Abstract
Rituximab (MabThera, Rituxan) is a chimeric IgG1 monoclonal antibody that specifically targets the CD20 surface antigen expressed on normal and neoplastic B-lymphoid cells. Rituximab is currently used in the treatment of both follicular and aggressive B-cell non-Hodgkin lymphomas. Despite its demonstrated clinical effectiveness, its in vivo mechanisms of action remain unknown and could differ by subtype of lymphoma. Rituximab has been shown to induce apoptosis, complement-mediated lysis, and antibody-dependent cellular cytotoxicity in vitro, and some evidence points toward an involvement of these mechanisms in vivo. Rituximab also has a delayed therapeutic effect as well as a potential “vaccinal” effect. Here, we review the current understanding of the mechanism of action of rituximab and discuss approaches that could increase its clinical activity. A better understanding of how rituximab acts in vivo should make it possible to develop new and more effective therapeutic strategies. (Blood. 2004;104:2635-2642)
Introduction
Rituximab is a chimeric monoclonal antibody (mAb) produced by recombinant technology. It binds specifically to CD20, an antigen expressed by most human B lymphocytes. Rituximab was created by fusing the light- and heavy-chain variable domains of 2B8, a murine monoclonal anti-CD20 antibody and human κ light-chain and γ1 heavy-chain constant regions.1 Rituximab is currently indicated in both follicular and aggressive B-cell non-Hodgkin lymphomas (NHL). It is the first approved targeted treatment in the field of oncology and its impact on the treatment of NHL is evidenced by the short interval between its initial description1 and its approval by both American and European authorities (1997 and 1998, respectively). Rituximab has some therapeutic activity in antibody-based autoimmune diseases,2 and its indications are likely to be extended in the next few years. Rituximab has become the hallmark of anticancer mAbs, which offer a new approach to the treatment of B-cell lymphoproliferative disorders.
Although the clinical effectiveness of rituximab is no longer in question, its in vivo mechanisms of action have yet to be elucidated. In vitro data suggest that it induces apoptosis, complement-mediated lysis (complement-dependent cytotoxicity [CDC]), and antibody-dependent cellular cytotoxicity (ADCC) and some studies seem to confirm the involvement of these mechanisms in vivo. Other studies have suggested that rituximab may have a “vaccinal” effect. Most of these studies were performed with CD20+ cell lines in vitro and in murine models and in some cases with fresh neoplastic B cells in vitro. It is therefore difficult to evaluate the respective contribution of each of these effects that, moreover, could have different contributions according to lymphoma subtype. Several recent studies on CD20 structure and downstream signaling pathways have put forward key arguments that have helped to elucidate the in vivo mechanisms of action of rituximab. A better understanding of rituximab-induced cell killing will allow the development of new, more effective antibodies derived from rituximab as well as innovative treatment modalities designed to optimize its mechanisms of action through selection of the most sensitive tumors, potentiation of cytotoxicity mechanisms or neutralization of resistance, design of optimal dosing schedules, and finding the best synergies. This review focuses on the current understanding of the mechanism of action of rituximab and suggests ways to improve its clinical efficacy.
The CD20 antigen
CD20 (or membrane-spanning 4-domain, group A, member 1 [MS4A1]) is a nonglycosylated protein of 33 to 35 kDa expressed on the surface of human B lymphocytes and also weakly on a small subset of T cells.3 The MS4A1 gene is located on chromosome 11q12-13. It is switched on at the pre-B-cell stage of B-cell development, expressed throughout B-cell maturation, and lost during final maturation to plasma cells. Because CD20 antigen is not expressed by either plasma cells or B-lymphoid stem cells, rituximab does not affect significantly immunoglobulin serum concentrations, and after a conventional 4-dose course of rituximab therapy, B-lymphocyte counts recover in 9 to 12 months.4,5 The MS4A1 promoter contains potential sites for several transcription factors such as Oct-1 and Oct-2, which bind to the BAT box,6 and PU.1 and Pip, which bind to the -160 site.7 Pip is a member of the interferon regulatory factor family and deletion of the BAT box and the -160 site results in an inactive promoter.6,8 Some evidence indicates that various cytokines including interferon α (IFN-α), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-4 (IL-4), and tumor necrosis factor α (TNF-α) up-regulate CD20 expression on lymphoma cells in vitro and perhaps in vivo.9 However, the mechanism of this up-regulation is unknown. Although some of these cytokines may increase the therapeutic activity of rituximab,10,11 there is no direct evidence that this is related to increased CD20 expression.
The CD20 protein has 4 membrane-spanning domains with both the amino and carboxy termini of the protein located within the cytoplasm (Figure 1), and only a short extracellular segment of about 43 residues between the third and fourth transmembrane regions.12 This configuration, deeply anchored in the membrane, protects from antigen shedding. Circulating “soluble forms” of CD20 have been detected in the serum of patients with chronic lymphocytic leukemia and high leukemic cell counts.13 These may originate from microparticles comprising CD20 inserted in their membrane bilayers. Human CD20 is 73% identical to murine CD20 with the transmembrane regions showing the greatest similarity of amino acid sequence.12,14 The extracellular domain differs in 16 of the approximate 43 amino acids,14 which may partly explain why rituximab does not bind to murine CD20. In the plasma membrane, the CD20 protein is probably not expressed in monomeric form, but organized into heterogeneous supramolecular complexes associating CD20 dimers or tetramers.15 CD20 has been reported to be closely associated with other proteins, in particular the transmembrane adapter protein p75/80, also named C-terminal src kinase-binding protein (Cbp),14,16,17 CD40, and major histocompatibility complex class II proteins (MHC II).18 Targeting CD20 with mAbs may therefore interfere with functions associated with CD20 partners such as CD40.18 Until recently, it was assumed that the extracellular loop bears only 2 groups of epitopes, one recognized by the majority of CD20 mAbs (such as B1, 2H7, rituximab), which induce inhibitory effects on B-cell proliferation, and another recognized only by the 1F5 mAb with unusual activating properties.19-23 However, Polyack et al14 have since demonstrated a high degree of epitope diversity among CD20 mAbs, which might explain the variety of biochemical and biologic effects of different anti-CD20 mAbs observed in vitro.24 The extracellular alanine and proline at positions 170 and 172, respectively, are critical in determining the rituximab epitope, although neighboring residues as well as the overall state of the oligomeric complex seem to be important.
CD20 antigen structure and organization into membrane bilayers. (A) The CD20 protein has 4 cell membrane-spanning domains with both the amino and carboxy termini of the protein located within the cytoplasm. CD20 is closely associated with other proteins, in particular the C-terminal src kinase-binding protein (Cbp), CD40, and major histocompatibility complex class II proteins (MHC II). (B) Antibody binding to CD20 induces rapid translocation of the molecule to lipid rafts. Cbp can concentrate the C-terminal Src kinases (Csk) such as Lyn, Flyn, or Lck, which phosphorylate Cbp, leading to induction of kinase activity.
CD20 antigen structure and organization into membrane bilayers. (A) The CD20 protein has 4 cell membrane-spanning domains with both the amino and carboxy termini of the protein located within the cytoplasm. CD20 is closely associated with other proteins, in particular the C-terminal src kinase-binding protein (Cbp), CD40, and major histocompatibility complex class II proteins (MHC II). (B) Antibody binding to CD20 induces rapid translocation of the molecule to lipid rafts. Cbp can concentrate the C-terminal Src kinases (Csk) such as Lyn, Flyn, or Lck, which phosphorylate Cbp, leading to induction of kinase activity.
Antibody binding to CD20 induces rapid translocation of the molecule to lipid rafts,16,25 which are membrane microdomains enriched in cholesterol and sphingolipids (Figure 1). The association between CD20 and lipid rafts is dependent on cholesterol and on a short membrane-proximal cytoplasmic sequence (residues 219-225), which differs between murine and human CD20.25 These lipid rafts are platforms for signal transduction, allowing the colocalization of receptors and signaling effectors. The likely association of CD20 with tyrosine kinases is supported by the observation that several of the biologic effects of anti-CD20 antibodies (homotypic aggregation, c-myc induction by 1F5 antibody) are dependent on tyrosine kinase.26,27 The presence of Cbp in association with CD20 in lipid rafts could concentrate a C-terminal Src kinase (Csk) such as Lyn, Fyn, or Lck,17 which phosphorylates Cbp, allowing autophosphorylation of Csk and thus inducing kinase activity.28,29 Recent data also suggest a role of acid sphingomyelinase and ceramide in CD20 signaling.30 These recent studies have helped to elucidate CD20 antigen structure and localization in lipid rafts, providing insight into the diversity of anti-CD20 mAbs and their various biologic effects.
CD20 function and agonist effects of rituximab
No CD20 ligand has been described and CD20 does not display the usual structure of a receptor. CD20-deficient mice do not have any obvious defect of B-cell function.31 Therefore, the exact in vivo function of CD20 remains largely unknown. Recently, some data indicated that CD20 could play an important role in Ca2+ influx across plasma membranes, sustaining intracellular Ca2+ concentration and allowing activation of B cells.32 The usual, but artificial, technique to assess CD20-activated signaling events is based on the use of anti-CD20 agonist mAbs. From these studies, it appears that anti-CD20 mAbs regulate the cell cycle and also induce a wide variety of other biologic responses including an increase in the expression of MHC II or adhesion molecules (lymphocyte function-associated antigen [LFA-1, LFA-3]),27 shedding of the B cell-expressed CD23,33 down-regulation of the B-cell receptor,34 and induction of apoptotis29,35-38 or, conversely, rescue from apoptosis.39
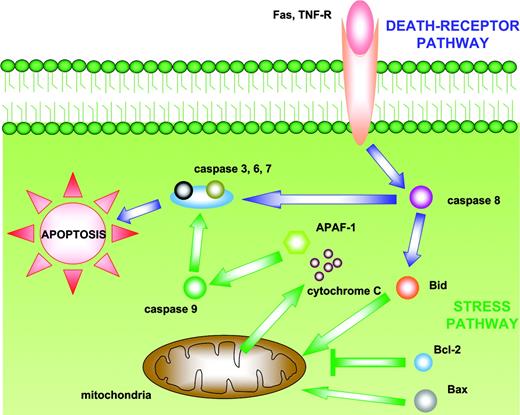
The possibility that CD20 plays a crucial role in regulating cell cycle progression, at least in normal B cells, is supported by the fact that the mAb 1F5 induces cell cycle transition from G0 to G1, whereas B1-like mAbs inhibit B-cell progression from the G1 to the S/G2 plus M phases.19-21 Rituximab has been shown to induce apoptosis in numerous B-cell lines.29,36-38 However, induction of apoptosis in vivo frequently requires hypercross-linking, that is, cross-linking of antigen-bound anti-CD20 antibodies with secondary antibodies.29,36-38 Apoptosis is mediated by 2 main pathways leading to activation of cytosolic cysteine-proteases, named caspases (Figure 2). Intracellular stress signals induce apoptosis through the bcl-2 family of proteins and subsequent activation of caspase-9 (mitochondrial pathway), whereas the death-receptor pathway (Fas, TNF-related apoptosis-inducing ligand [TRAIL], and tumor necrosis factor [TNF] receptors) is induced by external signals and involves the activation of caspase-8. Caspases-8 and -9 then activate downstream caspases such as caspase-3, resulting in cell death. It has been reported that CD20-mediated Src activation leads to rapid phosphorylation of phospholipase C-γ1 (PLC-γ1) and PLC-γ2,26 which induces Ca2+ influx and caspase-3 activation.29,40 CD20-induced apoptosis is significantly reduced by Csk inhibitors,29 caspase inhibitors, and calcium chelation,29,36,37 supporting the model in which CD20 ligation induces Csk activity, leading to PLC-γ phosphorylation, increased cytoplasmic Ca2+, and then caspase-3 activation.28,29 An important feature of apoptosis is its modulation by proapoptotic and antiapoptotic members of the bcl-2 gene family. The bcl-2 protein breaks the cell death machinery by blocking caspase activity, regulating Ca2+ flux, or by exerting an antioxidative activity.41-43 Byrd et al44 have demonstrated that a proportion of patients with B-cell chronic lymphocytic leukemia (B-CLL) treated with rituximab had in vivo activation of caspases-9 and -3, which correlated with depletion of B lymphocytes. This activation might be induced directly by rituximab but also by other mechanisms such as granzymes released by natural killer (NK) cells after binding of rituximab to Fc receptors.45 Two recent studies have questioned the nature and role of caspases in apoptosis induction by anti-CD20 antibodies.46,47 These studies have shown that CD20-induced apoptosis is associated with membrane changes (phosphoserine translocation and mitochondrial permeability changes) but not with DNA fragmentation and chromatin condensation, unless CD20 hypercross-linking is present. Apoptosis is not blocked by caspase-specific concentrations of inhibitors and therefore appears caspase-independent. Moreover, bcl-2 overexpression blocks only mitochondrial permeability changes, but not apoptosis.46,47 These data are important in view of the role of bcl-2 in B-cell NHL, which overexpresses this protein, and considering the observation that rituximab associated with chemotherapy is able to overcome the adverse prognostic influence of bcl-2 overexpression by diffuse large B-cell lymphoma cells.48
The 2 main apoptotic pathways. The “intrinsic” (mitochondrial) pathway is induced by intracellular stress signals leading to translocation of bcl-2 family members into the mitochondria. This induces alterations in mitochondrial permeability and consequent release of cytochrome C, which in association with apoptotic protease-activating factor 1 (APAF-1) activates caspase-9. The “extrinsic” (death-receptor) pathway is activated by the binding of ligands to tumor necrosis factor receptor (TNF-R) or FAS, inducing activation of caspase 8. Both caspase 8 and caspase-9 subsequently activate caspases-3, -6, and -7, which cleave substrates involved in the regulation of cell death.
The 2 main apoptotic pathways. The “intrinsic” (mitochondrial) pathway is induced by intracellular stress signals leading to translocation of bcl-2 family members into the mitochondria. This induces alterations in mitochondrial permeability and consequent release of cytochrome C, which in association with apoptotic protease-activating factor 1 (APAF-1) activates caspase-9. The “extrinsic” (death-receptor) pathway is activated by the binding of ligands to tumor necrosis factor receptor (TNF-R) or FAS, inducing activation of caspase 8. Both caspase 8 and caspase-9 subsequently activate caspases-3, -6, and -7, which cleave substrates involved in the regulation of cell death.
Recent clinical and in vitro data suggest that rituximab synergizes with chemotherapy by sensitizing malignant cells to the cytotoxic and apoptotic effects of drugs such as doxorubicin, cisplatin, dexamethasone, fludarabine, or retinoids.49-53 Rituximab has also been reported to down-regulate IL-10 in some lymphoma cell lines. IL-10 normally promotes bcl-2 expression in B cells, making them more susceptible to apoptotic stimuli.54,55 The in vivo relevance of apoptosis induction by rituximab remains unclear. This antibody is a poor inducer of apoptosis in fresh normal, chronic lymphocytic leukemic, or B-lymphoma cells, in clear contrast to B-cell lines. Finally, 2B8-IgG4 mAb displays the same cross-linking as rituximab but has no effector functions and does not induce B-cell depletion in primates.56 The in vivo reality of the apoptotic effects described for rituximab on cell lines still remains to be demonstrated in human lymphoma tumors.
CDC and CDCC
CDC is an important effector mechanism in the eradication of foreign agents and neoplastic cells. The complement system consists of classical, lectin, and alternative pathways that converge and ultimately generate the same set of effector molecules (Figure 3). The classical pathway requires immunoglobulins, whereas the lectin and alternative pathways are activated primarily by microbial components. The first step in activation of the classical pathway is the binding of the C1q component to Fc portions of IgG or IgM. This binding triggers a proteolytic cascade resulting in the generation of large amounts of C3b, the main effector molecule of the complement system. C3b molecules act as opsonins but also bind to the C3 convertase to form a C5 convertase, leading to the generation of the membrane attack complex (MAC) that kills target cells by disrupting the cell membrane. C3 is abundant in plasma and newly generated C3b is rapidly inactivated unless bound to the C5 convertase complex. C3b can also bind to complement receptors (CRs) expressed on effector cells such as granulocytes, macrophages, or NK cells and induce cell-mediated lysis or phagocytosis, depending on the effector cell complement-dependent cellular cytotoxicity (CDCC).
CDC classical pathway (IgG1, IgG3, IgM). The binding of the C1q component to Fc portions of IgG or IgM triggers a proteolytic cascade, resulting in the generation of large amounts of C3b. C3b molecules act as opsonins but also bind to the C3 convertase to form a C5 convertase, leading to the generation of the membrane attack complex (MAC), which kills the target.
CDC classical pathway (IgG1, IgG3, IgM). The binding of the C1q component to Fc portions of IgG or IgM triggers a proteolytic cascade, resulting in the generation of large amounts of C3b. C3b molecules act as opsonins but also bind to the C3 convertase to form a C5 convertase, leading to the generation of the membrane attack complex (MAC), which kills the target.
Several in vitro studies have demonstrated that rituximab induces CDC in B-lymphoma cell lines1,35,57 and fresh B-lymphoma cells,38,58-60 and this complement-mediated lysis correlates in part with the level of CD20 antigen expression.58,59,61 The sensitivity to CDC induced by rituximab could be different by subtype of lymphoma.59,61 Follicular and mantle cell lymphoma subtypes exhibited better responses to CDC than B-CLL.59,61 The ability of different anti-CD20 mAbs to induce complement-mediated lysis correlates with their capacity to translocate CD20 into lipid rafts,24 an effect that could be dependent on the epitope recognized by the mAb. The increase in complement activation products observed during rituximab treatment62 and its correlation with the side effects observed after the first infusion62 confirm that complement is indeed activated in vivo, but evidence is still lacking in humans to establish a relationship between complement activation and therapeutic efficacy. The recent demonstration that rituximab is unable to cure C1q-deficient mice inoculated with syngenic lymphoma cells (EL4) transduced with human CD20 provides the first in vivo argument showing that complement activation is prerequisite to rituximab action.63 Similar results have also been obtained in a complement-depleted xenograft model of lymphoma.64 Finally, in agreement with the requirement for CDC and its dependence on CD20 expression levels is the observation that in cynomolgus monkeys, normal circulating B cells expressing low levels of CD20 antigen are more resistant than high expressers to rituximab treatment in vivo.65
To prevent host tissue damage, a series of complement regulatory proteins (CtRPs) inhibits this system, among which glycosylphosphatidylinositol (GPI)-anchored proteins CD46, CD55, and CD59 are the most important (Figure 3). CD55 (decay-accelerating factor [DAF]) accelerates the decay of C3 and C5 convertase, and CD46 (membrane cofactor protein [MCP]) acts as a cofactor for the cleavage of C3b and C4b. CD59 prevents pore formation by the MAC. Convertase formation can also be prevented by cleaving C3b to its inactive form C3bi by factor I in conjunction with CD55 and CD46. The CtRPs are expressed by malignant B cells58,59 which might allow them to escape complement attack. Several reports have focused on CtRPs, particularly CD46, CD55, and CD59, in an attempt to relate their levels of expression with CDC. Although a correlation between CD20 expression level and CDC58,59 has been observed, the relationship between CD20/CtRP expression and rituximab-induced CDC has not been established,35,58-60,66 and the correlation with clinical response to rituximab still remains to be demonstrated.60 Manches et al61 have recently shown that CDC in vitro correlates with the ratio of CD20 and CtRP intensities (CD46, CD55, CD59). The neutralization of CD59 and to some extent of CD55 and CD46 by blocking mAbs in vitro increases CDC.57,59,66 Therefore, concomitant targeting of CD20 and neutralization of CD59 by a bispecific mAb could be tested in rituximab-resistant B-cell lymphoid malignancies. Another way to act on CDC would be to improve the binding of C1q on the Fc portion. The C1q-binding site on human IgG1 has recently been mapped67 and a mutant IgG1 with enhanced C1q binding has been created.68 This mutant IgG1 has increased CDC activity but weakened ADCC in vitro.
Although rituximab is usually well tolerated, the first infusion may be complicated by severe first-dose side effects.4 These events are correlated with complement activation and with the number of circulating B cells.62 In some patients, rituximab infusion was associated with severe side effects described as “cytokine-release syndrome,”69 which was fatal in a very limited number of cases.70 However, the levels of cytokines assessed in these patients did not correlate with adverse events62,69 and complement activation could also be responsible for the severity of this side effect. Because complement activation is probably required for the therapeutic effect, the use of anticomplement medications should be avoided. Instead, a reduction of the infusion rate is usually effective in controlling “cytokine-release syndrome.”71 To summarize, in vitro and in vivo data suggest that CDC is an important mechanism underlying the action of rituximab and also some of its adverse reaction and future studies should be directed at determining its clinical significance as well as the role of interactions between complement components and effector cells in rituximab activity.
Recruitment of Fcγ receptor-dependent functions
The CD20 protein is not shed from the cell surface and does not internalize on antibody binding and therefore appears to be an ideal target for the recruitment of effector cells expressing Fcγ receptors (FcγRs). ADCC is an important effector mechanism in the eradication of intracellular pathogens and tumor cells. Binding of IgG to target cell antigens allows the recruitment of effector cells such as NK cells and macrophages, which express Fc receptors. These cells are then directed toward target cells, inducing either phagocytosis or release of their cytotoxic granules to promote cell killing. Immunoglobulins interact with effector cells through their Fc portion, which binds to specific receptors (FcγR) expressed by nearly all the cells of the immune system (Table 1). There are 3 classes of FcγR: FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16). FcγRIa, FcγRIIa, FcγRIIc, and FcγRIIIa are activating receptors due to the presence of an immunoreceptor tyrosine-based activation motif (ITAM) either in the cytoplasmic domain (FcγRIIa, FcγRIIc) or in the accessory signaling γ chain (FcγRIa and FcγRIIIa). Cross-linking of activating FcγR by IgG-sensitized cells or IgG immune complexes induces tyrosine phosphorylation of ITAM by Src tyrosine kinases. Downstream signaling events eventually result in cellular responses such as phagocytosis, granule exocytosis, ADCC, or cytokine synthesis. FcγRIIb contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic domain and is the only inhibitory Fc receptor. Cocross-linking of FcγRIIb and BCR by an antigen already complexed to specific IgG results in tyrosine phosphorylation of ITIM, activation of phosphatases, and inhibition of BCR signals and B-cell activation. Most FcγRs, including FcγRIIb, are expressed by macrophages. Therefore, immune complexes can engage activating and inhibitory FcγR on the same cell, providing an opportunity to fine-tune cell activation. NK cells only express the activating receptors, FcγRIIIa and FcγRIIc, whereas polymorphonuclear cells express FcγRIIa and FcγRIIIb, which synergize for phagocytosis.
Fcγ receptor expression according to cell type
. | FcγRI . | FcγRIIa . | FcγRIIb . | FcγRIIc . | FcγRIIIa . | FcγRIIIb . |
|---|---|---|---|---|---|---|
| Monocytes/macrophages | + | + | + | — | + | — |
| NK cells | — | — | — | + | + | — |
| Neutrophils | +/− | + | — | — | — | + |
| B lymphocytes | — | — | + | — | — | — |
| Dendritic cells | + | + | + | — | + | — |
| Mast cells | +/− | + | + | — | — | — |
| Platelets | — | + | — | — | — | — |
. | FcγRI . | FcγRIIa . | FcγRIIb . | FcγRIIc . | FcγRIIIa . | FcγRIIIb . |
|---|---|---|---|---|---|---|
| Monocytes/macrophages | + | + | + | — | + | — |
| NK cells | — | — | — | + | + | — |
| Neutrophils | +/− | + | — | — | — | + |
| B lymphocytes | — | — | + | — | — | — |
| Dendritic cells | + | + | + | — | + | — |
| Mast cells | +/− | + | + | — | — | — |
| Platelets | — | + | — | — | — | — |
In vitro studies have shown that rituximab induces ADCC in human lymphoma cell lines.1,35 In a xenograft model of lymphoma, the role of ADCC was clearly demonstrated using FcR-γ chain-deficient mice,72 which do not express activating FcγRI and FcγRIIIa, as well as in a model using a neutralizing antibody against murine FcγR.22 More recently, the role of both NK and polymorphonuclear neutrophils has been suggested in a disseminated lymphoma xenograft in severe combined immunodeficiency (SCID) mice, using antibody-dependent depletion of these cell types.73 However, activating FcγRs are probably not the only receptors implicated in the antitumor response because FcγRIIb-deficient mice display a better tumoral reduction.72 These observations indicate that Fc receptor pathways are not restricted to an activating system such as expressed on NK cells, but also involve inhibitory pathways with FcγRIIb counterbalancing the activator receptors.
Human FCGR3A displays a nucleotide polymorphism at nucleotide 559 resulting in an amino acid substitution at position 158 with either a phenylalanine (F) or a valine (V). This substitution influences the affinity of IgG1 for the FcγRIIIa receptor with a higher affinity for FcγRIIIa-158VV NK cells.74 We have demonstrated a correlation between the FcγRIIIa genotype and the clinical and molecular responses to rituximab in untreated follicular lymphoma; patients homozygous for FcγRIIIa-158VV have the best clinical and molecular responses.75 These clinical results, recently confirmed by others,76,77 strongly support the hypothesis that ADCC is one of the in vivo mechanisms of rituximab action in follicular lymphoma. The lack of association in B-CLL78 could suggest that the extent of ADCC in rituximab activity could be dependent of lymphoma proliferation subtype. Phagocytosis of human rituximab-opsonized lymphoma cells by macrophages has recently been demonstrated in vitro.61 This phagocytosis is mediated by FcγRIIa, indicating that other FcγR-expressing cells may be also involved in the action of rituximab.61
The importance of the interaction between the Fc portion and FcγR highlights a potential way to increase rituximab activity. By in vitro molecular engineering of the Fc region, it is possible to improve the affinity of mAbs for FcγRIIIa and thereby to enhance ADCC.79 Glycosylation of IgG is necessary for effector functions and particularly for the interaction with FcγR.80 Modifications of glycosylation could influence the ability of mAbs to activate ADCC. For instance, addition of bisecting N-acetylglucosamine or a lack of fucose on IgG1 N-linked oligosaccharide chain can dramatically increase ADCC.81-83
As shown in the pivotal study,4,84 the pharmacokinetic profile of rituximab differs between responders and nonresponders. Moreover, normal B-cell depletion appears to correlate with circulating levels of rituximab.85 It has been suggested that the FCGR3A genotype affects rituximab pharmacokinetics, but this requires confirmation in a larger number of patients.
Cytokines such as IL-2, IL-12, IFN-α, GM-CSF, and granulocyte colony-stimulating factor (G-CSF) are known to increase ADCC/phagocytosis by stimulation or expansion of NK cells and macrophages.86-89 For example, IL-2 increases ADCC by NK cell expansion and activation88 and a correlation between NK cell counts and clinical response was found in a clinical trial combining IL-2 and rituximab in patients with NHL.90 IL-12 enhances the lytic activity of NK cells and induces IFN-γ.89 This cytokine was recently used in combination with rituximab in patients with recurrent NHL with a promising objective response rate.91 IFN-α has also been used in combination with rituximab in patients with relapsed NHL. The response rates were similar to those observed in trials where rituximab was given alone, although IFN-α increased time to treatment failure in responding patients.10,92 GM-CSF induces monocyte differentiation and can activate polynuclear neutrophils.86 The combination of rituximab with GM-CSF has been used in patients with relapsed/refractory NHL and preliminary results suggested a complete remission rate higher than that in patients treated with rituximab alone.11 In patients who received high-dose therapy and an autologous stem cell transplant, the period after grafting, which is associated with rapid NK cell count recovery,93 could be an optimal window for rituximab administration.94 In contrast, steroids have a dual impact on lymphoma cell lines. They increase apoptosis of lymphoma lines but also lead to decreased ADCC.52 Thus, the available evidence strongly favors a role for ADCC in the clinical activity of rituximab, paving the way to future studies to better define strategies for maximizing ADCC through the use of anti-CD20 mAbs.
Other mechanisms of action and “vaccinal” effect of rituximab
Several studies have indicated that maximal clinical and molecular responses to rituximab therapy may take several months, suggesting that short-term cytolytic mechanisms such as apoptosis, CDC, and ADCC are not the only ones involved (Figure 4). Rituximab-promoted lysis of lymphoma cells through any of these latter mechanisms may promote uptake and cross-presentation of lymphoma cell-derived peptides by dendritic cells, inducing their maturation and allowing the generation of specific cytotoxic T lymphocytes.95 It is difficult to demonstrate such an immune reaction in humans, but murine experiments clearly indicate that passive immunotherapy with mAbs can induce a specific antitumor immunity96,97 (not directed against the target antigen but against several unknown tumor-derived antigens). Induction of this specific immunity requires myeloid-derived dendritic cells,97,98 suggesting that adjuvant therapy promoting this pathway (GM-CSF, Flt-3 ligand, and IFN-α) might be of benefit. In this context, it is noteworthy that the combination of rituximab with GM-CSF yielded promising results in a phase 2 trial.11 Clinical studies have shown that retreatment with rituximab in patients who had a relapse after an initial response increased the time to progression99 and that maintenance therapy given to patients in response to rituximab increased the response rates.100 Together, these results strongly suggest that rituximab may have a “vaccinal” effect and may therefore be useful as maintenance therapy. Randomized clinical trials are needed to confirm the clinical impact of this approach.
Main mechanisms of action of rituximab and ways to increase its clinical efficacy. FAMP indicates fludarabine monophosphate; CR, complement receptor.
Main mechanisms of action of rituximab and ways to increase its clinical efficacy. FAMP indicates fludarabine monophosphate; CR, complement receptor.
Conclusion
A large body of evidence shows that rituximab induces cell death by different pathways, in particular ADCC, CDC, and apoptosis. Whereas some of these pathways are well described in vitro, others, including CDCC, phagocytosis, and cytotoxic T lymphocyte (CTL) generation, require further documentation. Moreover, the mechanisms by which rituximab actually works in vivo still need to be elucidated to design the best therapeutic strategies and to increase its activity. For instance, there are strong arguments to support the in vivo implication of CDC63 and ADCC,75 but although it is conceivable that complement pathways can be rapidly induced by rituximab, the recruitment of cell death effectors (macrophages, NK cells) and the interaction between their FcR and Fc portions are likely to take more time. Thus, the timing of additional therapies or adjuvants relative to the rituximab infusion may be crucial and would also depend on which mechanism is to be favored. Furthermore, the relative importance of the different mechanisms may vary according to lymphoma subtype or localization. The possibility of tailoring new anti-CD20 mAbs displaying an improved interaction with the host immune system, together with a better understanding of rituximab pharmacokinetics, will offer new opportunities to develop anti-CD20 strategies. The optimal use of rituximab has become an important challenge.
Prepublished online as Blood First Edition Paper, June 29, 2004; DOI 10.1182/blood-2004-03-1110.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank the CANCEN Association for its action in improving patient hospitalization conditions and their financial support in cancer research.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal