Abstract
Annexin A5 (A5) forms 2-dimensional crystals over phospholipid bilayers, blocking their availability for coagulation reactions. Recently, human antiphospholipid (aPL) monoclonal antibodies (mAbs) have been demonstrated by atomic force microscopy (AFM) to disrupt this crystallization and accelerate coagulation. We therefore performed a study with small, well-defined groups of patients to investigate whether these effects on A5 binding and activity are also detectable in plasmas from patients with the aPL syndrome. A5 binding to phospholipid was significantly reduced by plasmas of patients with the aPL syndrome and thromboembolism compared with healthy controls (mean ± SD, 26.7 ± 4.3 ng/well [n = 25] vs 30.5 ± 3.1 ng/well [n = 20], P < .01) and the non-aPL thromboembolism group (29.9 ± 3.2 ng/well [n = 15], P < .05). A5 anticoagulant activity was reduced by plasmas of patients with aPL syndrome and thromboembolism compared with aPL antibodies without thrombosis (182 ± 31% [n = 25] vs 210 ± 35% [n = 26], P < .01), non-aPL thromboembolism (229 ± 16% [n = 15], P < .001), and healthy controls (231 ± 14% [n = 30], P < .001). In conclusion, in accordance with recent AFM data with monoclonal human aPL antibodies, plasmas from patients with aPL antibodies with thromboembolism reduce both A5 binding to phospholipid and A5 anticoagulant activity. This “annexin A5 resistance” identifies a novel mechanism for thrombosis in the aPL syndrome. (Blood. 2004;104: 2783-2790)
Introduction
The antiphospholipid (aPL) syndrome is a disorder in which thrombosis and recurrent pregnancy losses are associated with the presence of antibodies directed against anionic phospholipids and putative cofactors.1,2 The pathophysiologic mechanism(s) for thrombosis in this disorder has not yet been established (for a recent review see Rand3 ). Annexin A5 (previously known as annexin-V) is a potent anticoagulant protein whose anticoagulant properties are a consequence of its high affinity for anionic phospholipid.4,5 The protein forms 2-dimensional crystals on phospholipid surfaces6-8 that shield the phospholipids from availability for the phospholipid-dependent coagulation reactions.9 Annexin A5 is highly expressed by human endothelial cells10 and trophoblasts11 and is present on the surfaces of these cells.11,12 Annexin A5 is required for the maintenance of placental integrity in mice.13 Infusion of anti-annexin A5 immunoglobulin G (IgG) antibodies into pregnant animals decreased the availability of annexin A5 to bind to the trophoblast surfaces and caused placental thrombosis, necrosis, and fetal loss. However, it is interesting to note that a transgenic annexin A5-deficient mouse was found to be fertile.14
We recently showed, through atomic force microscopic imaging, that aPL antibodies can disrupt the crystallization of annexin A5 over phospholipid membranes, expose underlying phospholipid membranes for availability for coagulation reactions, and thereby reduce the anticoagulant effects of annexin A5.15 These findings led us to investigate whether disruption of annexin A5 binding and reduction of its anticoagulant effect could be observed in plasmas from patients with aPL antibodies.
We therefore performed a small study with well-defined groups of patients to determine the effects of their plasmas on these parameters. Plasma-based assays were devised for this purpose and were validated with control plasmas spiked with purified aPL IgG and with plasmas from patients with the aPL syndrome. We then applied these assays to investigate plasmas from 3 well-defined groups: (1) patients with the aPL syndrome with histories of thromboembolic events, (2) patients with the aPL antibodies without previous histories of thrombosis or embolism, and (3) patients with thromboembolism without any evidence for aPL antibodies—compared with healthy control donors.
Patients, materials, and methods
Proteins and antibodies
Annexin A5 was purified from human placentas according to the method of Funakoshi et al.4 Oregon green-conjugated annexin A5 (OG-annexin A5; Molecular Probes, Eugene, OR) was used for annexin A5 binding assays. Human β2 glycoprotein I (β2GPI) was purified as previously described.16 Human factor Xa, bovine factor Va, and human prothrombin were generous gifts from Dr Yale Nemerson (Division of Thrombosis, Mount Sinai School of Medicine, New York, NY). Recombinant human tissue factor (TF; amino acids 1-243) was a gift from Genentech (San Francisco, CA). IgG antibodies were isolated from sera of 3 patients with severe antiphospholipid syndrome and 3 healthy subjects with protein G-Sepharose (Amersham Pharmacia Biotech, Uppsala, Sweden), as previously described.17 A previously characterized monoclonal aPL antibody IS4 was derived from a patient with the primary aPL syndrome.18 The antibody is an IgG3 that recognized human β2GPI-cardiolipin complexes, human β2GPI alone, and, to a small degree, cardiolipin alone.18 A monoclonal IgG3 from a patient with a monoclonal gammopathy (Sigma, St Louis, MO) was used as its control.
Plasma specimens
After institutional review board approval, blood specimens were collected under informed consent from patients who were recruited through the Rheumatology and the High-Risk Thrombosis Clinics at Duke University Medical Center. The samples were prepared by double centrifugation. Lupus anticoagulants were identified using the criteria proposed by the Subcommittee on Lupus Anticoagulants/Antiphospholipid Antibodies of the International Society of Thrombosis and Haemostasis,19 as previously described.20 Anticardiolipin antibody IgG and IgM levels were determined by enzyme-linked immunosorbent assay using an in-house test developed in the Clinical Immunology lab of Duke University Medical Center, as described by Loizou et al.21 Antiphospholipid antibody testing was performed on one or more occasions at a time when the patient was clinically stable. Repeat anticardiolipin antibody analysis was performed on all patient samples. The dilute Russell viper venom time (DRVVT)/Confirm test was done with a commercially available method (Diagnostica Stago, Parsippany, NJ). The prothrombin time (PT) and activated partial thromboplastin time (aPTT) tests were run using an MDA-180 instrument (Organon Teknika, Research Triangle Park, NC) with their standard reagents (Simplastin L and Platelin L, respectively).
There were 51 patients with aPL antibodies enrolled into the studies and subclassified into 2 groups: (1) aPL syndrome with thromboembolism and (2) aPL antibodies without thrombosis history.
The aPL syndrome with thromboembolism group contained 25 patients. All of the patients satisfied the Sapporo investigational criteria for the diagnosis of the aPL syndrome.22 These patients (13 male, 12 female) had sustained one or more thromboembolic events 3 or more months prior to analysis. There were 18 patients who sustained a venous thromboembolic event, 4 of whom also sustained an arterial thromboembolic event. In this group, 7 patients sustained isolated thromboembolic cerebrovascular events (mean age, 37 years; range, 18-49 years). With one exception, all of these patients were on oral anticoagulant therapy at the time of testing.
The aPL antibodies without thrombosis history group contained 26 patients with aPL antibodies who had not sustained a thromboembolic event (7 male, 19 female), although 4 of these patients had sustained one or more miscarriages. Of these 26 patients, 1 was on oral anticoagulant therapy for a prosthetic mitral valve.
Composing the non-aPL thromboembolism group were 15 additional patients (7 male, 8 female) who sustained a thromboembolic event (14 venous, 1 arterial) but who had no evidence of an aPL antibody. Of these 15 patients, 4 were heterozygous for factor V Leiden, 1 was heterozygous for the prothrombin 20210G>A polymorphism, and 1 was homozygous for the thermolabile variant of methylene tetrahydrofolate reductase. At the time of enrollment into the studies, 14 of these patients were on oral anticoagulant therapy. In addition, 30 plasma specimens were obtained from healthy blood bank donors at Mount Sinai Medical Center.
Annexin A5 binding studies
The annexin A5 binding and coagulation methods are outlined in Figure 1. We first performed quantitative assessment of effects of aPL IgG on the binding of annexin A5 to phospholipid by exposing the phospholipid to test plasma and then to labeled annexin A5 as previously described,17 with minor modifications. aPTT reagent-phospholipid (50 μL, Actin FS; Dade Behring, Newark, DE) was incubated with 50 μL citrated plasma that was spiked with aPL or normal IgG (7.5 mg/mL) for 10 minutes at 25°C. The mixture of aPTT reagent-phospholipid and IgG-spiked plasma was sedimented with a microcentrifuge (Eppendorf Centrifuge 5417R; Brinkmann Instrument, Westbury, NY) for 15 minutes at 20 800g at 25°C. The IgG-spiked plasma-treated phospholipid pellets were washed 3 times with HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-buffered saline (HBS) (0.01 M HEPES, 0.14 M NaCl, pH 7.5) and resuspended in 50 μL HBS containing 5 mM CaCl2. OG-annexin A5 (32.5 ng) in the calcium-containing buffer (50 μL) was then added into the phospholipid suspension and incubated for 5 minutes at 25°C. After additional centrifugation, the phospholipid pellets were resuspended in 100 μL HBS containing 10 mM EDTA (ethylenediaminetetraacetic acid) and sedimented with the microcentrifuge. The supernatant was removed to a microtiterplate (Fluoro-Nunc; Fisher Scientific, Pittsburgh, PA) filled with 100 μL/well HBS containing 10 mM EDTA and 0.05% bovine serum albumin (BSA). The quantity of phospholipid-associated annexin A5 in the supernatant was then detected by measuring wavelengths of excitation at 485 nM and emission at 535 nm using a microplate spectrofluorometer (Molecular Devices, Sunnyvale, CA). The results of the assay were expressed as nanogram of labeled annexin A5 per aliquot (50 μL) of aPTT reagent-phospholipid (ng/aliquot of phospholipid [PL]), as previously described.17
We then determined whether depleting plasmas of IgG would alter the observed effect on annexin A5 binding. Plasma (120 μL) from aPL patients or healthy controls was incubated with 200 μL protein G-Sepharose or Sepharose 2B (Amersham Pharmacia Biotech) overnight at 4°C. The beads were sedimented with the microcentrifuge for 10 minutes at 20 800g at 25°C and removed. The treatment with protein G-Sepharose does not leave any detectable IgG in the supernatants. aPTT reagent-phospholipid (50 μL) was incubated with 50 μL protein G-Sepharose-pretreated plasma or Sepharose 2B-pretreated plasma and incubated for 10 minutes at 25°C. The mixture of aPTT reagent-phospholipid and Sepharose-pretreated plasma was sedimented with the microcentrifuge for 15 minutes at 20 800g at 25°C. The pellets were washed 3 times with HBS and resuspended in 50 μL 5-mM calcium-containing HBS. OG-annexin A5 (32.5 ng) in the calcium-containing buffer (50 μL) was then added to the suspension and incubated for 5 minutes at 25°C. After centrifugation for 15 minutes at 20 800g, the phospholipid pellets were resuspended in 100 μL 10-mM EDTA containing HBS and then sedimented with the microcentrifuge. The phospholipid-associated annexin A5 that was released into the supernatant was then determined using the spectrofluorometer.
The effect of the aPL plasma concentration on annexin A5 binding was also determined. Citrated plasmas from 2 different patients with the aPL syndrome were serially diluted in normal plasma at 1%, 2%, 5%, 10%, 20%, 50%, and 100% ratios. aPTT reagent-phospholipid (50 μL) was incubated with 50 μL of the plasma for 10 minutes at 25°C. The mixture of aPTT reagent-phospholipid and the plasma was sedimented, washed, and resuspended in 50 μL of the calcium-containing HBS, followed by addition of 32.5 ng OG-annexin A5 in the buffer (50 μL). After incubation and centrifugation, the phospholipid pellets were resuspended in 100 μL 10-mM EDTA containing HBS and then sedimented, the phospholipid pellets were resuspended in 100 μL of the EDTA buffer and sedimented, and the phospholipid-associated annexin A5 was then detected using the spectrofluorometer.
Annexin A5 binding assays for patient plasma groups
Annexin A5 binding assays were performed for plasmas from the 3 groups of well-defined patients and healthy controls using phospholipid-coated plates.17 Microtiter plates (Nunc-Immuno Plate, MaxiSorp Surface; Fisher Scientific) were coated with phospholipids composed of 50 μM 30% 1,2-dioleoyl-sn-glycero-3-phospho-l-serine (PS) and 70% 1,2-dioleoyl-sn-glycero-3-phosphocholine (PC) (Avanti Polar-Lipids, Alabaster, AL) as previously described.17 The composition of phospholipid that was used was identical to that used for the atomic force imaging studies of aPL antibodies and annexin A5.15 Citrated plasma (50 μL/well) was added and incubated for 10 minutes at 37°C. The plate was washed 3 times with HBS. OG-annexin A5 (50 ng) in HBS containing 5 mM CaCl2 (50 μL) was then added and incubated for 90 minutes at 37°C. The plate was washed 3 times with HBS containing 5 mM CaCl2 and 0.1% BSA, followed by 100 μL 10-mM EDTA in HBS with 0.1% BSA. The quantity of annexin A5 dissociated from the phospholipid-coated wells was determined with the microplate spectrofluorometer. The results were reported as nanogram/well. To determine the precision and accuracy of the annexin A5 binding assay, we tested plasma from a healthy control 15 times in the same test-run and 3 times over a period of 3 weeks. The coefficient of variation (CV) for the assay was between 2.6% and 4.5%, and the interassay CV was 3.7%.
Annexin A5 resistance studies
The effects of a previously characterized monoclonal aPL antibody IS418 on annexin A5 anticoagulant activity were measured using the prothrombinase reaction and then confirmed in plasma. Experiments were performed at room temperature. Microtiter plates were coated with 30% PS/70% PC as previously described.17 This phospholipid composition is identical to that which had been used for the atomic force imaging studies15 and was validated in pilot studies performed with microtiter plates coated with variable proportions of PS/PC. HBS containing 0.1% BSA (350 μL) was added to the wells and incubated for 60 minutes. The wells were then filled with 300 μL HBS containing 1.25 mM CaCl2 (a concentration that is in the physiologic range of free calcium ion concentration in plasma) and 0.1% BSA, aPL monoclonal antibody (mAb, IS4) or an isotype-matched control mAb IgG3 (80 μg/mL) in triplicate, together with or without human β2GPI (15 μg/mL) and incubated for 30 minutes. The wells were washed 4 times with the buffer, followed by addition of annexin A5 (7 μg/mL) in the buffer, and incubated for 60 minutes. After washing 4 times with the buffer, factor Xa (final concentration 1.5 nM) and factor Va (1.5 nM) were added and incubated for 10 minutes. Prothrombin (34 nM) was then added to reach a total volume of 350 μL. The enzyme mixture in the well was serially aliquoted (50 μL/well) into another microtiter plate (DYNEX Technologies, Chantilly, VA) filled with 100 μL/well of bicine buffer (50 mM bicine, 0.1 M NaCl, 0.1% BSA, 25 mM EDTA, pH 8.5). Chromogenic substrate S-2238 (DiaPharma, West Chester, OH) was then added (0.57 mM). The thrombin generation rate (TGR) was measured using a SPECTRAmax Plus 384 microplate reader (Molecular Devices, Menlo Park, CA) at 405 nM and 490 nM dual beam at 35°C.
The effects of the aPL mAb on plasma coagulation and annexin A5 anticoagulant activity were studied as follows. Phospholipid vesicles, consisting of 30% PS and 70% PC, were incorporated with 1-243 TF using octyl glucoside as previously described.23,24 The aPL IS4 or control IgG3 mAb (50 μg/mL) was incubated with the relipidated TF (TF/PL, 3.05 nM tissue factor and 13.4 μM phospholipids) (a total of 100 μL) for 15 minutes at room temperature. HBS (150 μL) was added to increase the volume of the mixture to 250 μL. The mixture (50 μL) was then incubated with 50 μL pooled normal plasma for 20 seconds at 37°C in an ST4 Coagulation Instrument (American Bioproducts, Parsippany, NJ), after which 0.02 M CaCl2 or 0.02 M CaCl2 (50 μL) containing annexin A5 (30 μg/mL) was added. Duplicate tests, with and without annexin A5, were done. The coagulation times were measured and the mean times of the duplicate tests were recorded. The anticoagulant activity of annexin A5 was expressed as the “annexin A5 anticoagulant ratio” as follows: annexin A5 anticoagulant ratio = (coagulation time in the presence of annexin A5/coagulation time in the absence of annexin A5) × 100%.
The effects of aPL IgGs on annexin A5 anticoagulant activity were then determined using a 2-stage method (Figure 1), which exposed phospholipid to test plasma followed by an exposure to normal pooled plasma, as previously described, with modifications.17 One vial of lyophilized recombinant human TF (Innovin; Dade Behring) was reconstituted with 10 mL suspension of aPTT reagent (Actin FSL; Dade Behring). The mixture of TF and aPTT reagent-phospholipid was washed twice with HBS by sedimentation and resuspension with the microcentrifuge for 15 minutes at 20 800g at 25°C. The pellet was resuspended in a total of 10 mL HBS. Of this washed TF-aPTT reagent phospholipid, 100 μL was incubated with 50 μL normal plasma that was spiked with aPL or normal IgG to a final concentration of 7.5 mg/mL for 5 minutes at room temperature. The mixture of TF-aPTT-phospholipid and IgG-spiked plasma was sedimented with the microcentrifuge. The pellets were washed once with HBS and resuspended in 220 μL of the buffer. The pretreated phospholipid (50 μL) was then incubated with 50 μL pooled normal plasma for 20 seconds at 37°C in the ST4 Coagulation Instrument, which was then recalcified with 50 μL 0.02-M CaCl2, with and without annexin A5 (30 μg/mL); the annexin A5 anticoagulant ratios of the duplicate tests were determined.
The effects of depleting aPL IgG from plasmas on annexin A5 anticoagulant activity were then investigated. Plasmas from aPL patients or healthy controls were pretreated with protein G-Sepharose or Sepharose 2B as for the annexin A5 binding studies described above. The washed TF-aPTT-phospholipid reagent (100 μL), prepared as described above, was incubated with 50 μL protein G-Sepharose-pretreated plasma or Sepharose 2B-pretreated plasma for 5 minutes at room temperature. The mixtures were pelleted with the microcentrifuge, washed once in HBS, and resuspended in 220 μL of the buffer. The phospholipid (50 μL) was then incubated with 50 μL pooled normal plasma for 20 seconds at 37°C in the ST4 Coagulation Instrument, which was then recalcified with 50 μL 0.02-M CaCl2, with and without added annexin A5 (30 μg/mL), and the annexin A5 anticoagulant ratios were determined.
To observe the effects of varying concentrations of aPL plasma on annexin A5 anticoagulant activity, aPL plasma was serially diluted in a normal control plasma. The washed TF-aPTT reagent-phospholipid (100 μL), described above, was incubated with 50 μL of the plasma for 5 minutes, and the annexin A5 anticoagulant ratios were measured as described above.
Annexin A5 resistance assays for patient plasma groups
Annexin A5 resistance assays for the plasmas from the 4 groups described above were tested using prothrombin time (PT) reagent (tissue factor-phospholipid complex) (Thromboplastin-C Plus; Baxter Diagnostics, Deer-field, IL). A vial of PT reagent was washed twice with HBS by sedimentation and resuspension with the microcentrifuge as for TF-aPTT reagent phospholipid described above. The pellet of the washed PT reagent-phospholipid was resuspended in 10 mL HBS; aliquots (50 μL) were then incubated with 50 μL test plasma for 5 minutes at room temperature, washed, and resuspended in 220 μL HBS. A portion of this (50 μL) was incubated with 50 μL pooled normal plasma in the ST4 Coagulation Instrument, which was then recalcified with 50 μL 0.02-M CaCl2, with and without added annexin A5 (30 μg/mL); the annexin A5 anticoagulant ratio was determined. The precision and accuracy of the annexin A5 resistance assay was determined in the same manner as described above for the annexin A5 binding assay. The intra-assay CV was between 2.0% and 3.8%, and the interassay CV was 2.7%.
Statistical analysis
Differences between aPL and control IgGs were assessed using unpaired 2-tailed t test. Because of concern that the patient samples for the annexin A5 binding and resistance studies might have been drawn from nonnormal distributions, pairwise comparisons of patient groups were assessed by Kruskal-Wallis test.
Correlation coefficient (r) and 95% coefficient intervals between annexin A5 binding assay and annexin A5 resistance assay were evaluated by Spearman rank correlation within all groups. The 95% prediction intervals was generated by SigmaPlot software (SPSS, Chicago, IL).
Results
Annexin A5 binding studies
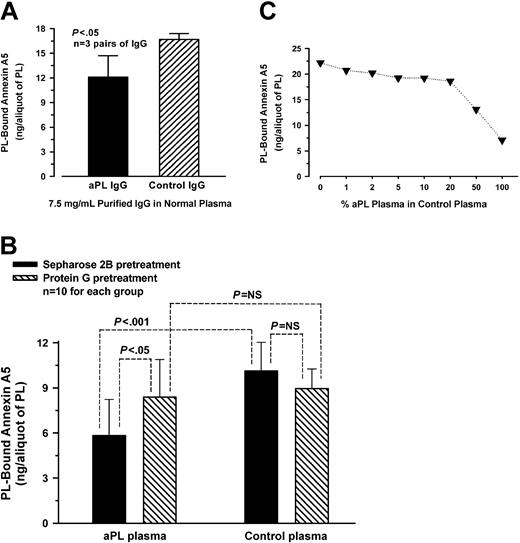
Quantitative assessment of annexin A5 binding to aPTT reagent-phospholipid showed that phospholipid preincubated with aPL IgG-spiked plasma bound significantly less labeled annexin A5 (mean ± SD, 12.1 ± 2.6 ng/aliquot of PL) than phospholipid preincubated with control IgG-spiked plasma (16.7 ± 0.7 ng/aliquot of PL, P < .05) (n = 3 pairs of different aPL and control IgGs) (Figure 2A). In separate experiments, depletion of aPL IgG antibodies from plasmas by protein G-Sepharose significantly increased the binding of annexin A5 to phospholipid (8.4 ± 2.5 ng/aliquot of PL) compared with aPL plasmas pretreated with Sepharose 2B (5.8 ± 2.4 ng/aliquot of PL, P < .05) (Figure 2B); in control plasmas, there was no significant difference in the ability to bind annexin A5 between plasmas pretreated with protein G-Sepharose (9.0 ± 1.3 ng) and plasmas pretreated with Sepharose 2B (10.1 ± 1.9 ng) (n = 10 different plasmas for each group) (Figure 2B). Dose-response studies on the effects of varying concentrations of aPL plasmas on annexin A5 binding showed that the phospholipid-bound annexin A5 progressively decreased as the concentration of aPL plasma was increased in a normal plasma (Figure 2C).
Annexin A5 binding studies. (A) Effects of spiking plasmas with aPL IgG on annexin A5 binding to phospholipid. aPL or control IgG was added to a normal plasma to a final concentration of 7.5 mg/mL and incubated with aPTT reagent-phospholipid. The phospholipid preincubated with aPL IgG-spiked plasma bound significantly less labeled annexin A5 (mean ± SD, 12.1 ± 2.6 ng/aliquot of PL) compared with the phospholipid preincubated with control IgG-spiked plasma (16.7 ± 0.7 ng/aliquot of PL, P < .05) (n = 3 pairs of different IgG). (B) Effects of depleting aPL IgG from plasmas on annexin A5 binding. aPL plasmas that were pretreated with protein G-Sepharose significantly increased the ability of annexin A5 binding to phospholipid (mean ± SD, 8.4 ± 2.5 ng/aliquot of PL) compared with aPL plasmas preincubated with Sepharose 2B (5.8 ± 2.4 ng/aliquot of PL, P < .05). In control plasmas, no significant difference on the binding of annexin A5 was observed between plasmas preincubated with protein G-Sepharose (9.0 ± 1.3 ng) and plasmas pretreated with Sepharose 2B (10.1 ± 1.9 ng) (n = 10 plasmas for each group). NS indicates not significant. (C) Effects of varying concentrations of aPL plasmas on annexin A5 binding. aPL plasmas were serially diluted in a normal plasma and incubated with aPTT reagent-phospholipid. The quantity of annexin A5 bound to phospholipid decreased progressively as the concentration of aPL plasma, diluted in a normal control plasma, was increased (each point shows the mean of 2 aPL patient plasmas).
Annexin A5 binding studies. (A) Effects of spiking plasmas with aPL IgG on annexin A5 binding to phospholipid. aPL or control IgG was added to a normal plasma to a final concentration of 7.5 mg/mL and incubated with aPTT reagent-phospholipid. The phospholipid preincubated with aPL IgG-spiked plasma bound significantly less labeled annexin A5 (mean ± SD, 12.1 ± 2.6 ng/aliquot of PL) compared with the phospholipid preincubated with control IgG-spiked plasma (16.7 ± 0.7 ng/aliquot of PL, P < .05) (n = 3 pairs of different IgG). (B) Effects of depleting aPL IgG from plasmas on annexin A5 binding. aPL plasmas that were pretreated with protein G-Sepharose significantly increased the ability of annexin A5 binding to phospholipid (mean ± SD, 8.4 ± 2.5 ng/aliquot of PL) compared with aPL plasmas preincubated with Sepharose 2B (5.8 ± 2.4 ng/aliquot of PL, P < .05). In control plasmas, no significant difference on the binding of annexin A5 was observed between plasmas preincubated with protein G-Sepharose (9.0 ± 1.3 ng) and plasmas pretreated with Sepharose 2B (10.1 ± 1.9 ng) (n = 10 plasmas for each group). NS indicates not significant. (C) Effects of varying concentrations of aPL plasmas on annexin A5 binding. aPL plasmas were serially diluted in a normal plasma and incubated with aPTT reagent-phospholipid. The quantity of annexin A5 bound to phospholipid decreased progressively as the concentration of aPL plasma, diluted in a normal control plasma, was increased (each point shows the mean of 2 aPL patient plasmas).
Annexin A5 binding assays for patient plasma groups
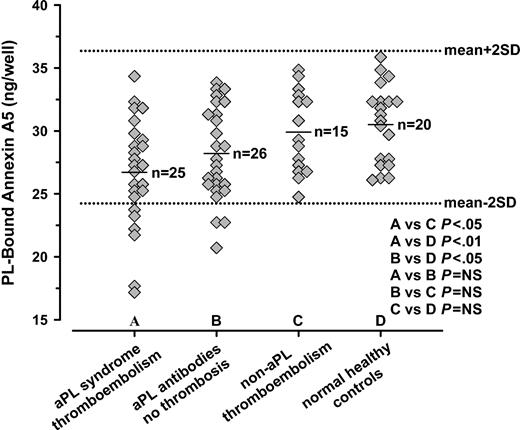
Annexin A5 binding assays for plasmas from the well-defined 3 groups of patients and healthy controls showed that phospholipid-coated wells preincubated with plasmas from the aPL syndrome with thromboembolism group bound significantly less annexin A5 (mean ± SD, 26.7 ± 4.3 ng/well; n = 25; range, 17.2-34.3 ng/well; median, 27.3 ng/well) than wells preincubated with plasmas from the healthy control group (30.5 ± 3.1 ng/well; n = 20; range, 26.1-35.9 ng/well; median, 31.1 ng/well; P < .01) and the non-aPL thromboembolism group (29.9 ± 3.2 ng/well; n = 15; range, 24.8-34.9 ng/well; median, 29.3 ng/well; P < .05) (Figure 3). There were 4 patients with aPL antibodies without thrombosis who had miscarriages; 2 of these showed annexin A5 binding levels that were near the mean and median of the controls, with values of 31.3 and 33.3 ng/well, respectively, while the other 2 had levels that were 24.7 and 25.8 ng/well, respectively. The wells pretreated with plasmas from the group of patients with aPL antibodies without thrombosis also bound significantly less annexin A5 (28.2 ± 3.7 ng/well; n = 26; range, 20.7-33.8 ng/well; median, 27.5 ng/well) than the healthy controls (P < .05). There were no significant differences in the binding of annexin A5 between the wells pretreated with plasmas from the aPL syndrome with thromboembolism and the aPL antibodies without thrombosis groups, and plasmas from the non-aPL thromboembolism and the healthy controls (Figure 3).
Annexin A5 binding assays for patient plasma groups. PS/PC-coated wells that had been preincubated with plasmas from the aPL syndrome with thromboembolism group (A) bound significantly less annexin A5 (mean ± SD, 26.7 ± 4.3 ng/well, n = 25) than wells preincubated with plasmas from the healthy control group (D) (30.5 ± 3.1 ng/well, n = 20, P < .01) and plasmas from non-aPL thromboembolism group (C) (29.9 ± 3.2 ng/well, n = 15, P < .05). Wells pretreated with plasmas from the aPL antibodies without thrombosis group (B) also bound significantly less annexin A5 (28.2 ± 3.7 ng/well, n = 26) than the healthy controls (D) (P < .05). There were no significant differences in the binding of annexin A5 between the wells pretreated with plasmas from the aPL syndrome with thromboembolism (A) and the aPL antibodies without thrombosis (B) groups, and plasmas from the non-aPL thromboembolism (C) and the healthy controls (D). Error bars are shown for mean ± 2 SD of healthy controls (D).
Annexin A5 binding assays for patient plasma groups. PS/PC-coated wells that had been preincubated with plasmas from the aPL syndrome with thromboembolism group (A) bound significantly less annexin A5 (mean ± SD, 26.7 ± 4.3 ng/well, n = 25) than wells preincubated with plasmas from the healthy control group (D) (30.5 ± 3.1 ng/well, n = 20, P < .01) and plasmas from non-aPL thromboembolism group (C) (29.9 ± 3.2 ng/well, n = 15, P < .05). Wells pretreated with plasmas from the aPL antibodies without thrombosis group (B) also bound significantly less annexin A5 (28.2 ± 3.7 ng/well, n = 26) than the healthy controls (D) (P < .05). There were no significant differences in the binding of annexin A5 between the wells pretreated with plasmas from the aPL syndrome with thromboembolism (A) and the aPL antibodies without thrombosis (B) groups, and plasmas from the non-aPL thromboembolism (C) and the healthy controls (D). Error bars are shown for mean ± 2 SD of healthy controls (D).
Annexin A5 resistance studies
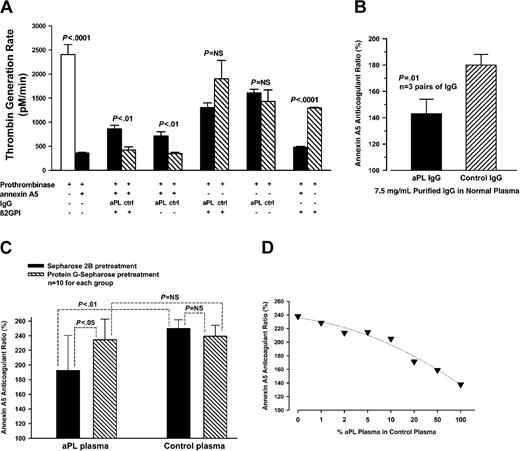
We examined the effects of a previously characterized monoclonal aPL antibody, IS4,18 on the anticoagulant activity of annexin A5 using the prothrombinase reaction. We then determined whether the aPL mAb-mediated blunting of annexin A5 anticoagulant activity also occurs in plasma. The addition of factor Xa, factor Va, and prothrombin to PS/PC-coated wells yielded a baseline thrombin generation rate (TGR) of 2402 ± 210 pM/min (mean ± SD). Addition of 7 μg/mL (final concentration) annexin A5 significantly reduced the TGR to 358 ± 18 pM/min (P < .0001) (Figure 4A). In the absence of annexin A5, the addition of aPL or control mAb alone to the system showed no significant difference on the TGR (1605 ± 78 pM/min vs 1431 ± 236 pM/min); the addition of the aPL mAb together with β2GPI (15 μg/mL) showed a nonstatistically significant reduction of the TGR compared with the control mAb (1302 ± 96 pM/min vs 1900 ± 384 pM/min). In contrast, in the presence of annexin A5, the aPL mAb significantly increased the TGR (713 ± 87 pM/min) compared with the control mAb (347 ± 28 pM/min, P < .01). The TGR was further increased by the addition of β2GPI (859 ± 77 pM/min, compared with 421 ± 64 pM/min for the control mAb, P < .01). When β2GPI alone was added to the prothrombinase system, the TGR was 1296 ± 11 pM/min and was 478 ± 18 pM/min for β2GPI added together with annexin A5 (P < .0001) (Figure 4A).
Annexin A5 resistance studies. (A) Effect of aPL mAb on inhibition of prothrombinase reaction by annexin A5. Factor Xa, factor Va, and prothrombin added to the PL bilayer yielded a baseline thrombin generation rate (TGR) of 2402 ± 210 pM/min (mean ± SD). Addition of annexin A5 significantly reduced the TGR to 358 ± 18 pM/min (P < .0001). Addition of the aPL mAb to the system containing the annexin A5 reversed the anticoagulant effect of annexin A5 and increased the TGR (713 ± 87 pM/min compared with 347 ± 28 pM/min for the control mAb, P < .01), a reversal that was further enhanced by the addition of β2GPI (859 ± 77 pM/min compared with 421 ± 64 pM/min for the control mAb, P < .01). (B) Effects of spiking plasma with aPL IgG on annexin A5 anticoagulant activity. aPL or control IgG was added to a normal plasma. The annexin A5 anticoagulant ratio for the phospholipid preincubated with aPL IgG and β2GPI was significantly less (mean ± SD, 143 ± 11%) than the phospholipid preincubated with control IgG and β2GPI (180 ± 8%, P = .01) (n = 3 pairs of different IgG). (C) Effects of depleting aPL IgG from aPL plasmas on annexin A5 anticoagulant activity. aPL plasmas that were pretreated with protein G-Sepharose significantly increased annexin A5 anticoagulant ratios (mean ± SD, 235 ± 28%) compared with plasmas pretreated with Sepharose 2B (193 ± 48%, P < .05). In control plasmas, no significant difference on annexin A5 anticoagulant activity was observed between plasmas pretreated with protein G-Sepharose (239 ± 15%) and plasmas pretreated with Sepharose 2B (250 ± 12%) (n = 10 pairs of aPL and control plasmas). (D) Effects of varying aPL plasma concentration on annexin A5 anticoagulant activity. Varying dilutions of aPL plasma in control plasma were incubated with TF-aPTT reagent-phospholipid. The annexin A5 anticoagulant ratio progressively decreased as the concentration of aPL plasma was increased in control plasma. (Each point is the mean of 2 aPL patient plasmas.)
Annexin A5 resistance studies. (A) Effect of aPL mAb on inhibition of prothrombinase reaction by annexin A5. Factor Xa, factor Va, and prothrombin added to the PL bilayer yielded a baseline thrombin generation rate (TGR) of 2402 ± 210 pM/min (mean ± SD). Addition of annexin A5 significantly reduced the TGR to 358 ± 18 pM/min (P < .0001). Addition of the aPL mAb to the system containing the annexin A5 reversed the anticoagulant effect of annexin A5 and increased the TGR (713 ± 87 pM/min compared with 347 ± 28 pM/min for the control mAb, P < .01), a reversal that was further enhanced by the addition of β2GPI (859 ± 77 pM/min compared with 421 ± 64 pM/min for the control mAb, P < .01). (B) Effects of spiking plasma with aPL IgG on annexin A5 anticoagulant activity. aPL or control IgG was added to a normal plasma. The annexin A5 anticoagulant ratio for the phospholipid preincubated with aPL IgG and β2GPI was significantly less (mean ± SD, 143 ± 11%) than the phospholipid preincubated with control IgG and β2GPI (180 ± 8%, P = .01) (n = 3 pairs of different IgG). (C) Effects of depleting aPL IgG from aPL plasmas on annexin A5 anticoagulant activity. aPL plasmas that were pretreated with protein G-Sepharose significantly increased annexin A5 anticoagulant ratios (mean ± SD, 235 ± 28%) compared with plasmas pretreated with Sepharose 2B (193 ± 48%, P < .05). In control plasmas, no significant difference on annexin A5 anticoagulant activity was observed between plasmas pretreated with protein G-Sepharose (239 ± 15%) and plasmas pretreated with Sepharose 2B (250 ± 12%) (n = 10 pairs of aPL and control plasmas). (D) Effects of varying aPL plasma concentration on annexin A5 anticoagulant activity. Varying dilutions of aPL plasma in control plasma were incubated with TF-aPTT reagent-phospholipid. The annexin A5 anticoagulant ratio progressively decreased as the concentration of aPL plasma was increased in control plasma. (Each point is the mean of 2 aPL patient plasmas.)
The effects of the aPL mAb on blunting annexin A5 anticoagulant activity in the prothrombin system were confirmed in plasma. Addition of TF/PL that had been incubated with the aPL mAb to plasma resulted in a coagulation time of 39.3 ± 4.0 seconds versus 28.0 ± 1.2 seconds (mean ± SD) for TF/PL treated with control mAb (n = 4, P < .01). When the plasma was recalcified together with annexin A5, the coagulation time with the aPL mAb-treated TF/PL was significantly less prolonged, to 62.9 ± 5.6 seconds, compared with prolongation to 73.0 ± 5.1 seconds for the control IgG3 (n = 4, P < .05). Thus, compared with the control mAb, the aPL mAb significantly prolonged the coagulation time in the absence of annexin A5 but accelerated the coagulation time in the presence of annexin A5, thereby reducing the annexin A5 anticoagulant ratio to 156 ± 12%, versus 278 ± 2.1% for the control mAb (n = 4, P < .0001).
We investigated whether aPL IgGs isolated from aPL patient sera might have an effect on annexin A5 anticoagulant activity by monitoring the coagulation times. We found that TF-aPTT reagent-phospholipid that had been preincubated with aPL IgG-spiked plasma significantly reduced annexin A5 anticoagulant activity, expressed as annexin A5 anticoagulant ratios (mean ± SD, 143 ± 11%), compared with the phospholipids preincubated with control IgG-spiked plasma (180 ± 8%, P = .01) (n = 3 pairs of different IgGs) (Figure 4B). In separate experiments, aPL plasmas that were depleted of IgG by pretreatment with protein G-Sepharose significantly increased annexin A5 anticoagulant activity (235 ± 28%) compared with aPL plasmas that were pretreated with Sepharose 2B without protein G (193 ± 48%, P < .05); in control plasmas, there was no significant difference in annexin A5 anticoagulant activity between plasmas pretreated with protein G-Sepharose (239 ± 15%) and plasmas pretreated with Sepharose 2B alone (250 ± 12%) (n = 10 pairs of different aPL and control plasmas) (Figure 4C). Dose-response studies on the effects of varying concentrations of aPL plasmas on annexin A5 anticoagulant activity showed that the annexin A5 anticoagulant ratio progressively decreased as the concentration of aPL plasmas was increased in a normal plasma (Figure 4D).
Annexin A5 resistance assays for patient plasma groups
Annexin A5 resistance assays were performed for plasmas from the 3 groups of well-defined patients and healthy controls using PT reagent-phospholipid. Pilot studies to examine the influence of oral anticoagulant therapy on annexin A5 anticoagulant activity showed that there was no significant difference in the annexin A5 anticoagulant ratio between phospholipid pretreated with plasmas from non-aPL patients who were on oral anticoagulant for prosthetic heart valves (mean ± SD, 230 ± 13%, n = 10) and plasmas from healthy controls (228 ± 12%, n = 10). The assays for plasmas from the 4 patient groups showed that plasmas from the aPL syndrome with thromboembolism group had a significant reduced annexin A5 anticoagulant ratio (182 ± 31%; n = 25; range, 120%-226%; median, 187%) compared with plasmas from the aPL antibodies without thrombosis group (210 ± 35%; n = 26; range, 112%-250%; median, 220%; P < .01), non-aPL thromboembolism group (229 ± 16%; n = 15; range, 205%-275%; median, 229%; P < .001), and the healthy control group (231 ± 14%; n = 30; range, 208%-268%; median, 234%; P < .001) (Figure 5). Plasmas from the aPL antibodies without thrombosis group also showed a significantly reduced annexin A5 anticoagulant ratio compared with the healthy control group (P < .05) (Figure 5). Of the 4 patients with miscarriages in this group, the 2 who had the lower levels of annexin A5 binding also showed low annexin A5 anticoagulant ratios (137% and 178%), while the other 2 patients had levels (226% and 235%) that were near the mean and median values of the healthy controls. There was no significant difference between the non-aPL thromboembolism and healthy control groups (Figure 5).
Annexin A5 resistance assays for patient plasma groups. The annexin A5 anticoagulant ratio for the aPL syndrome with thromboembolism group (A) was significantly decreased (mean ± SD, 182 ± 31%, n = 25) compared with the aPL antibodies without thrombosis history group (B) (210 ± 35% [n = 26], P < .01), the non-aPL thromboembolism group (C) (229 ± 16% [n = 15], P < .001), and the healthy control group (D) (231 ± 14% [n = 30], P < .001). The ratio for the plasmas from the aPL antibodies without thrombosis history group (B) also was significantly reduced compared with the healthy control group (D) (P < .05). There were no significant differences in annexin A5 anticoagulant ratio for the non-aPL thromboembolism group (C) compared with the aPL antibodies without thrombosis history group (B) and the healthy control group (D). Error bars are shown for mean ± 2 SD of normal healthy controls (D).
Annexin A5 resistance assays for patient plasma groups. The annexin A5 anticoagulant ratio for the aPL syndrome with thromboembolism group (A) was significantly decreased (mean ± SD, 182 ± 31%, n = 25) compared with the aPL antibodies without thrombosis history group (B) (210 ± 35% [n = 26], P < .01), the non-aPL thromboembolism group (C) (229 ± 16% [n = 15], P < .001), and the healthy control group (D) (231 ± 14% [n = 30], P < .001). The ratio for the plasmas from the aPL antibodies without thrombosis history group (B) also was significantly reduced compared with the healthy control group (D) (P < .05). There were no significant differences in annexin A5 anticoagulant ratio for the non-aPL thromboembolism group (C) compared with the aPL antibodies without thrombosis history group (B) and the healthy control group (D). Error bars are shown for mean ± 2 SD of normal healthy controls (D).
Correlations
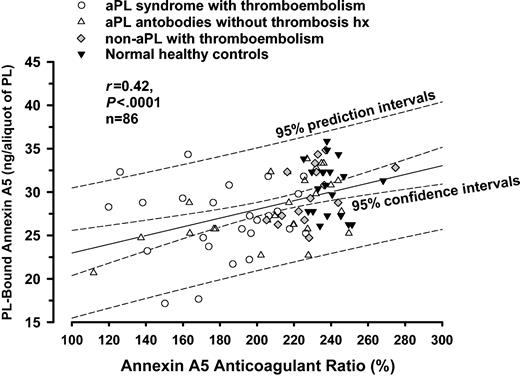
There was a weak but statistically significant correlation between the quantity of phospholipid-bound annexin A5 and the annexin A5 anticoagulant ratio (r = 0.42; 95% confidence intervals, 0.23-0.59; P < .0001; n = 86) (Figure 6).
Correlation analysis for annexin A5 resistance and annexin A5 binding assays. There was a weak but statistically significant correlation between the quantity of PL-bound annexin A5 and the annexin A5 anticoagulant ratio (r = 0.42, P < .0001, n = 86).
Correlation analysis for annexin A5 resistance and annexin A5 binding assays. There was a weak but statistically significant correlation between the quantity of PL-bound annexin A5 and the annexin A5 anticoagulant ratio (r = 0.42, P < .0001, n = 86).
Discussion
There is significant evidence to support the hypothesis that aPL antibodies may promote thrombosis in the aPL syndrome by disruption of the anticoagulant annexin A5 shield. IgG fractions from patients with the aPL syndrome reduce the quantity of phospholipid-bound annexin A5 on the apical surfaces of cultured trophoblasts and endothelial cells, and accelerate coagulation of plasma added to these cells.12 IgG fractions from aPL patients reduce annexin A5 on noncellular phospholipid surfaces17,25 and accelerate plasma coagulation and thrombin generation.17,26 Sera from aPL patients reduce the quantity of annexin A5 on platelets.27 We recently provided morphologic evidence with atomic force microscopy that human monoclonal aPL antibodies disrupt the ordered crystallization of annexin A5 and expose phospholipid for availability for coagulation reactions, and showed that the antibodies accelerate thrombin generation.15
In the present study, using fluorescence-labeled annexin A5, we demonstrated that plasmas that had been spiked with purified IgG fractions from patients with the aPL syndrome reduce annexin A5 binding to phospholipid; the reduction of annexin A5 binding is mediated by aPL IgG as evidenced by the effects of depleting IgG from plasmas with protein G-Sepharose. This reduction occurs in aPL plasmas in a concentration-dependent manner. Measurement of the effects upon the annexin A5 binding for the patient groups showed a statistically significant reduction of annexin A5 using plasmas from patients with a clinical history for the aPL syndrome with thrombosis compared with healthy controls and the non-aPL thromboembolism patients. We also showed that an aPL mAb blunted the anticoagulant effect of annexin A5 on a purified prothrombinase reaction, and that this effect could also be demonstrated in plasma by measuring coagulation times with tissue factor-phospholipid complex that had been exposed to the aPL mAb. It is interesting that this mAb reduced the anticoagulant effect of annexin A5 even in the absence of β2GPI—an effect that had been described for some other aPL mAbs.15 It is also interesting to note that although this aPL mAb was not previously found to have lupus anticoagulant activity in a dilute Russell viper venom time (DRVVT) assay,18 it did prolong tissue factor-mediated coagulation in this system.
In order to assay patient samples for resistance to annexin A5 anticoagulant activity, we designed a 2-stage coagulation assay that uses pooled normal plasma for the coagulation phase of the test. This was done in order to address the variable baseline coagulation times of individual patients. Plasmas spiked with aPL IgGs reduce the anticoagulant activity of annexin A5 on the coagulation time. The reduction of annexin A5 anticoagulation activity is mediated by aPL IgG as evidenced by loss of this effect after plasmas were depleted of IgG by protein G-Sepharose. This effect occurs in a manner that is dependent upon the concentration of aPL plasma. Measurements of the effects of plasmas upon the anticoagulant activity of annexin A5 showed a statistically significant reduction with plasmas from patients with a clinical history for the aPL syndrome with thrombosis. A remarkable aspect of this study, which stands in contrast to the paradoxical “lupus anticoagulant” effect, is the aPL antibody-mediated acceleration of coagulation that occurs in this system containing annexin A5.
We observed that the differences between the aPL syndrome group and the other groups were more significant with the annexin A5 resistance assay than the binding assay. Remarkably, 18 of the 25 aPL syndrome patients had annexin A5 anticoagulant ratios that were lower than the lowest measurements obtained from the healthy controls and the non-aPL thromboembolism group and also below 2 standard deviations of the mean of the healthy controls. As to the difference in annexin A5 results between the symptomatic and asymptomatic groups of aPL patients, we hypothesize that reduced annexin A5 binding and anticoagulant activity reflect increased antibody avidity. However, future studies will be needed to investigate this. Interestingly, 6 of the 26 patients with aPL antibodies, but without a thrombosis history, also had similar low levels of annexin A5 anticoagulant ratios. We speculate that these asymptomatic individuals may be at high risk for thrombosis. Future studies would be needed to address this possibility. We also speculate that the coagulation assay reports the quantity of unshielded phospholipid available for coagulation reactions more precisely than does the annexin A5 binding assay.
These are model systems that, like all model systems, are subject to limitations. It may be argued that the sequence of addition, with plasma containing the antibodies added before the annexin A5, and the absence of calcium in the first plasma incubation favor the competition of the antibodies over the annexin A5. These were necessary on practical grounds since a first incubation with annexin A5 would require calcium concentrations that would already clot the patient plasma used for the first stage and would not enable us to do a second stage with the pooled normal plasma. We previously addressed the question of order of addition in the atomic force imaging study, where we found that either order of addition (annexin A5 first or antibodies cofactors first) resulted in disruption of annexin A5 crystallization, and where the effect occurred in the presence of a physiologic concentration of calcium.15
Analysis of the relationship between annexin A5 binding assays and annexin A5 resistance assays showed a weak but statistically significant positive correlation between these 2 assays. Larger studies are planned to further evaluate the relationships between these types of assays and clinical conditions. It was recently reported that annexin A5 levels are increased in the plasmas of patients with systemic lupus erythematosus and primary antiphospholipid syndrome compared with healthy controls but that this was not correlated with thrombosis28 ; however, conclusions about the displacement of annexin A5 cannot be drawn from those data since neither annexin A5 binding nor anticoagulant activity was measured in those patients.
The data presented here demonstrate that aPL antibody-mediated blunting of annexin A5 binding to phospholipid and of the anticoagulant activity of annexin A5 can be detected in plasmas from aPL patients. These data are consistent with the hypothesis that disruption of the annexin A5 antithrombotic shield is a mechanism for thrombosis in the aPL syndrome. Also, our data indicate that the 2 types of assays—the annexin A5 binding assay and the annexin A5 resistance assay—may identify patients who are at risk for thrombosis with aPL syndrome. Although one group had previously concluded through ellipsometry that aPL antibodies “unambiguously” do not displace annexin A5,29 the current data with patient plasmas extend the atomic force microscopic imaging data with human aPL mAbs15 to support the conclusion that aPL antibodies can indeed interfere with annexin A5 crystallization and with its anticoagulant activity. This concept is also supported by the work of others using phospholipid-coated microtiter plates25 and freeze-thawed platelets.27
Several other mechanisms have been described to explain thrombosis in the aPL syndrome (for recent reviews see Rand2 and Riboldi et al30 ). Among these are mechanisms in which the antibodies have been proposed to interfere with endogenous anticoagulant mechanisms, such as the protein C-protein S system31-33 and tissue factor pathway inhibitor,34 and also mechanisms in which the antibodies promote the expression of proinflammatory and proadhesive phenotypes such as stimulation of tissue factor expression and cell adhesion molecules.30,35
While this study provides data that support a role for aPL antibodies in disrupting annexin A5 binding and anticoagulant activity, it does not establish whether this mechanism occurs in vivo. It remains possible that antibody-mediated interference with annexin A5 binding and anticoagulant activity correlate with thrombosis in the aPL syndrome because they are surrogate markers for high-avidity antibodies that actually have other pathologic effects. At this time, one can only speculate about whether one, or several, of the proposed thrombogenic mechanisms acting in concert are involved in the pathophysiology of the aPL syndrome. In any event, this type of study that translates a basic science observation with monoclonal antibodies, purified proteins, and phospholipids into clinical assays using blood from small groups of well-defined patients is a significant step in further defining the mechanism(s) of this syndrome in humans.
In conclusion, we have demonstrated that plasmas from patients with the aPL syndrome show inhibition of annexin A5 binding and resistance to annexin A5 anticoagulant activity. These results are in accordance with recent atomic force imaging results with human monoclonal aPL antibodies. The inhibition of annexin A5 binding and the resistance to its anticoagulant activity are attributable to the aPL antibodies in plasma and stand in contrast to the paradoxical “lupus anticoagulant” effect that has been extensively described. Plasma tests for reduction of annexin A5 binding and resistance to annexin A5 anticoagulant activity correlate with thrombosis in the aPL syndrome and appear to identify a pathophysiologically relevant mechanism for thrombosis in these patients.
Prepublished online as Blood First Edition Paper, July 8, 2004; DOI 10.1182/blood-2004-01-0203.
Supported by grant HL-61331 from the National Institutes of Health/the National Heart Lung and Blood Institute; grant M01-RR-30 from the National Institutes of Health/the National Center for Research Resources and the General Clinical Research Centers Program; grant K24 AI01603-01 from Mid-Career Investigator Award in Patient-Oriented Research; a research grant from the Arthritis Foundation; and a research award from the Alliance for Lupus Research.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Karen Moore for performing the anticardiolipin assays, and Ms Elizabeth Thames for patient identification and recruitment. This paper is dedicated to the memory of Dr Peter C. Harpel whose support contributed to the development of this work.


![Figure 5. Annexin A5 resistance assays for patient plasma groups. The annexin A5 anticoagulant ratio for the aPL syndrome with thromboembolism group (A) was significantly decreased (mean ± SD, 182 ± 31%, n = 25) compared with the aPL antibodies without thrombosis history group (B) (210 ± 35% [n = 26], P < .01), the non-aPL thromboembolism group (C) (229 ± 16% [n = 15], P < .001), and the healthy control group (D) (231 ± 14% [n = 30], P < .001). The ratio for the plasmas from the aPL antibodies without thrombosis history group (B) also was significantly reduced compared with the healthy control group (D) (P < .05). There were no significant differences in annexin A5 anticoagulant ratio for the non-aPL thromboembolism group (C) compared with the aPL antibodies without thrombosis history group (B) and the healthy control group (D). Error bars are shown for mean ± 2 SD of normal healthy controls (D).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/9/10.1182_blood-2004-01-0203/6/m_zh80210468890005.jpeg?Expires=1767727576&Signature=aegmOyIK7qBbB4PrJjmgHrq4ANdpGZMgWd1H--b~x-27IGd6Yc-Ob1SQQuVickRdOMr-QdCvzRvC0A1kn5kK14EdfyN8QDMFLj-gIDpqSg5yE4tVU49q6IqCdUELRP2EdjAGLptSuZZydL4u~HIinPO~HY7wkNol29TiC9EM~O~GM6ddwtCdY62vPhuZKazfW4~8uIRSY3PXYUbaHYqxiNkM9HX3g-yFiQAtffufVPjaR5Cod0Yn2mubCAebLfszJfxOCF-Sm1b7m65qOngMd8rqpiCoBFVHhRg9ToyKPHjFZk4ZWHiD2oWfgzIBYtq1mcin0kGhYDxAuieshmH5nQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal