Abstract
Pharmacokinetic interactions between chemotherapy and highly active antiretroviral therapy (HAART) are described, but there are few data on their clinical relevance. Patients with systemic AIDS-related non-Hodgkin lymphoma (ARL) were treated with concomitant HAART and infusional cyclophosphamide-doxorubicin-etoposide (CDE) chemotherapy. We compared neutropenia according to whether patients received protease inhibitor (PI)-based HAART or non-PI regimens. Differences in survival, response rates, immunologic parameters, and virologic parameters were also investigated. The day-10 (Mann-Whitney U test; P = .012) and day-14 (P = .025) neutrophil counts were significantly lower in patients receiving PIs, though there were no differences in the number of days of granulocyte colony-stimulating factor (G-CSF) administered between groups (P = .16). Grade 3 or 4 infections requiring hospitalization were recorded for a total of 58 (31%) of 190 cycles of CDE: 23 (48%) of 48 when prescribed PIs and 35 (25%) of 142 with concomitant PI-sparing HAART (χ2 test; P = .0025). There were no statistically significant differences in the response rates, relapse-free survival, or disease-free survival between patients receiving PIs and those not receiving PIs. PI-based HAART appears to significantly potentiate the myelotoxicity of CDE chemotherapy. This potentiation may be a consequence of microsomal enzyme inhibition reducing the metabolism of cytotoxics in this regimen.
Introduction
HIV-1 infection is associated with a greatly increased risk for cerebral and systemic non-Hodgkin lymphoma (NHL), and the incidence of each increases with decreasing CD4 cell counts and progressive immunodeficiency. Effective highly active antiretroviral therapy (HAART) has reduced this incidence, though NHL remains a common cause of morbidity and mortality during the course of infection with HIV-1 and the second most common tumor observed in HIV-1 patients.1-6
Treatment of AIDS-related non-Hodgkin lymphoma (ARL) first involved standard conventional chemotherapy schedules. Recently, infusional treatment has been advocated and adopted by many larger treatment centers.7,8 Infusional chemotherapy for systemic NHL was first reported in the pre-HAART era using cyclophosphamide-doxorubicin-etoposide (CDE) administered as a 96-hour continuous infusion for up to 6 courses, together with granulocyte colony-stimulating factor (G-CSF).9 However, additional studies have suggested interactions between HAART and chemotherapy. When CDE was combined with the protease inhibitor (PI) saquinavir, mucositis was observed in 67% of patients.10 When it was given with the nucleoside analog didanosine, mucositis was observed in 12% of patients.11
A multicenter phase-2 trial, conducted by the Eastern Cooperative Oncology Group, of infusional CDE in 48 patients with ARL not receiving HAART demonstrated less impressive results (complete remission rate [CR], 46%; median survival, 8 months).12 This particular schedule has been used with concomitant HAART, and we have previously demonstrated the effects on lymphocyte subsets and HIV viral load.13 At the National Cancer Institute, etoposide-prednisolone-vincristine-cyclophosphamide-doxorubicin (EPOCH), a dose-adjusted infusional chemotherapy schedule, has been developed, and it, too, omits all HAART for the duration of the chemotherapy.14 Initial reports have been encouraging, with a CR rate of 79%. However, there was a dramatic decrease in the CD4 cell count during chemotherapy; even with the reinstitution of HAART at the end of chemotherapy, baseline levels were not recovered for another 12 months.15 This study has been expanded to 39 selected patients (CR rate, 74%)16 and is under investigation in a multicenter study under the auspices of the AIDS Malignancy Consortium.
Although initial studies demonstrating the efficacy of HAART were performed with PI-based therapies,17 recent data have indicated that non-nucleoside reverse transcriptase inhibitor (NNRTI)-based HAART is at least as effective at controlling immunologic and viral load parameters, has a lower pill burden, and is better tolerated than PI-based HAART.5,18-20 Much of the controversy surrounding the concomitant use of HAART with chemotherapy centers on the potential for drug interactions.21 Both major categories of HAART, NNRTIs and PIs, are extensively metabolized by the cytochrome P450 system; hence, there may be competitive drug interactions when they are administered concomitantly with other drugs metabolized through this pathway.22,23 PIs may modify the metabolism of cytotoxic drugs by inhibiting the CYP3A4 enzyme. Indeed, pharmacokinetic studies have demonstrated a modest delay in the clearance of cyclophosphamide in patients receiving indinavir compared with historical controls, though no increase in hematologic toxicity was observed.24 Another potential interaction occurs because protease inhibitors are substrates and inhibitors of the drug transporter P-glycoprotein, an efflux pump that transports a wide range of structurally unrelated drugs, such as PIs and anthracyclines.25-29 One application of these interactions now used in HIV therapy is pharmacokinetic boosting, whereby metabolic interactions are exploited to reduce peaks and troughs.30
To investigate further the interactions between the 2 major categories of HAART and chemotherapy, we compared PI- and non-PI-containing HAART regimens on the incidence of neutropenia in a cohort of patients with ARL. The finding of significantly increased neutropenia in patients receiving CDE and PIs supports previous findings of interactions between these drugs and underscores the need for caution and regular monitoring when prescribing them together.
Study design
Patients
The Chelsea and Westminster cohort of HIV-positive patients is one of the largest in Europe, and we prospectively collect data on the patients who enter the study. We investigated patients in whom ARL was diagnosed during the HAART era, from 1999 to 2003. During this time, 46 patients with newly diagnosed ARL were treated with concomitant HAART and infusional cyclophosphamide (200 mg/m2 per day on days 1-4), doxorubicin (12.5 mg/m2 per day on days 1-4), and etoposide (60 mg/m2 per day on days 1-4) chemotherapy (CDE at 4 weekly cycles through a Hickman line for 6 cycles). All patients received prophylaxis with 960 mg co-trimoxazole daily, 1250 mg azithromycin once a week, and either 200 mg itraconazole or 50 mg fluconazole daily. Three hundred micrograms G-CSF administered subcutaneously once a day was initiated 24 hours after the completion of the 96-hour infusion of chemotherapy and continued until a neutrophil count of more than 1.5 × 106/L was recorded. This study was performed with appropriate ethical approval (from the Chelsea and Westminster Hospital) and informed consent in accordance with the Declaration of Helsinki.
Concomitant HAART therapy was prescribed in accordance with current published guidelines.31-33 Total leukocyte and neutrophil counts were measured on days 1, 7, 10, and 14 of each cycle, and the number of days of G-CSF was recorded for each cycle. CD4 lymphocyte subset analysis was performed using whole blood stained with murine anti-human monoclonal antibodies (TetraOne; Beckman Coulter, High Wycombe, United Kingdom). Response rates were recorded according to standard criteria,34 as were International Prognostic Index (IPI) scores35 and toxicity to chemotherapy.
Statistical methods
Variables between groups were compared using the χ2 test for nominal variables and the Mann-Whitney U test for non-parametric variables. Survival was calculated from the day of diagnosis until death or the date of last follow-up. Overall survival duration curves were plotted according to the method of Kaplan and Meier.36 The log rank method was used to test for the significance of differences in survival distributions.37
Results and discussion
Patient demographics
Demographic details of the cohort of 46 patients with ARL are shown in Table 1. Most (93%) of these patients were men, and they underwent a total of 190 cycles of CDE, 48 with PI HAART regimens (11 patients) and 142 (35 patients) with PI-sparing HAART regimens. The concomitant HAART treatment was triple nucleoside therapy (n = 3), NNRTI with dual nucleoside (n = 32), PI with dual nucleoside (n = 9), and both NNRTI and PI with nucleosides (n = 2). Twelve patients received zidovudine as part of HAART (1 with a PI regimen and 11 with a PI-sparing regimen χ2 test; P = .14).
Clinicopathologic details of patients
. | PI-sparing HAART (%) . | PI-based HAART. (%) . | Total . | P . |
|---|---|---|---|---|
| No. patients | 35 | 11 | 46 | NA |
| Previous ADI | 1 (3) | 5 (45) | 6 (13) | .003* |
| Median CD4 [range] | 199 [0-466] | 153 [31-636] | 190 [0-636] | .41† |
| Median VL | 19 000 | 78 | 16 855 | .38† |
| Stage greater than 2 | 27 (77) | 7 (64) | 34 (74) | .37* |
| BM involvement | 8 (23) | 3 (27) | 11 (24) | .76* |
| Burkitt lymphoma | 4 (11) | 2 (18) | 6 (13) | .56* |
| Meningeal involvement | 8 (23) | 1 (9) | 9 (20) | .32* |
| Low IPI score | 10 (29) | 3 (27) | 13 (28) | .55* |
| Low-intermediate IPI score | 9 (26) | 3 (27) | 12 (26) | — |
| High-intermediate IPI score | 9 (26) | 3 (27) | 12 (26) | — |
| High IPI score | 7 (20) | 1 (9) | 8 (17) | — |
. | PI-sparing HAART (%) . | PI-based HAART. (%) . | Total . | P . |
|---|---|---|---|---|
| No. patients | 35 | 11 | 46 | NA |
| Previous ADI | 1 (3) | 5 (45) | 6 (13) | .003* |
| Median CD4 [range] | 199 [0-466] | 153 [31-636] | 190 [0-636] | .41† |
| Median VL | 19 000 | 78 | 16 855 | .38† |
| Stage greater than 2 | 27 (77) | 7 (64) | 34 (74) | .37* |
| BM involvement | 8 (23) | 3 (27) | 11 (24) | .76* |
| Burkitt lymphoma | 4 (11) | 2 (18) | 6 (13) | .56* |
| Meningeal involvement | 8 (23) | 1 (9) | 9 (20) | .32* |
| Low IPI score | 10 (29) | 3 (27) | 13 (28) | .55* |
| Low-intermediate IPI score | 9 (26) | 3 (27) | 12 (26) | — |
| High-intermediate IPI score | 9 (26) | 3 (27) | 12 (26) | — |
| High IPI score | 7 (20) | 1 (9) | 8 (17) | — |
NA indicates not applicable; ADI, AIDS-defining infection; BM, bone marrow; VL, viral load; and —, not assessed.
χ2 test
Mann-Whitney U test
Patients receiving a PI (n = 11) were compared with patients on HAART regimens that did not contain PIs (n = 35). There were no statistically significant differences in CD4 cell count (Mann-Whitney U test; P = .41) or in HIV-1 viral load (Mann-Whitney U test; P = .38) at NHL diagnosis between the 2 groups. There was also no significant difference in histologic subtype of NHL (χ2 test; P = .56), NHL stage distribution (χ2 test; P = .37), bone marrow infiltration (χ2 test; P = .76), meningeal disease at presentation (χ2 test; P = .32), or IPI38 group (χ2 test; P = .55).
Lymphoma outcome
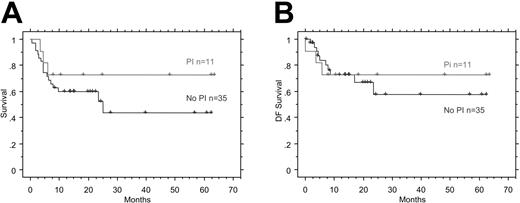
Three patients (1 on PI-based HAART) were not evaluable for response to CDE because they died before completing 2 courses of chemotherapy. The overall response rate for the cohort was 74%: 50% CRs (5 of 11 patients on PIs, 18 of 35 patients on non-PIs) and 24% partial responses (PRs) (4 of 11 patients on PIs, 7 of 35 patients on non-PIs). The median survival for the cohort was 26 months, and the 2-year overall survival rate is 61% (95% confidence interval [CI], 47-76). There was no statistically significant difference in the overall survival rate or the CR rate between patients receiving a PI and those not (CR, 18 of 35 and 5 of 11; PR, 7 of 35 and 4 of 11). There were no significant differences in overall survival (P = .28; Figure 1A) or in disease-free survival (P = .74; Figure 1B) between those receiving either category of HAART. The trend toward an increased overall survival is likely a consequence of the small sample size at the ends of the Kaplan-Meier curves.
Survival after HAART. (A) Kaplan-Meier overall survival duration curves comparing patients treated with and without a PI as part of concomitant HAART therapy. χ2 = 1.176; DF = 1; P = .2781. (B) Kaplan-Meier disease-free survival curves comparing patients treated with or treated without a PI as part of concomitant HAART therapy. χ2 = .108; DF = 1; P = .7426.
Survival after HAART. (A) Kaplan-Meier overall survival duration curves comparing patients treated with and without a PI as part of concomitant HAART therapy. χ2 = 1.176; DF = 1; P = .2781. (B) Kaplan-Meier disease-free survival curves comparing patients treated with or treated without a PI as part of concomitant HAART therapy. χ2 = .108; DF = 1; P = .7426.
Treatment-related toxicity
Cancer Toxicity Criteria (CTC) grade 3 or 4 infections necessitating hospitalization were recorded for 58 (31%) of 190 cycles of CDE; 23 (48%) of 48 patients were prescribed PIs, and 35 (25%) of 142 were prescribed concomitant PI-sparing HAART (χ2 test; P = .0025). CTC grade 4 neutropenia was recorded for 79 (42%) of 190 cycles; 26 (54%) of 48 patients were prescribed PIs, and 53 (38%) of 138 were prescribed concomitant PI-sparing HAART (χ2 test; P = .05).
The median neutrophil counts at days 1, 7, and 10 for all cycles were 3.5 × 106/L (range, 0.3-31 × 106/L), 4.7 × 106/L (range, 0-28 × 106/L), and 0.6 × 106/L (range, 0-24 × 106/L). Values for patients receiving either a PI-based or a PI-sparing HAART regimen are shown in Table 2. Day-10 and -14 neutrophil counts were significantly lower in patients receiving concomitant protease inhibitors. Altogether, 20 (10%) of 190 cycles of chemotherapy were delayed, 7 (16%) of 48 of those when a PI-based schedule was administered and 13 (9%) of 142 when a PI-sparing schedule was used (χ2 test; P = .29). One patient who had stage Ib ethmoidal diffuse large B-cell lymphoma and who was receiving a PI-based regimen (nelfinavir, didanosine, and stavudine) died of neutropenic sepsis after his third cycle of chemotherapy. At the time of his death, he had achieved PR to treatment.
Neutrophil counts and G-CSF use in patients receiving PI-based or PI-sparing HAART
. | PI-sparing HAART median (range) . | PI-based HAART median (range) . | P . |
|---|---|---|---|
| D1 neutrophil count, ×106/L | 3.4 (0.3-31) | 3.5 (0.8-15) | .35 |
| D7 neutrophil count, ×106/L | 4.0 (0-22) | 5.0 (0-28) | .14 |
| D10 Neutrophil count, ×106/L | 0.7 (0-24) | 0.2 (0-19) | .012 |
| D14 Neutrophil count, ×106/L | 3.9 (0-49) | 2.2 (0-24) | .025 |
| No. d on G-CSF | 7 (5-20) | 7 (5-10) | .16 |
. | PI-sparing HAART median (range) . | PI-based HAART median (range) . | P . |
|---|---|---|---|
| D1 neutrophil count, ×106/L | 3.4 (0.3-31) | 3.5 (0.8-15) | .35 |
| D7 neutrophil count, ×106/L | 4.0 (0-22) | 5.0 (0-28) | .14 |
| D10 Neutrophil count, ×106/L | 0.7 (0-24) | 0.2 (0-19) | .012 |
| D14 Neutrophil count, ×106/L | 3.9 (0-49) | 2.2 (0-24) | .025 |
| No. d on G-CSF | 7 (5-20) | 7 (5-10) | .16 |
P values were determined using the Mann-Whitney U test.
In those for whom data are available, PI- and NNRTI-based HAART has reduced morbidity and mortality associated with HIV, mainly by reducing the incidence of opportunistic infections.17 Its protective effects have also been shown to result in a decreased incidence of AIDS-defining malignancies, such as NHL39 and Kaposi sarcoma,20 with PI- and NNRTI-based HAART demonstrating equivalent efficacies. Here, we observed that protease inhibitors significantly enhanced neutropenia induced by infusional CDE chemotherapy in patients with ARL, evidenced by statistically significant decreases in day-10 and day-14 neutrophil counts in patients receiving PI-containing HAART compared with those who received HAART that did not contain PIs. Importantly, grade 3 or 4 infections necessitating hospitalization occurred in significantly more patients receiving PIs than in those not receiving PIs (48% vs 25%; χ2 test; P = .0025). Other between-group comparisons, however, were not statistically significant.
Previously, the concomitant use of antiretroviral agents with chemotherapy had been considered standard clinical practice, with the possible exception of zidovudine, which significantly added to the myelosuppression of combination chemotherapy, and didanosine, which may worsen the peripheral neuropathy caused by taxones and vinca alkaloids.40,41 There are few data regarding the pharmacokinetic interactions of PIs and NNRTIs with chemotherapy, though the inhibition of cytochrome p450/CYP3A enzyme system by certain PIs22,42,43 x may reduce hepatic metabolism of cyclophosphamide and anthracycline. Such inhibition and subsequent increased levels of cytotoxics may explain the significantly increased neutropenia observed here with PIs.
Because modification of the p-glycoprotein efflux pump is an important underlying mechanism of drug interaction,44,45 another possibility is that the well-described inhibition of this transporter by PIs46,47 leads to increased intracellular levels of cytotoxics. Interestingly, PI-containing HAART, non-PI (NNRTI-containing)-based HAART, and cytotoxics such as doxorubicin can increase the levels of expression of p-glycoprotein48-50 though the functional consequences of increased expression are unclear.
A study of concomitant HAART and chemotherapy in patients with ARL has demonstrated that the CD4 cell count declines by 50% during chemotherapy but recovers rapidly within 1 month of the completion of chemotherapy.51 These findings support the use of concomitant HAART during chemotherapy to maintain immune function and during chemotherapy when patients are at high risk for infection. Recent work has highlighted the fact that PIs may have clinically important antitumor effects. These experiments, conducted more than 3 decades after Folkman's, showed that tumor growth was dependent on the formation of new blood vessels52 and that systemic administration of the PIs saquinavir and indinavir to nude mice induced regression of proliferative lesions promoted by angiogenic cytokines,53 with a potency similar to that of paclitaxel. Ritonavir has also been observed to have specific antitumor effects in vitro and in mouse xenotransplantation models.54 However, data from large cohort studies has found that NNRTI-based HAART is as effective as PI-based regimens at preventing NHL and Kaposi sarcoma during the course of HIV-1 infection.5,20,55
The potentiation of myelosuppression by PIs that we observed is likely caused by a pharmacokinetic interaction with the p450 microsomal enzyme system or inhibition of the P-glycoprotein efflux pump. However, there is no observed increase in response rate with PI-based HAART, suggesting that there is no significant dose-response gradient at this threshold even if there is a statistically significant dose-toxicity relationship. Patients with ARL have increased susceptibility to the consequences of immunosuppression, and our data suggest that physicians prescribing infusional chemotherapy to patients with ARL may want to consider alternative antiretroviral regimens to PIs. Recent data indicating the potency and durability of NNRTI-based HAART should provide reassurance to clinicians in this setting.
Prepublished online as Blood First Edition Paper, July 6, 2004; DOI 10.1182/blood-2004-05-1747.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal