Abstract
St Jude Total Therapy Study XIIIB for childhood acute lymphoblastic leukemia (ALL) incorporated more stringent risk classification, early intensification of intrathecal chemotherapy, reinduction treatment, and the addition of dexamethasone to postremission therapy to increase the proportion of event-free survivors without jeopardizing their quality of life. Cranial irradiation was reserved for the 12% of patients who had T-cell ALL and a presenting leukocyte count of 100 × 109/L or more, or CNS-3 (5 or more leukocytes/μL with identifiable blast cells in an atraumatic sample or the presence of cranial nerve palsy) status. Among the 247 consecutive patients enrolled in the study, 117 were classified as having lower-risk leukemia and received mainly antimetabolite-based continuation therapy; the 130 cases with higher-risk leukemia received more intensive continuation chemotherapy with multiple drug pairs administered in weekly rotation. The 5-year event-free survival estimate was 80.8% ± 2.6% (SE); the 8-year rate was 78.6% ± 5.8%. The 5-year cumulative risk of an isolated central nervous system (CNS) relapse was 1.7% ± 0.8%, and that of isolated plus combined CNS relapse was 3.0% ± 1.1%. The 5-year cumulative risks of etoposide-related myeloid malignancies were 1.8% ± 1.3% in the lower-risk patients who received a cumulative dose of 1.2 g/m2 and 5.0% ± 2.0% in the higher-risk patients who received a cumulative dose of up to 14.4 g/m2 (P = .18). Independent adverse prognostic features included the presence of MLL-AF4 or BCR-ABL fusion gene and minimal residual leukemia of 0.01% or more at the end of the 6-week remission induction phase. Our results suggest the efficacy of early intensification of intrathecal chemotherapy and provide the basis for studies omitting cranial irradiation altogether. (Blood. 2004;104:2690-2696)
Introduction
As the cure rate for childhood acute lymphoblastic leukemia (ALL) approaches 80%,1-5 the focus of contemporary treatment programs has turned increasingly to improving the quality of life of long-term survivors. In Total Therapy Study XIIIA at St Jude Children's Research Hospital, early intensification of intrathecal chemotherapy for all patients with cranial irradiation administered only to high-risk cases (22% of the total study population) reduced the risk of central nervous system (CNS) relapse to an exceedingly low level (∼ 1%) and boosted the 5-year event-free survival rate to 80%.6 In the subsequent study, XIIIB, several treatment modifications were made in an effort to improve clinical outcome further without increasing long-term sequelae, and to study the pharmacodynamics of intravenous mercaptopurine with or without preceding methotrexate administered intravenously in high doses or orally in fractionated (lower) doses.
Among the treatment modifications, cranial irradiation was limited to patients with T-cell ALL and a leukocyte count of 100 × 109/L or more, CNS-3 status (as described in “Patients”), or both (12% of all patients), a subgroup with a particularly high risk of CNS relapse.7,8 Dexamethasone was used instead of prednisone in postremission therapy to improve both CNS and systemic control of leukemia.9 l-asparaginase was deleted from the etoposide-containing continuation treatment to decrease the risk of therapy-related acute myeloid leukemia, based on data suggesting that l-asparaginase can potentiate the leukemogenic effect of etoposide.10,11 Reinduction treatment was introduced earlier in therapy to forestall relapse. Finally, the risk classification system was revised so that a greater proportion of patients would receive postremission treatment for lower-risk ALL, a modification that may decrease the risk of long-term sequelae. In this article, we report the outcome of Study XIIIB, excluding the pharmacologic results, which are described in earlier publications.12,13
Patients, materials, and methods
Patients
From August 1994 to July 1998, 247 consecutive children and adolescents, 18 years of age or younger, with newly diagnosed ALL were enrolled in Total Therapy Study XIIIB at St Jude Children's Research Hospital. All patients were evaluable for the study. The treatment protocol was approved by the institutional review board, and signed informed consent was obtained from the patients' parents or guardians.
The diagnosis of ALL was based on morphologic and cytochemical evaluation of bone marrow smears as well as immunophenotyping and cytogenetic and molecular genetic analysis of leukemic blast cells. Depending on the pattern of blast cell reactivity to a panel of monoclonal antibodies, cases were classified as T-cell or B-cell precursor, as previously described.14 Flow cytometric determination of blast-cell DNA content was part of the routine evaluation; cases were classified according to the DNA index (ratio of DNA content in leukemic cells versus normal diploid G0/G1 cells) as less than 1.16 or more than or equal to 1.16. All patients underwent lumbar puncture at the time of diagnostic bone marrow aspiration, and the cerebrospinal fluid was examined as previously described.15,16 The CNS status of each patient was defined as follows: (i) CNS-1, no identifiable blast cells in an atraumatic sample; (ii) CNS-2, less than 5 leukocytes/μL with identifiable blast cells in an atraumatic sample; (iii) CNS-3, 5 or more leukocytes/μL with identifiable blast cells in an atraumatic sample or the presence of cranial nerve palsy; (iv) traumatic lumbar puncture without blasts, 10 or more erythrocytes/μL without identifiable blasts; and (v) traumatic lumbar puncture with blasts, 10 or more erythrocytes/μL with identifiable blasts. Minimal residual disease was determined by flow cytometry, as previously described.17
Risk classification
Patients were assigned to a higher- or lower-risk group on the basis of their presenting clinical features, the biologic features of their leukemic cells, and their early response to remission-induction treatment. Patients were considered to have lower-risk ALL if they were 1 to 9 years old with a presenting leukocyte count less than 50 × 109/L or had a DNA index of 1.16 or more; however, they could not have a CNS-3 status; testicular leukemia (documented by ultrasonographic examination); a T-cell immunophenotype, the t(9;22), t(4;11), t(1;19) associated with a pre-B immunophenotype, an MLL gene rearrangement, or near-haploidy; nor could their bone marrow contain 5% or more leukemic blasts on day 15 of remission induction (day 19 after the start of up-front therapy). All other patients were classified as higher risk.
Treatment
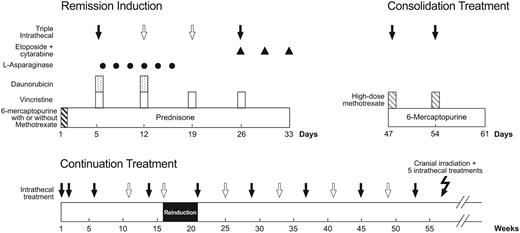
Patients were stratified by age, leukocyte count, immunophenotype, and sex, and randomized to receive 1 of 3 up-front therapies: intravenous mercaptopurine alone (1 g/m2 over 6 hours); low-dose oral methotrexate (30 mg/m2 every 6 hours for a total of 6 doses) followed by intravenous mercaptopurine (1 g/m2 over 6 hours); or high-dose intravenous methotrexate (1 g/m2 over 24 hours) immediately followed by intravenous mercaptopurine (1 g/m2 over 6 hours). Conventional remission induction therapy was started 4 days after the initiation of up-front therapy with prednisone (40 mg/m2 per day for 28 days), vincristine (1.5 mg/m2 on days 1, 8, 15, and 22), daunorubicin (25 mg/m2 on days 1 and 8), l-asparaginase (10 000 units/m2 on days 2, 4, 6, 8, 10, and 12, with added doses on days 15, 17, and 19 in patients with 5% or more blasts in day-15 marrow), and etoposide (300 mg/m2) plus cytarabine (300 mg/m2) on days 22, 25, and 29 (Figure 1). Upon attaining complete remission (approximately 2 weeks after the completion of remission-induction therapy), all patients received consolidation therapy with 2 weekly doses of high-dose methotrexate (2 g/m2 over 2 hours, followed by leucovorin rescue beginning 42 hours later) and mercaptopurine (75 mg/m2 per night for 2 weeks).
Schema of remission induction, consolidation treatment, and continuation therapy for the first year. Solid arrows indicate triple intrathecal treatment that was administered to all patients and open arrows, additional doses that were administered to patients with an increased risk of CNS relapse. Cranial irradiation plus 5 triple intrathecal treatments were administered only to patients with T-cell immunophenotype and an initial leukocyte count of 100 × 109/L or more or a CNS-3 status. Other details are provided in “Patients, materials, and methods.”
Schema of remission induction, consolidation treatment, and continuation therapy for the first year. Solid arrows indicate triple intrathecal treatment that was administered to all patients and open arrows, additional doses that were administered to patients with an increased risk of CNS relapse. Cranial irradiation plus 5 triple intrathecal treatments were administered only to patients with T-cell immunophenotype and an initial leukocyte count of 100 × 109/L or more or a CNS-3 status. Other details are provided in “Patients, materials, and methods.”
Postremission therapy (120 weeks) for lower-risk cases consisted of daily mercaptopurine (75 mg/m2) and weekly intravenous methotrexate (40 mg/m2), reinforced by high-dose methotrexate and mercaptopurine (same doses as used in consolidation therapy) every 8 weeks for the first year, and dexamethasone (8 mg/m2 per day for 7 days) plus vincristine (1.5 mg/m2) pulse every 4 weeks. Reinduction therapy (similar to the initial 4-week remission induction with only one dose of etoposide plus cytarabine on day 22, followed by 2 weekly doses of high-dose methotrexate plus daily mercaptopurine) was administered from weeks 16 to 21 after remission. Altogether, 10 courses of high-dose methotrexate were given.
Postremission therapy (120 weeks) for higher-risk cases consisted of drug pairs administered in weekly rotation (Table 1). Reinduction treatment was the same as that used in lower-risk cases. A total of 10 courses of high-dose methotrexate were given. Allogeneic hematopoietic stem cell transplantation in first remission was performed in 11 patients with matched unrelated (n = 9) or matched related (n = 2) donors; 4 underwent transplantation for t(4;11)+ ALL, 4 for Philadelphia chromosome-positive ALL, and 1 each for t(11;19)+ B-cell precursor ALL, hemophagocytic syndrome, and delayed remission (failure to achieve remission at the end of remission induction).
Postremission therapy for higher-risk ALL
Week . | Drug pair . |
|---|---|
| 1 | Etoposide 300 mg/m2 IV + cyclophosphamide 300 mg/m2 IV |
| 2 | Methotrexate 40 mg/m2 IV* + mercaptopurine 75 mg/m2 PO every evening |
| 3 | Methotrexate 40 mg/m2 IV* + cytarabine 300 mg/m2 IV |
| 4 | Vincristine 1.5 mg/m2 IV† + dexamethasone 8 mg/m2 PO per day in 3 divided doses |
| 5 | Etoposide 300 mg/m2 IV + cyclophosphamide 300 mg/m2 IV |
| 6 | Methotrexate 2000 mg/m2 IV* over 24 hours + mercaptopurine 75 mg/m2 PO every evening |
| 7 | Etoposide 300 mg/m2 IV + cytarabine 300 mg/m2 IV |
| 8 | Vincristine 1.5 mg/m2 IV† + dexamethasone 8 mg/m2 PO per day in 3 divided doses |
Week . | Drug pair . |
|---|---|
| 1 | Etoposide 300 mg/m2 IV + cyclophosphamide 300 mg/m2 IV |
| 2 | Methotrexate 40 mg/m2 IV* + mercaptopurine 75 mg/m2 PO every evening |
| 3 | Methotrexate 40 mg/m2 IV* + cytarabine 300 mg/m2 IV |
| 4 | Vincristine 1.5 mg/m2 IV† + dexamethasone 8 mg/m2 PO per day in 3 divided doses |
| 5 | Etoposide 300 mg/m2 IV + cyclophosphamide 300 mg/m2 IV |
| 6 | Methotrexate 2000 mg/m2 IV* over 24 hours + mercaptopurine 75 mg/m2 PO every evening |
| 7 | Etoposide 300 mg/m2 IV + cytarabine 300 mg/m2 IV |
| 8 | Vincristine 1.5 mg/m2 IV† + dexamethasone 8 mg/m2 PO per day in 3 divided doses |
Drug pairs were administered in weekly rotation, interrupted by reinduction therapy from weeks 16 to 21.
IV indicates intravenously; PO, orally.
Each high-dose methotrexate was followed by 5 doses of leucovorin 10 mg/m2 per dose administered every 6 hours at hours 44, 50, 56, 62, and 68 from the start of methotrexate infusion. In patients who received cranial irradiation (from weeks 55 to 57), methotrexate was given intramuscularly instead of intravenously after week 57.
The last high-dose methotrexate was given on week 53, after which it was replaced by regular-dose methotrexate.
Infants younger than 1 year received a dose of 0.05 mg/kg. The maximum dose was 2 mg.
CNS-directed therapy was given according to presenting risk features and CNS status. All patients received triple intrathecal chemotherapy (simultaneously administered methotrexate, hydrocortisone, and cytarabine), in age-appropriate doses, on days 5 and 26 of remission induction (Figure 1). On days 12 and 19, 2 additional intrathecal treatments were given to patients with CNS-2, CNS-3, or a traumatic lumbar puncture with identifiable blasts. Upon attaining complete remission, all patients received triple intrathecal therapy for 4 weekly doses (the first 2 treatments were given on the same dates of high-dose methotrexate therapy as consolidation therapy), and then every 6 to 8 weeks during the first 53 to 55 weeks of continuation treatment. Subgroups of patients with leukocyte counts of 100 × 109/L or more, T-cell ALL and a leukocyte count of 50 × 109/L or more, or the t(9;22) received triple intrathecal therapy every 4 weeks during the first year of continuation treatment. Cranial irradiation was administered to 30 patients with T-cell ALL and a leukocyte count of 100 × 109/L or more or CNS-3 status at diagnosis (18 Gy in 12 fractions and 24 Gy in 16 fractions, respectively, plus 5 intrathecal treatments from weeks 56 to 59). Intrathecal treatments were followed in 24 hours by leucovorin rescue and were discontinued in all patients after the first year of continuation treatment. The total number of intrathecal treatments ranged from 13 in lower-risk patients with a CNS-1 status to 26 in patients with T-cell ALL, leukocyte count of 100 × 109/L or more and CNS-2, CNS-3, or traumatic lumbar puncture with identifiable blasts.
A trimethoprim and sulfamethoxazole combination was given to all patients twice daily for 3 consecutive days per week from day 19 of remission induction to 6 weeks after completion of all chemotherapy as prophylaxis against Pneumocystis carinii pneumonia.
Statistical analysis
The duration of event-free survival (EFS) was defined as the time from diagnosis until the date of failure (induction failure, relapse, death, or the development of a second malignancy) or until the date of last contact for all event-free survivors. Patients who did not attain a complete remission were considered failures at time zero. EFS rates were estimated by the method of Kaplan and Meier and were compared with the Mantel-Haenszel test.18 The Cox proportional hazards model was used to identify independent prognostic factors with respect to event-free survival. All analyses were performed on the basis of “intent-to-treat”; only patients who remained failure-free were censored on the date of last contact.
For patients who achieved complete remission, cumulative incidence functions of isolated CNS or isolated plus combined CNS relapse, as well as therapy-related myeloid malignancies, were constructed by the method of Kalbfleisch and Prentice,19 and the functions were compared with Gray's test.20 An isolated CNS relapse was defined as one without simultaneous relapse at another site, while a combined CNS relapse was one in the CNS accompanied by relapse in the bone marrow or any other extramedullary site. All other failures were considered competing events in the estimation of cumulative incidence functions. The database last updated on February 6, 2004, was used for analysis. The median follow-up time for patients remaining in continuous complete remission was 6.6 years (range, 3.7 to 9.3 years). At the time of analyses, 94% of survivors had been seen within 2 years; 2 patients were apparently lost to follow-up.
Results
Patient characteristics
The presenting clinical and laboratory characteristics of the 247 patients (144 males and 103 females) are reported in Table 2. The median age at diagnosis was 5.99 years (range, 0.08 to 18.79 years) and the median leukocyte count was 11.9 × 109/L (range, 0.4 to 906 × 109/L). Black children constituted a relatively large fraction (18.2%) of the patients treated in the study; consequently, there were increased proportions of patients with T-cell ALL (17.6%) or pre-B cell ALL with t(1;19)/E2A-PBX1 fusion (4.7%).2 Of the 10 infant cases, 5 have t(4;11)(q21;q23) and 3 have t(11;19)(q23;13.3).
Treatment outcome according to selected clinical and biologic features
Features . | No. of patients (%) . | No. obtaining complete remission (%) . | 5-year event-free survival in % (SE) . | P value according to Mantel-Haenszel test . |
|---|---|---|---|---|
| Risk | ||||
| Lower | 117 (47.4) | 116 (99.1) | 88.1 (3.8) | .003 |
| Higher | 130 (52.6) | 126 (96.9) | 73.0 (5.5) | |
| NCI/Rome risk (B-lineage ALL) | ||||
| Standard | 112 (55.4) | 111 (99.1) | 87.3 (3.2) | .054 |
| High | 90 (44.6) | 88 (97.8) | 76.7 (4.6) | |
| Age | ||||
| Younger than 1 y | 10 (4.0) | 10 (100) | 70.0 (14.5) | .08 |
| 1 to 10 y | 161 (65.2) | 160 (99.4) | 84.3 (2.9) | |
| Older than 10 y | 76 (30.8) | 72 (94.7) | 74.9 (5.2) | |
| Sex | ||||
| Female | 103 (41.7) | 101 (98.1) | 83.3 (3.8) | .31 |
| Male | 144 (58.3) | 141 (97.9) | 79.1 (3.5) | |
| Race | ||||
| White | 199 (80.6) | 194 (97.5) | 79.3 (3.0) | .45 |
| Black | 45 (18.2) | 45 (100) | 86.5 (5.1) | |
| Other | 3 (1.22) | 3 (100) | 100 (0.0) | |
| Leukocyte count, | ||||
| Lower than 10 × 109/L | 111 (44.9) | 108 (97.3) | 82.7 (3.8) | .02 |
| 10 to 49 × 109/L | 70 (28.3) | 70 (100) | 88.6 (3.9) | |
| 50 to 99 × 109/L | 28 (11.3) | 27 (96.4) | 78.6 (7.8) | |
| 100 × 109/L or higher | 38 (15.4) | 37 (97.4) | 63.0 (7.8) | |
| CNS status | ||||
| CNS-1 + TLP− | 145 (58.7) | 142(97.9) | 81.3 (3.4) | .96 |
| CNS-2 | 78 (31.6) | 76 (97.5) | 80.6 (4.6) | |
| CNS-3 | 7 (2.8) | 7 (100) | 71.4 (15.6) | |
| TLP+ | 17 (6.9) | 17 (100) | 82.4 (9.2) | |
| Immunophenotype | ||||
| B-cell precursor | 202 (82.4) | 199 (98.5) | 82.6 (2.8) | .17 |
| T-cell | 43 (17.6) | 41 (95.3) | 71.9 (6.8) | |
| DNA index | ||||
| 1.16 or more | 46 (18.6) | 45 (97.8) | 91.2 (4.3) | .03 |
| Less than 1.16 | 201 (81.4) | 197 (98.0) | 78.5 (3.0) | |
| t(9;22)/BCR-ABL | ||||
| Absent | 232 (97.1) | 228 (98.3) | 82.6 (2.6) | <.0001 |
| Present | 7 (2.9) | 6 (85.7) | 28.6 (13.9) | |
| t(4;11)/MLL-AF4 | ||||
| Absent | 204 (96.7) | 199 (97.5) | 82.2 (2.8) | .003 |
| Present | 7 (3.3) | 7 (100) | 42.9 (16.2) | |
| t(1;19)/E2A-PBX1 | ||||
| Absent | 201 (95.3) | 196 (97.5) | 81.0 (2.9) | .99 |
| Present | 10 (4.7) | 10 (100) | 80.0 (11.9) | |
| TEL-AML1 | ||||
| Present | 39 (22.7) | 39 (100) | 84.5 (6.2) | .41 |
| Absent | 133 (77.3) | 129 (97.0) | 78.8 (3.7) | |
| Day 19 marrow | ||||
| Less than 5% blasts | 220 (92.4) | 219 (99.6) | 83.5 (2.6) | <.001 |
| 5% or more blasts | 18 (7.6) | 15 (83.3) | 55.6 (11.2) | |
| Minimal residual leukemia on remission date | ||||
| Less than 0.01% | 86 (72.9) | 86 (100) | 84.8 (4.5) | .004 |
| 0.01% or more | 32 (27.1) | 32 (100) | 65.5 (10.3) |
Features . | No. of patients (%) . | No. obtaining complete remission (%) . | 5-year event-free survival in % (SE) . | P value according to Mantel-Haenszel test . |
|---|---|---|---|---|
| Risk | ||||
| Lower | 117 (47.4) | 116 (99.1) | 88.1 (3.8) | .003 |
| Higher | 130 (52.6) | 126 (96.9) | 73.0 (5.5) | |
| NCI/Rome risk (B-lineage ALL) | ||||
| Standard | 112 (55.4) | 111 (99.1) | 87.3 (3.2) | .054 |
| High | 90 (44.6) | 88 (97.8) | 76.7 (4.6) | |
| Age | ||||
| Younger than 1 y | 10 (4.0) | 10 (100) | 70.0 (14.5) | .08 |
| 1 to 10 y | 161 (65.2) | 160 (99.4) | 84.3 (2.9) | |
| Older than 10 y | 76 (30.8) | 72 (94.7) | 74.9 (5.2) | |
| Sex | ||||
| Female | 103 (41.7) | 101 (98.1) | 83.3 (3.8) | .31 |
| Male | 144 (58.3) | 141 (97.9) | 79.1 (3.5) | |
| Race | ||||
| White | 199 (80.6) | 194 (97.5) | 79.3 (3.0) | .45 |
| Black | 45 (18.2) | 45 (100) | 86.5 (5.1) | |
| Other | 3 (1.22) | 3 (100) | 100 (0.0) | |
| Leukocyte count, | ||||
| Lower than 10 × 109/L | 111 (44.9) | 108 (97.3) | 82.7 (3.8) | .02 |
| 10 to 49 × 109/L | 70 (28.3) | 70 (100) | 88.6 (3.9) | |
| 50 to 99 × 109/L | 28 (11.3) | 27 (96.4) | 78.6 (7.8) | |
| 100 × 109/L or higher | 38 (15.4) | 37 (97.4) | 63.0 (7.8) | |
| CNS status | ||||
| CNS-1 + TLP− | 145 (58.7) | 142(97.9) | 81.3 (3.4) | .96 |
| CNS-2 | 78 (31.6) | 76 (97.5) | 80.6 (4.6) | |
| CNS-3 | 7 (2.8) | 7 (100) | 71.4 (15.6) | |
| TLP+ | 17 (6.9) | 17 (100) | 82.4 (9.2) | |
| Immunophenotype | ||||
| B-cell precursor | 202 (82.4) | 199 (98.5) | 82.6 (2.8) | .17 |
| T-cell | 43 (17.6) | 41 (95.3) | 71.9 (6.8) | |
| DNA index | ||||
| 1.16 or more | 46 (18.6) | 45 (97.8) | 91.2 (4.3) | .03 |
| Less than 1.16 | 201 (81.4) | 197 (98.0) | 78.5 (3.0) | |
| t(9;22)/BCR-ABL | ||||
| Absent | 232 (97.1) | 228 (98.3) | 82.6 (2.6) | <.0001 |
| Present | 7 (2.9) | 6 (85.7) | 28.6 (13.9) | |
| t(4;11)/MLL-AF4 | ||||
| Absent | 204 (96.7) | 199 (97.5) | 82.2 (2.8) | .003 |
| Present | 7 (3.3) | 7 (100) | 42.9 (16.2) | |
| t(1;19)/E2A-PBX1 | ||||
| Absent | 201 (95.3) | 196 (97.5) | 81.0 (2.9) | .99 |
| Present | 10 (4.7) | 10 (100) | 80.0 (11.9) | |
| TEL-AML1 | ||||
| Present | 39 (22.7) | 39 (100) | 84.5 (6.2) | .41 |
| Absent | 133 (77.3) | 129 (97.0) | 78.8 (3.7) | |
| Day 19 marrow | ||||
| Less than 5% blasts | 220 (92.4) | 219 (99.6) | 83.5 (2.6) | <.001 |
| 5% or more blasts | 18 (7.6) | 15 (83.3) | 55.6 (11.2) | |
| Minimal residual leukemia on remission date | ||||
| Less than 0.01% | 86 (72.9) | 86 (100) | 84.8 (4.5) | .004 |
| 0.01% or more | 32 (27.1) | 32 (100) | 65.5 (10.3) |
Data missing for some features.
TLP− indicates traumatic lumbar puncture without blasts; TLP+, traumatic lumber puncture with blast.
Treatment outcome
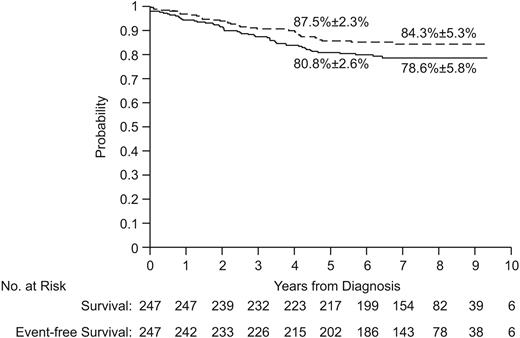
Of the 247 patients, 242 (98%) entered complete remission (99% of the lower-risk group and 97% of the higher-risk group). Of the 5 patients who did not achieve complete remission, 2 had refractory leukemia (one T-cell and the other Philadelphia chromosome-positive), 2 died of fungal infection (candidiasis and aspergillosis), and 1 died of cerebral hemorrhage. The 5-year event-free survival estimates (± SE) were 80.8% ± 2.6% for all 247 patients (Figure 2), 88.7% ± 3.0% for lower-risk patients, and 73.8% ± 4.0% for higher-risk patients. The 8-year estimates were 78.6% ± 5.8%, 86.5% ± 6.6%, and 71.5% ± 9.3%, respectively. Of the 11 patients who underwent allogeneic transplantation, 5 are alive and in remission at 5.4 to 8.6 years of follow-up.
Event-free survival (solid line) and survival (dashed lines) of all patients. The 5-year and 8-year estimates are means ± SE.
Event-free survival (solid line) and survival (dashed lines) of all patients. The 5-year and 8-year estimates are means ± SE.
Table 3 summarizes the types of failure according to the treatment arm. There were 5 induction failures, 19 hematologic relapses, 4 isolated CNS relapses, 1 isolated testicular relapse, 5 other relapses (combined with CNS relapse in 4 cases), 8 second malignancies (4 acute myeloid leukemia, 3 myelodysplastic syndrome, and 1 chronic myeloid leukemia), and 7 deaths in remission (4 after hematopoietic stem cell transplantation, 1 from Clostridium sepsis, 1 from Pseudomonas sepsis, and 1 from liver failure in association with cystic fibrosis). The 5-year cumulative risk of isolated CNS relapse (± SE) was 1.7% ± 0.8%, and that for isolated plus combined CNS relapse was 3.0% ± 1.1%; all 4 isolated relapses occurred within 3 years of diagnosis. The cumulative risk of any relapse was 11.6% ± 2.1% at 5 years and 14.0% ± 2.4% at 10 years. The 5-year cumulative risk of the development of therapy-related malignancies was 3.5% ± 1.2%; all 8 cases of myeloid malignancies occurred within the first 5 years after diagnosis. The cumulative risk of therapy-related myeloid malignancies in the higher-risk group was higher, but not significantly different from that in the lower-risk group (5.0% ± 2.0% vs 1.8% ± 1.3% at 5 years, P = .18).
Distribution of adverse events in 2 treatment groups
. | Lower-risk . | Higher-risk . |
|---|---|---|
| No. | 117 | 130 |
| Induction failure | 1 | 4 |
| Relapse | ||
| Bone marrow | 8 | 11 |
| CNS only | 1 | 3 |
| CNS + bone marrow | 1 | 1 |
| CNS + mediastinal | 0 | 1 |
| CNS + ocular | 0 | 1 |
| Testicular only | 0 | 1 |
| Ocular | 0 | 1 |
| Second malignancies | 2 | 6 |
| Deaths in remission | 2 | 5 |
| Patients in CCR | 102 | 96 |
. | Lower-risk . | Higher-risk . |
|---|---|---|
| No. | 117 | 130 |
| Induction failure | 1 | 4 |
| Relapse | ||
| Bone marrow | 8 | 11 |
| CNS only | 1 | 3 |
| CNS + bone marrow | 1 | 1 |
| CNS + mediastinal | 0 | 1 |
| CNS + ocular | 0 | 1 |
| Testicular only | 0 | 1 |
| Ocular | 0 | 1 |
| Second malignancies | 2 | 6 |
| Deaths in remission | 2 | 5 |
| Patients in CCR | 102 | 96 |
The 5-year survival rates were 85.7% ± 2.3% for the entire cohort of 247 patients (Figure 2), 92.1% ± 2.6% for lower-risk patients, and 79.9% ± 3.6% for higher-risk patients. The 8-year rates were 84.3% ± 5.3%, 90.4% ± 5.7%, and 78.9% ± 8.8%, respectively.
Prognostic factors
Among the clinical and biologic features analyzed, only a leukocyte count of 100 × 109/L or more, a DNA index less than 1.16, the presence of the t(9;22)/BCR-ABL or the t(4;11)/MLL-AF4, and a poor response to remission induction therapy (based on a bone marrow examination on day 19 or at the end of induction therapy) were predictive of an inferior outcome (Table 2). All 3 relapses in the infant group occurred in patients with the t(4;11). Sex, age, race, immunophenotype, CNS status, and the t(1;19)/E2A-PBX1 or TEL-AML1 lacked prognostic significance. By multivariate analysis applied to the entire cohort, only the t(4;11)/MLL-AF4 (hazard ratio, 6.38; 95% confidence interval, 1.73-23.34; P = .005) and the t(9;22)/BCR-ABL (hazard ratio, 4.39; 95% confidence interval, 1.45-13.27; P = .009) were independently associated with an inferior treatment outcome. These features also conferred a higher risk of failure in patients with B-cell precursor ALL, whereas patients with T-cell ALL (n = 43) lacked discernible risk factors (data not shown). When the multivariate analysis was limited to the 127 patients who were tested for minimal residual disease at the end of remission induction therapy, residual leukemia of 0.01% or more was the only independent adverse prognostic feature (hazard ratio, 2.43; 95% confidence interval, 1.11-5.3; P = .027).
Table 4 shows the presenting features of the 8 patients with isolated or combined CNS relapse. None of the features were significantly associated with the development of isolated or isolated plus combined CNS relapse (data not shown).
Presenting features of patients with CNS relapse
Patient no. . | Age, y . | Sex . | Leukocyte count, × 109/L . | Immunophenotype . | Genotype . | CNS status . |
|---|---|---|---|---|---|---|
| Isolated CNS relapse | ||||||
| 1 | 2 | Male | 565 | B-cell precursor | Pseudodiploid | CNS-2 |
| 2 | 6 | Male | 23.5 | T-cell | Pseudodiploid | CNS-2 |
| 3 | 7 | Male | 8 | B-cell precursor | Hyperdiploid > 50 | CNS-1 |
| 4 | 9 | Male | 2.4 | B-cell precursor | Near-haploid | CNS-2 |
| Combined CNS relapse | ||||||
| 5 | 5 | Male | 367.5 | T-cell | Normal | CNS-2 |
| 6 | 5 | Female | 6.8 | B-cell precursor | Hyperdiploid > 50 | CNS-1 |
| 7 | 13 | Male | 10.9 | B-cell precursor | — | CNS-1 |
| 8 | 13 | Male | 48.2 | T-cell | Pseudodiploid | CNS-1 |
Patient no. . | Age, y . | Sex . | Leukocyte count, × 109/L . | Immunophenotype . | Genotype . | CNS status . |
|---|---|---|---|---|---|---|
| Isolated CNS relapse | ||||||
| 1 | 2 | Male | 565 | B-cell precursor | Pseudodiploid | CNS-2 |
| 2 | 6 | Male | 23.5 | T-cell | Pseudodiploid | CNS-2 |
| 3 | 7 | Male | 8 | B-cell precursor | Hyperdiploid > 50 | CNS-1 |
| 4 | 9 | Male | 2.4 | B-cell precursor | Near-haploid | CNS-2 |
| Combined CNS relapse | ||||||
| 5 | 5 | Male | 367.5 | T-cell | Normal | CNS-2 |
| 6 | 5 | Female | 6.8 | B-cell precursor | Hyperdiploid > 50 | CNS-1 |
| 7 | 13 | Male | 10.9 | B-cell precursor | — | CNS-1 |
| 8 | 13 | Male | 48.2 | T-cell | Pseudodiploid | CNS-1 |
Toxicity
Grade 4 infections (not including disseminated fungal infections) developed in 13 patients during remission induction, 8 during reinduction treatment, and 15 during continuation treatment, with a cumulative risk of 13.8% ± 2.2% over the entire treatment period. Disseminated fungal infections (grade 3 or 4) were documented, mostly by diagnostic imaging, in 11 patients during remission induction, 2 during consolidation treatment, and 3 during continuation treatment, with a cumulative risk of 6.1% ± 1.5% over the entire course of treatment. As mentioned earlier, among the 6 toxic deaths not related to transplantation, disseminated fungal infection and bacterial sepsis accounted for 2 cases each.
Discussion
Study XIIIB was at least as effective as Study XIIIA (5-year event-free survival rate, 80.8% ± 2.6% vs 77.6% ± 3.2%), despite a smaller proportion of patients treated with intensified chemotherapy (47% vs 88%). We attribute this result in part to the substitution of dexamethasone for prednisone during the postremission phases of therapy,9 and in part to the more precise classification of risk features, including molecular genetic abnormalities, and the measurement of early treatment response.21 Importantly, the use of dexamethasone during postremission treatment did not result in excessive infections, with only 3 cases of disseminated fungal infection noted in this phase of treatment.
It is well recognized that cranial irradiation can cause many major late complications, including second cancers, neurocognitive deficits, and endocrinopathy. In fact, our recent long-term follow-up study of 10-year event-free survivors revealed a 20% cumulative risk of second neoplasms among patients who had received cranial irradiation, resulting in a higher-than-expected mortality rate; these survivors also had a high unemployment rate, and among females a low marital rate.22 Hence, the practice in modern clinical trials has been to limit the use of this treatment modality to patients with particularly high risk of CNS relapse. Investigators of the Berlin-Frankfurt-Münster consortium have reduced the dosage of cranial irradiation to 12 Gy in patients with T-cell or high-risk ALL and to 18 Gy in those with CNS-3 status.23 In the Pediatric Oncology Group studies, among patients with B-cell precursor ALL, only those with CNS-3 status received craniospinal irradiation (24 Gy cranial and 15 Gy spinal).24 In Study XIIIB, only 12% of the patients received cranial irradiation, compared with 22% of those in Study XIIIA. The cumulative risks of isolated CNS and isolated plus combined CNS relapse remained low in this study, compared with those in Study XIIIA: 1.7% versus 1.2%, and 3.0% versus 3.2%, respectively. To this end, treatment in Study XIIIB has largely eliminated the adverse prognostic impact of CNS-2 status and traumatic lumbar puncture with blasts, findings associated with 2- to 3-fold increased risk of CNS relapse in other studies.15,23,25-27 Hence, results of Study XIIIB provide further information on which to base a randomized clinical trial of CNS-directed therapy with or without cranial irradiation in patients at high risk of CNS relapse, including those with CNS-3 status. We contend that in the context of effective systemic and intrathecal chemotherapy, cranial irradiation can be eliminated in all patients.8
The rate of therapy-related myeloid malignancies was unacceptably high (3.5% ± 1.2%) in Study XIIIB, despite the use of a less leukemogenic treatment regimen that excluded l-asparaginase during etoposide-containing continuation treatment.28 The risk of therapy-related myeloid malignancies in lower-risk patients was lower than that in the higher-risk patients, but the difference did not attain statistical significance. Patients in the lower-risk arm received only 4 etoposide treatments (total dose, 1.2 g/m2), whereas those in the higher-risk arm received up to 44 treatments (total dose, 14.4 g/m2). This finding supports our previous observations that treatment schedule, coadministration of other therapy, and host susceptibility are more important than the cumulative dose of epipodophyllotoxins in the development of therapy-related leukemia.28 In this regard, we demonstrated the emergence of therapy-related leukemia fusion transcripts in an adolescent after 6 months of chemotherapy that included only 2 doses of etoposide and 1 dose of daunorubicin.29 Nonetheless, additional studies are needed to determine the relative importance of treatment schedule versus dosage in the pathogenesis of therapy-related leukemia because our data do not permit direct assessment of these factors. Results of a DNA microarray analysis suggested that the gene expression profile of the patients' de novo leukemic cells could be predictive of the development of therapy-related leukemia,30 but additional studies are needed to confirm this finding. Thus, until patients at risk for this complication can be reliably identified, we have omitted epipodophyllotoxins from our front-line ALL treatment programs. The 2 exceptions are patients who are candidates for allogeneic hematopoietic stem cell transplantation and those with relapsed leukemia. Preliminary results of our ongoing study suggest that elimination of epipodophyllotoxins has not compromised either event-free survival or CNS control (C.-H.P., unpublished observation, June 2004).
With the improved treatment in Study XIIIB, MLL-AF4 fusion and BCR-ABL fusion were the only 2 presenting features clearly associated with a poor prognosis. Once assigned an unfavorable risk status in treatment programs that used standard antimetabolite-based therapy,14 patients with the t(1;19)/E2A-PBX1 fusion actually had a high cure rate in this study (5-year EFS rate, 80% ± 11.9%). Notably, in a subset of patients studied for minimal residual leukemia, this early response measure emerged as the most important prognostic indicator, because it accounts for both the intrinsic drug sensitivity/resistance of the leukemic cells and the pharmacodynamics of chemotherapy affected by the pharmacogenetics of the host.31,32
In summary, this study provides data further supporting the importance of early intensification of intrathecal therapy, as well as a platform on which to test the feasibility of eliminating cranial irradiation altogether in patients with newly diagnosed ALL. It should be noted that intrathecal therapy and dexamethasone can also adversely affect neurocognitive function.33-35 Studies are ongoing to test neurocognitive performance of the long-term survivors treated in this study. Further refinement of therapy by incorporating minimal residual leukemia measurement and host pharmacogenetic determination into existing risk classification schemes may advance the cure rate further.1,21 The ultimate goal of modern leukemia therapy, to cure all patients with ALL, will not be realized until we learn to target therapy more effectively in patients with high-risk genetic abnormalities and in others with poor early responses. In this regard, studies are under way to test the efficacy of imatinib mesylate in cases with BCR-ABL fusion, and a phase 1 trial of a fms-like tyrosine kinase-3 (FLT-3) inhibitor is being planned for patients with MLL-rearranged leukemias.36
Prepublished online as Blood First Edition Paper, July 13, 2004; DOI 10.1182/blood-2004-04-1616.
Supported by grants CA-21765, CA-36401, CA-51001, CA-60419, CA-71907, CA-78224, GM61374, and GM61393 from the National Institutes of Health, a Center of Excellence grant from the State of Tennessee, and the American Lebanese Syrian Associated Charities. C.-H.P. is the American Cancer Society FM Kirby Clinical Research Professor.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal