA, not applicable; and ND, not done.
Amount of mα2, hγ2 present in the peripheral blood as a percentage of the total Hb.
Percentage of red cells staining for γ-globin expression as determined by FACS analysis.
Average vector copy number in PB leukocytes as determined by semiquantitative DNA PCR.
Statistically significant difference from mock group with P at < .01, one-sided.
Not statistically different from mock with P = .11, one-sided.
Statistically different from mock group with P < .05, one-sided.
Statistically significant difference from d432βΔγm group with P at least < .02, one-sided.
Not significantly different from d432βΔγm group with P = .098, 2-sided.
Statistically significant difference from d432βΔγm group with P < .03, one-sided.
The higher γ-globin expression of the mLARβΔγV5 vector was associated with an enhanced therapeutic effect, relative to the d432βΔγm vector (Table 2). The mLARβΔγV5 vector directed an output of 20 g/L (2.0 g/dL) of Hb per vector copy compared with the 8.5 g/L (0.85 g/dL) output per vector copy of the d432βΔγm vector (Figure 2B). This translated into an average 26 g/L (2.6 g/dL) improvement (compared with mock-transduced cells, P < .0003) in Hb concentration for animals that received transplants of mLARβΔγV5-transduced cells, which was higher than the average 17 g/L (1.7 g/dL) increment observed for mice that received transplants of d432βΔγm-transduced cells (Figure 2B, P = .029). Significant differences in the degree of correction of β-thalassemic red cell morphologic abnormalities and red cell indices (data not shown), reticulocyte counts, and extramedullary erythropoiesis, as judged by spleen size, were also observed between mice receiving cells transduced with the 2 vectors (Table 2). Despite the high-level production of HbF directed by the mLARβΔγV5 vector, complete hematologic cure did not occur (compared with mice transplanted with normal control HW80 BM cells, Table 2). This may reflect the possibility that expression of human γ-globin cannot fully complement the absence of murine β-globin, perhaps due to species-related effects regarding the production of the human globin chains in murine erythroid cells and their subsequent interaction with murine α-globin.18
Analysis of position effects on γ-globin vector expression in clonal CFU-S erythroid cells
Since we previously observed that expression of the d432βΔγm vector demonstrated significant susceptibility to chromosomal position effects,3 we hypothesized that the improved expression and efficacy of the mLARβΔγV5 vector could be due to resistance to these effects. To address this possibility, we assessed the probability of expression of each globin vector in unique clonal erythroblast populations containing single-copy vector insertions. BM cells were harvested from 4 of the primary recipients of mLARβΔγV5-transduced cells and d432βΔγm-transduced cells and injected into irradiated, secondary recipient mice. Thirteen days after transplantation, clonal splenic colonies derived from primitive CFU-S cells were obtained and TER119 brightly positive erythroblasts were assessed for γ-globin expression by FACS analysis. Concurrently, the presence, number, and pattern of vector integrations for each CFU-S clone were determined by Southern blot analysis (data not shown). DNA-positive colonies from the mLARβΔγV5 and d432βΔγm secondary mice having unique vector–genomic DNA junction fragments on Southern blot analysis were identified, with some clones in both groups occurring many times. The unique single-copy mLARβΔγV5 clones demonstrated a more than 2-fold-higher probability of γ-globin expression (60% versus 24%, P = .031, one-sided) than did the single-copy d432βΔγm clones (Figure 3A-B). In contrast, the proportion of TER119-positive cells (Figure 3A) did not differ between the 2 groups (mLARβΔγV5 clones 95% versus d432βΔγm clones 93%, P = .53, 2-sided), indicating that there were no significant differences in the erythroid composition of the clonal populations under comparison.19 The level of γ-globin expression, as represented by the mean fluorescence intensity (MFI), trended higher for the single-copy mLARβΔγV5 clones relative to the d432βΔγm clones (226 versus 152, P = .15, one-sided). These data suggested that the mLARβΔγV5 vector was less susceptible to position dependent effects on expression.
Expression of the mLARβΔγV5 γ-globin vector is less susceptible to stable position effects. (A) FACS analysis for TER119 and HbF expression in representative unique, single-copy secondary CFU-S clones derived from primary mice receiving cells transduced with the indicated vector. For the TER119 analysis, M1 represents the gate for cells staining above the isotype control background; the percentage of TER119-staining cells is indicated in each histogram. R2 designates the brightly staining TER119-positive cells that were analyzed for HbF expression. The percentage of HbF+ cells for each clone is indicated in the histogram to the right of the TER119 histogram. (B) The percentage of HbF+ cells in TER119 clonal erythroblast populations containing the indicated γ-globin vector was determined by FACS analysis. Each column of diamonds represents individual secondary CFU-S clones, derived from primary mice that underwent transplantation, harboring a unique, single integration of the indicated vector. The mean ± SEM of HbF+ cells for each group is shown, with the mean indicated by the solid horizontal bar. The P value indicates a statistically significant difference between the mean values of the 2 groups.
Expression of the mLARβΔγV5 γ-globin vector is less susceptible to stable position effects. (A) FACS analysis for TER119 and HbF expression in representative unique, single-copy secondary CFU-S clones derived from primary mice receiving cells transduced with the indicated vector. For the TER119 analysis, M1 represents the gate for cells staining above the isotype control background; the percentage of TER119-staining cells is indicated in each histogram. R2 designates the brightly staining TER119-positive cells that were analyzed for HbF expression. The percentage of HbF+ cells for each clone is indicated in the histogram to the right of the TER119 histogram. (B) The percentage of HbF+ cells in TER119 clonal erythroblast populations containing the indicated γ-globin vector was determined by FACS analysis. Each column of diamonds represents individual secondary CFU-S clones, derived from primary mice that underwent transplantation, harboring a unique, single integration of the indicated vector. The mean ± SEM of HbF+ cells for each group is shown, with the mean indicated by the solid horizontal bar. The P value indicates a statistically significant difference between the mean values of the 2 groups.
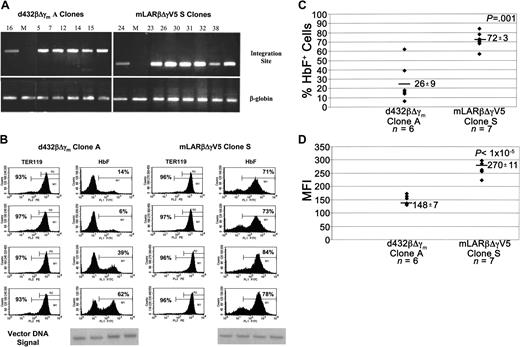
To ascertain the variability of vector expression in progeny derived from the same transduced clone (position effect variegation), individual mLARβΔγV5 and d432βΔγm spleen colony clones that were independently identified several times in the secondary transplant recipients were compared for HbF expression according to vector copy number. Southern blot analysis using 2 different enzymes that cut once within each vector identified a group of single-copy clones of each vector having identical integration patterns (data not shown). Utilizing sequence data obtained by LM-PCR to determine the site of genomic vector integration for one of these clones in each group (see “Materials and methods”), a PCR assay using integration site–specific primers unequivocally verified the presence of the unique insertion in all of the other clones having the same Southern blot banding pattern (Figure 4A). The single-copy type “S” mLARβΔγV5 clones displayed a significantly higher (72% versus 26%, P = .001, one-sided) probability of globin expression compared with the type “A” d432βΔγm clones (Figure 4B-C). Differences in expression were not due to differences in the number of transduced cells present in the populations analyzed, as judged by Southern blot analysis of the vector DNA signal (Figure 4B and data not shown) or differences in the proportions of TER119-positive cells (both with a mean of 95%). Additionally, the level of HbF expression for the mLARβΔγV5 clones, reflected by the MFI, was significantly higher relative to the d432βΔγm clones (Figure 4D).
Expression of the mLARβΔγV5 γ-globin vector in multiple isolates of a single-copy clone is less susceptible to variegating position effects. (A) Top panels: PCR analysis of genomic DNA for the different d432βΔγm clone A and mLARβΔγV5 clone S isolates, using integration site–specific primers. Bottom panels show PCR amplification using β-globin primers as a positive control. The numbers above the lanes represent the clone numbers, and M indicates a mock-transduced CFU-S isolate. (B) FACS analysis for TER119 and HbF expression in representative CFU-S isolates of d432βΔγm clone A and mLARβΔγV5 clone S. Analysis was as in Figure 3 with the percentage of TER119+ and HbF+ cells indicated in the histograms for each isolate. Shown below is the vector DNA signal for each clone as determined using Southern blot analysis. (C) The percentage of HbF+ cells as determined by FACS analysis is shown for each isolate of the indicated d432βΔγm and mLARβΔγV5 clones. The mean ± SEM of HbF+ cells for each group is shown, with the mean indicated by the solid horizontal bar. The P value indicates a statistically significant difference between the mean values of the 2 groups. (D) The MFI of staining with the HbF monoclonal antibody is shown for the different d432βΔγm clone A and mLARβΔγV5 clone S isolates. The mean ± SEM MFI values for each group are shown, with the horizontal solid bar indicating the mean value for each group of clones. The P value indicates a statistically significant difference between the mean values of the 2 groups.
Expression of the mLARβΔγV5 γ-globin vector in multiple isolates of a single-copy clone is less susceptible to variegating position effects. (A) Top panels: PCR analysis of genomic DNA for the different d432βΔγm clone A and mLARβΔγV5 clone S isolates, using integration site–specific primers. Bottom panels show PCR amplification using β-globin primers as a positive control. The numbers above the lanes represent the clone numbers, and M indicates a mock-transduced CFU-S isolate. (B) FACS analysis for TER119 and HbF expression in representative CFU-S isolates of d432βΔγm clone A and mLARβΔγV5 clone S. Analysis was as in Figure 3 with the percentage of TER119+ and HbF+ cells indicated in the histograms for each isolate. Shown below is the vector DNA signal for each clone as determined using Southern blot analysis. (C) The percentage of HbF+ cells as determined by FACS analysis is shown for each isolate of the indicated d432βΔγm and mLARβΔγV5 clones. The mean ± SEM of HbF+ cells for each group is shown, with the mean indicated by the solid horizontal bar. The P value indicates a statistically significant difference between the mean values of the 2 groups. (D) The MFI of staining with the HbF monoclonal antibody is shown for the different d432βΔγm clone A and mLARβΔγV5 clone S isolates. The mean ± SEM MFI values for each group are shown, with the horizontal solid bar indicating the mean value for each group of clones. The P value indicates a statistically significant difference between the mean values of the 2 groups.
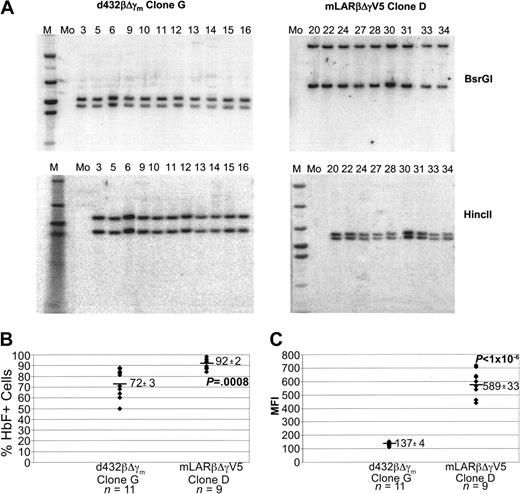
A second group of mLARβΔγV5 and d432βΔγm clones were identified having 2 vector copies. Southern blot analyses with 2 different enzymes that each cut once within the vector genomes demonstrated identical banding patterns for the clones within each group (Figure 5A). These data strongly suggested that the clones in each group were identical given the highly unlikely possibility of comigration of 2 hybridizing vector bands in each Southern blot. The type “D” mLARβΔγV5 clones showed a higher probability of globin expression compared with the type “G” d432βΔγm clones (Figure 5B), as well as a significantly higher MFI (Figure 5C).
Expression of the mLARβΔγV5 γ-globin vector in multiple isolates of a 2-copy clone is less susceptible to variegating position effects. (A) Southern blot analysis of DNAs from d432βΔγm and mLARβΔγV5 clones. DNAs were digested with BsrGI or HincII as indicated, liberating a viral vector–genomic DNA junction fragment. The lane numbers represent clone numbers, M represents lanes containing the molecular weight marker, and Mo indicates a sample from a mock-transduced CFU-S isolate. (B) The percentage of HbF+ cells as determined by FACS analysis is shown for each isolate of the indicated d432βΔγm G and mLARβΔγV5 D clones. The mean ± SEM of HbF+ cells for each group is shown, with the mean indicated by the solid horizontal bar. The P value indicates a statistically significant difference between the mean values of the 2 groups. (C) The MFI of staining with the HbF monoclonal antibody is shown for the different d432βΔγm clone G and mLARβΔγV5 clone D isolates. The mean ± SEM MFI values for each group are shown, with the horizontal solid bar indicating the mean value for each group of clones. The P value indicates a statistically significant difference between the mean values of the 2 groups.
Expression of the mLARβΔγV5 γ-globin vector in multiple isolates of a 2-copy clone is less susceptible to variegating position effects. (A) Southern blot analysis of DNAs from d432βΔγm and mLARβΔγV5 clones. DNAs were digested with BsrGI or HincII as indicated, liberating a viral vector–genomic DNA junction fragment. The lane numbers represent clone numbers, M represents lanes containing the molecular weight marker, and Mo indicates a sample from a mock-transduced CFU-S isolate. (B) The percentage of HbF+ cells as determined by FACS analysis is shown for each isolate of the indicated d432βΔγm G and mLARβΔγV5 D clones. The mean ± SEM of HbF+ cells for each group is shown, with the mean indicated by the solid horizontal bar. The P value indicates a statistically significant difference between the mean values of the 2 groups. (C) The MFI of staining with the HbF monoclonal antibody is shown for the different d432βΔγm clone G and mLARβΔγV5 clone D isolates. The mean ± SEM MFI values for each group are shown, with the horizontal solid bar indicating the mean value for each group of clones. The P value indicates a statistically significant difference between the mean values of the 2 groups.
Increased probability of γ-globin vector expression in intergenic vector insertion locations
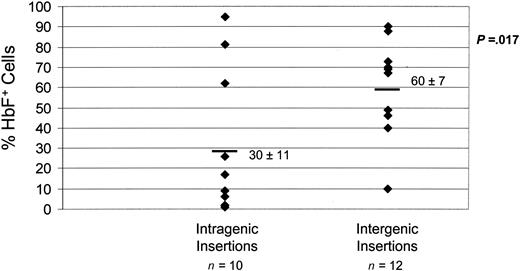
To determine in more detail how specific genomic sites of vector integration may influence globin vector expression, globin vector–genomic DNA junction sites were cloned by LM-PCR (see “Materials and methods”) and the insertion site location in the mouse genome identified for the majority of the single-copy, secondary CFU-S clones of both vectors (7 mLARβΔγV5 and 3 d432βΔγm clones) and for 12 additional single-copy, primary d432βΔγm CFU-S clones. Vector insertion sites were determined to be intragenic if they occurred within the exonic/intronic structure of a gene, or intergenic if they occurred outside of such structures. An insertion site location was identified for each of the 22 clones. Ten (45%) of 22 of the insertions occurred in intragenic locations within introns, consistent with the predilection of lentiviral integration into genes.12,20,21 When globin vector expression was assessed as a function of insertion type, intergenic insertion sites were observed to have a significantly higher probability of expression in progeny erythroblasts compared with insertions occurring in intragenic locations (Figure 6). Interestingly, for the intragenic vector insertions, vector orientation with respect to the transcriptional direction of the gene did not seem to influence the probability of vector expression (data not shown). In subgroup analysis, 2 of 3 intragenic mLARβΔγV5 insertions expressed well (> 50% HbF+ erythroblasts), whereas only 1 of 7 intragenic d432βΔγm insertions expressed well. Furthermore, whereas 3 of 4 intergenic mLARβΔγV5 insertions expressed well, only 4 of 8 intergenic d432βΔγm insertions expressed well. Thus, a significant proportion of globin vector integrations occurred within genes, which was overall associated with compromised expression. However, mLARβΔγV5 vector expression appeared to be influenced to a lesser degree by vector insertion location, likely accounting for its improved expression and efficacy.
Increased probability of γ-globin vector expression at intergenic sites of vector integration. The percentage of HbF+ cells as determined by FACS analysis is shown for 22 CFU-S clones as a function of the site of insertion (intragenic or intergenic) within the cellular genome. The mean ± SEM of HbF+ cells for each group is shown, with the mean indicated by the solid bar. The P value indicates a statistically significant difference between the mean values of the 2 groups.
Increased probability of γ-globin vector expression at intergenic sites of vector integration. The percentage of HbF+ cells as determined by FACS analysis is shown for 22 CFU-S clones as a function of the site of insertion (intragenic or intergenic) within the cellular genome. The mean ± SEM of HbF+ cells for each group is shown, with the mean indicated by the solid bar. The P value indicates a statistically significant difference between the mean values of the 2 groups.
Discussion
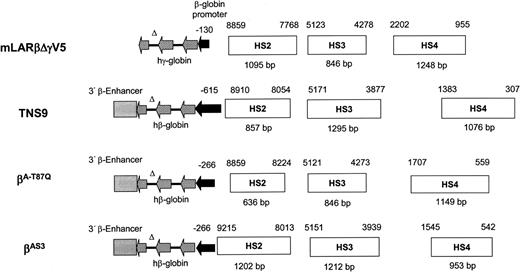
The use of vectors encoding γ-globin, rather than β-globin or β-globin variants, provides an attractive alternative therapeutic approach for gene therapy of β-thalassemia and sickle cell disease.3,22-27 Here, we show that incorporation of extended regulatory elements from the β-globin LCR into a γ-globin lentiviral vector significantly enhances the degree of therapeutic efficacy obtained through stem cell gene transfer in murine β-thalassemia. This improved efficacy was attributable to more consistent and higher levels of γ-globin expression at the clonal level. Mice that received transplants of stem cells transduced with mLARβΔγV5 had total HbF levels of 17% to 33%, which resulted in significant phenotypic improvement. In contrast, despite a higher average vector copy number, none of 5 mice treated with stem cells transduced with d432βΔγm had HbF levels more than 15%, and only 2 mice had levels more than 10%. The latter results are consistent with our previous work using d432βΔγm, in which about 25% of mice achieved significant HbF levels and phenotypic improvement.3 Compared with the d432βΔγm vector, we observed a 4-fold enhancement in output of HbF per vector copy with mLARβΔγV5, which translated to a more than 2-fold increase in the Hb increment per vector copy. Since the β-globin LCR HS3 element was similar in both vectors (Figure 1A), the enhancement in expression appears attributable to inclusion of additional sequences flanking the HS2 and HS4 cores. Indeed, a previous study using murine erythroleukemia cell clones containing single-copy expression cassettes at specific chromosomal loci showed that these sequences flanking the HS cores provide synergistic enhancement of expression.11 In this regard, the mLARβΔγV5 vector provided significant phenotypic correction with a relatively modest average vector copy of 1.3, whereas higher average vector copy numbers were required to achieve a significant therapeutic effect in other studies.2,4,5 In contrast, the β-globin lentiviral vector of Sadelain and colleagues, containing 3.2 kb of LCR sequences, performed similarly to our current vector, achieving substantial globin expression with an average vector copy number of 0.8.1 It is likely that the differences in vector performance observed in this and the other studies to date reflect the differences in the LCR sequences contained in the various reported vectors (Figure 7).
Schematic representation of HIV-1–based, lentiviral globin expression cassettes utilized to correct murine models of β-thalassemia and sickle cell disease. The mLARβΔγV5 globin expression cassette is compared with those contained in TNS9, βA-T87Q, and βAS.31,2,5 DNA fragments containing the HS sites and flanking sequences appear as open horizontal rectangles, the solid horizontal arrow indicates the β-globin promoter, and the hatched horizontal arrows indicate the genomic β- or γ-globin sequences contained in the mature mRNA. The solid lines connecting the 3 arrows represent intronic sequences; all of the vectors utilized a deletion in intron 2 to improve vector performance. The solid gray rectangle indicates the 3′β-globin enhancer sequence. The fragment sizes of the DNA elements derived from the β-globin LCR that contain the indicated HS sites are shown below each horizontal rectangle, whereas the GenBank coordinates (accession number U01317) of the sequences are shown above each rectangle.
Schematic representation of HIV-1–based, lentiviral globin expression cassettes utilized to correct murine models of β-thalassemia and sickle cell disease. The mLARβΔγV5 globin expression cassette is compared with those contained in TNS9, βA-T87Q, and βAS.31,2,5 DNA fragments containing the HS sites and flanking sequences appear as open horizontal rectangles, the solid horizontal arrow indicates the β-globin promoter, and the hatched horizontal arrows indicate the genomic β- or γ-globin sequences contained in the mature mRNA. The solid lines connecting the 3 arrows represent intronic sequences; all of the vectors utilized a deletion in intron 2 to improve vector performance. The solid gray rectangle indicates the 3′β-globin enhancer sequence. The fragment sizes of the DNA elements derived from the β-globin LCR that contain the indicated HS sites are shown below each horizontal rectangle, whereas the GenBank coordinates (accession number U01317) of the sequences are shown above each rectangle.
Previous studies of mice containing ectopic globin transgenes have shown that the site of transgene integration can affect both the probability and pattern of globin expression in clonal erythroid progeny.28-30 One novel aspect of our current study is the evaluation of both stable and variegating position effects on globin vector expression in clonal erythroblasts populations containing single-copy vector insertions. Expression of the mLARβΔγV5 vector was less susceptible to stable chromosomal position effects (Figure 3). Instances where a unique erythroblast clonal population was independently identified several times allowed us to also determine that the mLARβΔγV5 vector demonstrated less position effect variegation of expression (Figures 4 and 5). Interestingly, a deleterious effect on vector expression was observed when vector insertions occurred within genes (Figure 6). However, the mLARβΔγV5 vector showed a trend toward better expression, relative to the d432βΔγm vector, even in intragenic sites. Although further studies are required to investigate the mechanisms underlying the more variable globin vector expression observed in intragenic sites of integration, transcriptional interference is one possible factor that could compromise vector expression and partly underlie the basis for position effects.31 Consistent with this hypothesis are recent data indicating that highly expressed genes lie distant from their closest neighboring genes, suggesting that transcriptional interference is a contributing factor in determining the overall expression level of a gene.32
Of the clones with single-copy vector insertions, 45% were intronic, consistent with previous work using cell lines and rhesus macaques that underwent transplantation that showed a bias of lentiviral vectors to integrate within genes.12,20,21 This observation, coupled with our finding here that intragenic insertion sites can compromise vector expression, provides a possible explanation for the requirement of multiple globin vector copies per transduced cell to achieve therapeutic expression levels.2-5 Although it is unknown whether multiple vector insertions per cell may increase the risk for insertional mutagenesis in humans,9 available experimental data suggest a correlation between vector insertion number and mutagenesis.33,34 Thus, globin vector designs that promote consistent, high-level expression under single-copy conditions are desirable to minimize the need and potential risk of multiple vector insertions per cell.35
Despite the promise of lentiviral vectors to improve the efficiency of human HSC gene transfer, it is uncertain whether they will provide consistent stem cell gene transfer of the order needed for a therapeutic effect in humans with hemoglobin disorders. In this regard, a method to increase the proportion of genetically corrected stem cells will likely be necessary. Recently, we demonstrated that an in vivo selection system based on vectors encoding the methylguanine methyltransferase drug resistance protein could correct murine β-thalassemia through enrichment of transduced HSCs.36 Significant in vivo selection using this system in a canine transplantation model has also recently been demonstrated.37 This approach therefore appears promising as an avenue to increase corrected stem cell chimerism to therapeutic levels.
Our recent observation that simian immunodeficiency virus–based lentiviral vectors can efficiently transduce rhesus macaque hematopoietic repopulating cells12 will allow testing of globin lentiviral vectors in the rhesus macaque transplantation model. The evaluation of globin vector gene transfer efficiency, expression, and safety considerations in a large animal model that accurately reflects transplantation of autologous, vector-transduced stem cells in humans should prove valuable.38 The goal of such studies is to provide convincing preclinical efficacy and safety data prior to human clinical trials. We believe that this approach will maximize the likelihood of success in the context of an acceptable risk-to-benefit ratio for patients.
Prepublished online as Blood First Edition Paper, June 15, 2004; DOI 10.1182/blood-2004-03-0863.
Supported in part by the National Heart, Lung, and Blood Institute (NHLBI) grant KO8 HL04205 (D.A.P.), NHLBI grant HL070705 (D.A.P.), NHLBI Comprehensive Sickle Cell Center grant U54 HL70590 (D.A.P.), NHLBI Program Project PO1 HL53749 (A.W.N.), Cancer Center Support (CORE) grant CA-21765 (A.W.N.), and the American Lebanese Syrian Associated Charities.
A.W.N. and D.A.P. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the flow cytometry laboratory of Dr Richard Ashmun for expertise in FACS analysis. We thank Dr Mark Groudine for permission to use the LCR configuration contained in plasmid L1-432mLAR-bEGFP-1L, which was generously provided by Dr Ross Hardison. We also appreciate the advice of Dr Susan Imren in establishing the antibody staining procedure for the CFU-S assay. We appreciate the help of Ms Alicia Malone in manuscript preparation.

![Figure 2. Consistent, therapeutic, γ-globin expression of the mLARβΔγV5 lentiviral vector in β-thalassemic mice. (A) Cellulose acetate Hb electrophoresis gels were used to separate the different Hb species in red cell lysates from mice that underwent transplantation with β-thalassemic BM cells transduced with the indicated vectors. This β-thalassemic mouse strain has the “diffuse” Hb pattern characterized by an uppermost \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(\mathrm{m}{\alpha}_{2}\mathrm{m}{\beta}_{2}^{\mathrm{minor}}\) \end{document} species and a faster migrating \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(\mathrm{m}{\alpha}_{2}{\beta}_{2}^{\mathrm{maj}}\) \end{document} species. Chimeric mα2hγ2 molecules (termed HbF, indicated by the solid horizontal arrow) migrate faster than the endogenous murine Hb species. No endogenous murine “single” Hb molecules, which migrate between the “diffuse” and chimeric species, were observed, indicating full donor engraftment. The left panel shows samples from mice that underwent transplantation with BM cells transduced with the d432βΔγm vector, whereas the right panel shows samples from mice that underwent transplantation with mLARβΔγV5-transduced cells. Percent F equals the quantity of the chimeric mα2hγ2 species, estimated by densitometry, as a proportion of all Hb species. Vector copy number (VC no.) is the average copy number in PBLs as estimated by DNA PCR. The percent F per VC is the mean output of chimeric mα2hγ2 molecules per average vector copy number. (B) Mean Hb concentration (± standard error of the mean [SEM]) of mice that underwent transplantation with mock-transduced β-thalassemic BM cells, β-thalassemic BM transduced with the indicated γ-globin vectors, or mock-transduced wild-type (WT) BM cells. The mean Hb concentration improvement per vector copy is indicated where applicable. *Statistically significant difference in mean Hb value of mLARβΔγV5 mice versus d432βΔγm mice, P = .029, one-sided.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/8/10.1182_blood-2004-03-0863/6/m_zh80200468190002.jpeg?Expires=1764963619&Signature=zb4qibJjQBE2rBiopYzwkwJhWP~AJfeRYQ5LtR4t7nWDJfBpENsYi6iOffeRMFRUWcUXhjw6tsUHbvt798cYCn3MhXc0-szG7TRrC2K32S9~EFPoMgd3k8Pl68zbhmc5b3WfavZCaJsW8wWt0pdm24EifWLArR5TF0kPP5VLGiAS8UycWC0xVTXRDX4FhWqHg66uD9sPtGV71y-8xGfpFpkCEWyZnHX7qLNmFROewaEiKJSvsLn5TXtF0eqBkdJNnle~veBxfTCU8DYlxCKMqHKN6EKkCweBPcKzNy~4dPqUO7dJSYkMbnMhf-RqhmvSsXc3Sna2YBBLcrW9pC7dDw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal