Abstract
Interaction of the activating ligand H60 with NKG2D receptor constitutes a major stimulatory pathway for natural killer (NK) cells. The influence of inhibitory Ly49 receptors on NKG2D-mediated activation is not clearly understood. Here we show that the magnitude of NKG2D-mediated cytotoxicity is directly proportional to both the levels of H60 and the nature of major histocompatibility complex (MHC) class I molecules expressed on the target cells. The expression levels of H60 on the target cells determined the extent to which the inhibition by Ly49C/I receptors can be overridden. In contrast, even a higher expression of H60 molecule on the target cells failed to overcome the inhibition mediated by Ly49A/G receptors. Also, the level of interferon-γ (IFN-γ) and granulocyte-macrophage colony-stimulating factor (GM-CSF) generated by NK cells through anti-NKG2D monoclonal antibody (mAb)-mediated activation is significantly reduced by the presence of immobilized anti-Ly49A/G mAbs. Thus, NKG2D-mediated cytotoxicity and cytokine secretion results from the fine balance between activating and inhibitory receptors, thereby defining the NK cell-mediated immune responses. (Blood. 2005;105:233-240)
Introduction
Natural killer (NK) cells are important effector cells in many innate immune processes.1 The “missing-self” hypothesis proposes that NK cells lyse target cells that have lost expression of major histocompatibility complex (MHC) class I molecules (ie, that are “missing self”).2 Thus, in an unchallenged steady state, murine NK cells discriminate “self” cells from those that are “missing self” through an array of Ly49 receptors, which inhibit lysis upon recognition of classical MHC class I molecules on target cells.3-5 Loss of MHC class I molecules on target cells relieves the NK cell of Ly49-mediated inhibition, thus allowing the NK cells to mediate cytotoxicity.6 Under challenged conditions, however, target cells express a family of stress-inducible ligands, including UL16-binding protein (ULBP)7,8 and MHC class I chain-related genes (MICA/B) in humans9 and H60,10,11 Rae-1,12 and murine UL-16-binding protein-like transcript 1 (MULT-1)13,14 in mice, which are recognized by the activating receptor, NKG2D. NKG2D is expressed by most NK cells, and it associates with immunoreceptor tyrosine-based activation motif (ITAM)-containing DNAX protein 12 (DAP12)/killer cell activating receptor-associated protein (KARAP) or a non-ITAM-bearing DAP10 signal transducing subunits.15-17 Earlier studies indicated that ectopic expression of H60 or Rae-1 molecules on autologous tumor cells rendered them susceptible to NKG2D-mediated NK cell cytotoxicity despite the normal expression of self-MHC class I molecules.10,18 Although these findings demonstrated a nonrequirement for the down-regulation of MHC class I to mediate cytotoxicity by NK cells, the interplay between NKG2D-mediated activation and Ly49 receptor-mediated inhibition has not been clearly elucidated.
Here we show that the magnitude of activation through NKG2D is a function of altering the balance in the signaling strength between activating NKG2D and inhibitory Ly49 receptors. To determine if NK cell response is a function of an “altered balance” of activating and inhibiting ligands, we first varied the quantity of activating ligand, H60, on the target cells and quantified the magnitude of NK cell-mediated cytotoxicity. Secondly, we tested specific inhibitory Ly49 molecules for their ability to modulate the activation through NKG2D by blocking Ly49C/I interaction to cognate MHC class I molecules. Third, we examined the effect of an additional MHC class I molecule, H2-Dd, a ligand for inhibitory Ly49A/G receptors, on the NKG2D-mediated cytotoxicity. Fourth, using immobilized monoclonal antibodies (mAbs) to NKG2D and Ly49A or Ly49G receptors, we demonstrate that simultaneous engagement of inhibitory Ly49 receptor during NKG2D cross-linking resulted in a significant down-modulation of interferon-γ (IFN-γ) and granulocyte-macrophage colony-stimulating factor (GM-CSF) generation. Finally, to understand the mechanism by which the activating and inhibiting pathways interrelate during NK cell activation, we analyzed the nuclear translocation of nuclear factor-κB (NF-κB). Based on these results we propose an integrative hypothesis in which a balance in the signaling strength between inhibitory Ly49 and activating NKG2D receptors governs the effector functions of NK cells.
Materials and methods
Mice and cell lines
Inbred mouse strains C57BL/6, BALB/c, and B10.D2 were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred at the animal care facility at the Medical College of Wisconsin, Milwaukee. EL4 and RMA/S were a kind gift from Dr N. Shastri, University of California, Berkeley; C3 and YAC-1 cells were obtained from American Type Culture Collection ([ATCC] Rockville, MD). All cell cultures were grown at 37°C, 5% CO2, in RPMI 1640 medium (Gibco/Invitrogen, San Diego, CA) supplemented with l-glutamine, sodium pyruvate, 2-mercaptoethanol, antibiotics (penicillin/streptomycin), and 10% fetal calf serum (FCS).
NK-cell preparation and cytotoxicity assays
NK cells from indicated mouse strains were prepared from splenocytes using established methods.19 Briefly, single-cell suspensions from spleen were passed through nylon wool columns (Polysciences, Warrington, PA) for the depletion of adherent populations consisting of B cells and macrophages. Cells eluted from these columns were cultured with 1000 units of interleukin-2 (IL-2) (a kind gift from the National Cancer Institute Biological Resources Branch [NCI-BRB] Preclinical Repository, Frederick, MD). Nonadherent cells were removed on day 4, and the remaining adherent population was cultured with fresh medium and IL-2 for another 2 to 3 days before it was used. Purity of the NK cultures was checked, and preparations with more than 95% of NK1.1+ were used for the experiments. NK-mediated cytotoxicity was quantified using 51chromium (51Cr)-labeled target cells using established protocols.20 Briefly, effector cells were added to target cells in U-bottom plates at varied effector-target cell ratios. Percent specific lysis was calculated from the amount of 51Cr released into the culture supernatant. Antibody inhibition assays were done as follows: IL-2-activated NK cells were harvested on day 7 and incubated with anti-Ly49C/I (SW5E6) or anti-Ly49I (YLI-90) antibodies at 37°C for 45 minutes followed by the addition of 51Cr-labeled target cells. Effect of antibody blocking was analyzed in conventional 51Cr-release cytotoxicity assays. Antibody inhibition assays were performed either by varying the concentrations of indicated antibodies at a 20:1 effector-target (E/T) ratio or using a different E/T ratio while maintaining the antibodies at 2 μg per well.
Flow cytometry and cell sorting
The following hybridomas were obtained from ATCC: anti-Kb, Y3, anti-Db, B22.249.R5, anti-NK1.1 (PK136), anti-Dd, and HB47. Anti-Ly49A (A1), anti-Ly49C/I (SW5E6), and fluorescein isothiocyanate (FITC)-conjugated anti-Ly49G (4D11) were obtained from BD PharMingen, (San Diego, CA). Anti-Ly49I (YLI-90) was obtained from eBioscience, San Diego, CA. B6-derived, IL-2-activated NK cells are stained with appropriate mAbs and subjected to cell sorting using FACSAria (Becton Dickinson, Mountain View, CA). FITC-conjugated rat anti-mouse immunoglobulin (eBioscience) and phycoerythrin (PE)-conjugated goat anti-human Fc-specific immunoglobulin G (IgG) (Jackson ImmunoResearch Labs, West Grove, PA) were used as secondary antibodies. Stable cell clones and NK cells were stained in 1% FCS-phosphate-buffered saline (PBS) containing appropriate mAbs. To avoid nonspecific binding through Fc receptors, NK cells were pretreated with Fc-Block (2.4G2, anti-CD16/CD32) mAb (BD PharMingen) before adding specific mAbs. Standard flow cytometry analysis was carried out in FACScan using CELLQuestPro software (Becton Dickinson).
DNA constructs and fusion proteins
Polymerase chain reaction (PCR) products encoding full-length H60 cDNA were generated using forward 5′-ACCATGGCAAAGGGAGCCACCAGC-3′ and reverse 5′-TTATAGCTGGGAATGAGGACTGCA-3′ primers and cloned into pEAK eukaryotic expression vector (Edge Biosystems, Gaithersburg, MD) between HindIII and NotI restriction sites. The NKG2D-Ig construct was generated ligating PCR products encoding the Fc portion of the human IgG (kind gift from Dr P. Newman, Blood Research Institute [BRI]) and the ectodomain of NKG2D into pSecTag2B vector (Invitrogen, San Diego, CA). B6-derived mRNA was reverse transcribed, and NKG2D encoding cDNA was amplified using forward 5′-CCGGAATTCTTTCAGCCAGTATTGTGCAAC-3′ and reverse 5′-CGGCTCGAGTTACACCGCCCTTTTCATGCA-3′ primers and cloned between EcoRI and XhoI restriction sites. IgG-Fc encoding sequence was amplified using forward 5′-CCCAAGCTTCTAGAGGTAGAGCCCAAATCTTGT-3′ and reverse 5′-CCGGAATTCTTTACCCGGAGACAGGGAGAG-3′ primers and cloned upstream of the NKG2D sequence between HindIII and EcoRI restriction sites. Full-length H2-Dd cDNA was amplified using forward 5′-CCCAAGCTTATGGGGGCGATGGCTCCCCGC-3′ and reverse 5′-CGCGGATCCTCACACTTTACAATCTGGGAG-3′ primers from a cDNA library generated from BALB/c-derived mRNA (Edge Biosystems) and cloned into pCDNA3 expression vector (Invitrogen) between HindIII and BamHI restriction sites.
Generation of stable cell lines
EL4 cells (10 × 106) were electroporated with 20 to 50 μg linearized, gel-purified plasmid DNA using BioRad Gene Pulser II (BioRad, Richmond, CA) at 300 V. Stable clones were selected and cultured in 1 mg/mL G418 (Cellgro, Herndon, VA). H60-positive clones were selected based on their ability to bind to soluble NKG2D-Ig fusion protein or their ability to process and present an antigenic epitope, LTFNYRNL, on H2-Kb MHC class I and to stimulate BCZ103 T-cell hybridoma.21 H2-Dd-positive subclones of ST63 were characterized using anti-Dd antibody through flow cytometry.
Secretion and quantification of cytokines through ELISA
C57BL/6-derived, IL-2-cultured, Fc-blocked NK cells were activated with 5 μg/mL platebound anti-NKG2D mAb, A10 (eBioscience), in the presence or absence of titrating concentrations of anti-Ly49A (A1, BD PharMingen), anti-Ly49G2 (LGL-1; BD PharMingen), or both. Total concentrations of IFN-γ and GM-CSF were quantified in the supernatants of NK cells using enzyme-linked immunosorbent assay (ELISA) kits from eBioscience, following the manufacturer's instructions. Standard curves generated using recombinant murine IFN-γ and GM-CSF were used to calculate the concentrations of respective cytokines. IgG1 (BD PharMingen) or IgG2a (eBioscience) isotype controls were used in place of anti-Ly49 mAbs as experimental controls.
Gel mobility shift assays for NF-κB
NK cells (1 × 106) were resuspended in 1 mL of 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) pH 7.9, 10 mM KCl, 0.1 mM EDTA (ethylenediaminetetraacetic acid), 0.1 mM EGTA (ethylene glycol tetraacetic acid), and 1 mM dithiothreitol (DTT) along with complete protease inhibitors (Roche Diagnostics, Basel, Switzerland) and incubated on ice for 15 minutes. Nonidet P-40 (NP-40) was added to this mixture at a final concentration of 0.5%, vortexed, and the mixture centrifuged for 30 seconds. The nuclear pellets were resuspended in 10 μL of 20 mM HEPES pH 7.9, 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, and 1 mM DTT along with complete protease inhibitors (Roche) and kept on ice for 15 minutes. Nuclear extracts were collected by centrifugation and were incubated with 2 μg poly(dIdC) for 15 minutes followed by another 15 minutes of incubation with 1 ng T4 kinase-labeled DNA probe. The synthetic NF-κB probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was an exact copy of the NF-κB binding site in κ light chain gene enhancer. The NF-κB5 × binding buffer consists of 50 mM Tris (tris(hydroxymethyl)aminomethane)-HCl pH 7.5, 500 mM NaCl, 0.5 mM EDTA, 25% glycerol, 0.5% NP-40, and 5 mM DTT. Samples were run on a 4.5% polyacrylamide gel with 2.5% glycerol in 0.5 × Tris-borate-EDTA (TBE). Gels were dried and visualized by autoradiography. For supershifts, mAb against p50 (sc-114x; Santa Cruz Biotechnology, CA) was preincubated with cell extracts on ice for 30 minutes before addition of DNA probe.
Results
Expression levels of the H60 molecule on target cells form the critical basis in determining the magnitude of NKG2D receptor-mediated NK cell cytotoxicity
To investigate the effect of the quantity of activating ligand, we stably transfected EL4 cells with H60-encoding cDNA21,22 and selected 2 clones, ST61 and ST63, with differing levels of H60 expression. Both clones bound substantially to sNKG2D-Ig (Figure 1A) with clone ST61 exhibiting approximately 11-fold less binding than clone ST63. Thus, the selected clones represented either the “optimal” level of H60 expression (ST61), as in the case of normal splenocytes induced with lipopolysaccharide (LPS), or an “overexpression” of H60 (ST63) that was greater than that of several tumor cells (YAC-1, C3) (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Expression level of H60 is directly proportional to the level of NKG2D-mediated activation. (A) Expression levels of H60 on target cells were quantified by flow cytometry following staining with sNKG2D-Ig fusion protein. Soluble immunoglobulin Fc (Ig) or the secondary antibody alone (2°) was used to determine background staining levels. (B) Cytotoxicity of B6- and B10.D2-derived NK cells against EL4, ST61, and ST63. Increasing numbers of NK cells were titrated against a constant number of 51Cr-labeled target cells (5000 per well), and percent cytotoxicity was determined in a conventional 4-hour cytotoxicity assay. Data presented as the mean percent cytotoxicity ± standard deviation (SD) were determined from triplicate wells and are representative of 6 independent experiments. (C) Expression levels of MHC class I molecules in EL4, ST61, and ST63 were tested using anti-H2-Kb (α-Kb) and anti-H2-Db (α-Db) mAbs.
Expression level of H60 is directly proportional to the level of NKG2D-mediated activation. (A) Expression levels of H60 on target cells were quantified by flow cytometry following staining with sNKG2D-Ig fusion protein. Soluble immunoglobulin Fc (Ig) or the secondary antibody alone (2°) was used to determine background staining levels. (B) Cytotoxicity of B6- and B10.D2-derived NK cells against EL4, ST61, and ST63. Increasing numbers of NK cells were titrated against a constant number of 51Cr-labeled target cells (5000 per well), and percent cytotoxicity was determined in a conventional 4-hour cytotoxicity assay. Data presented as the mean percent cytotoxicity ± standard deviation (SD) were determined from triplicate wells and are representative of 6 independent experiments. (C) Expression levels of MHC class I molecules in EL4, ST61, and ST63 were tested using anti-H2-Kb (α-Kb) and anti-H2-Db (α-Db) mAbs.
To determine the effect of different levels of H60 expression on NK cell activation, nontransfected EL4 cells and ST61 and ST63 transfectants were used as targets in 51Cr-release assays. NK cells derived from C57BL/6 (B6) mice mediated cytotoxicity against both ST61 and ST63 targets but not against parental EL4 cells; however, ST63 targets, which expressed higher levels of H60, were lysed to a greater extent compared with ST61 (Figure 1B). We also measured the lysis of EL4, ST61, and ST63 (all H-2b) by alloreactive NK cells derived from B10.D2 (H-2d) mice. B10.D2-derived NK cells could be subjected to the “missing-self” rule because EL4, ST61, and ST63 do not express the self-MHC class I of B10.D2 (H-2d). As expected, NK cells from B10.D2 followed a similar pattern to that of B6, except the cytotoxicity of B10.D2-derived NK cells was significantly higher for all targets (Figure 1B). EL4, ST61, and ST63 cells expressed equivalent levels of the MHC class I molecules, H2-Kb and H2-Db (Figure 1C), and activated peptide-specific T-cell hybridomas to the same extent. This suggests that the higher level of H60 expression and not preferential loss of potentially inhibitory MHC class I molecules was responsible for more efficient lysis of ST63 versus ST61 targets by NK cells (Figure S2). We conclude from these observations that the level of expression of the NKG2D ligand, H60, influences the magnitude of target cell lysis by NK cells.
NK cell-mediated cytotoxicity is a function of altered balance between activation through NKG2D receptor and inhibition via Ly49C/I receptors
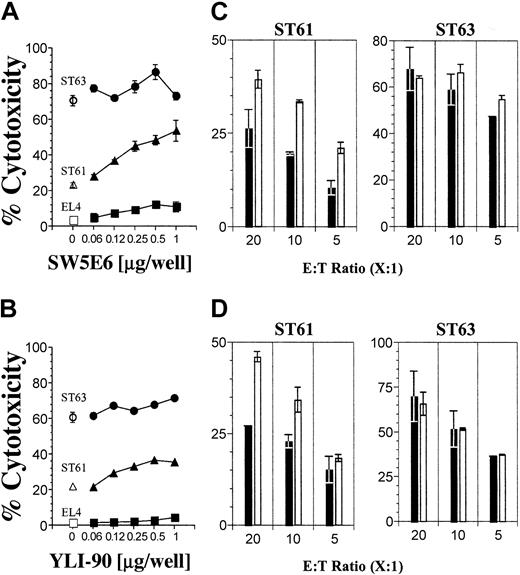
NK cells discriminate “self” from “nonself” using different Ly49 receptors that interact with specific MHC class I molecules. To determine whether these inhibitory receptors play a role in NKG2D-mediated activation, we first tested the role of Ly49C/I receptors that interact with H2-Kb. To block this interaction we used specific mAbs that reacted to both Ly49C and Ly49I (SW5E6) or only Ly49I (YLI-90) receptors.4 If the Ly49C+/I+ B6 NK cells were being inhibited by H2-Kb molecules on the EL4-derived targets, we should observe release of inhibition and, thus, increased cytotoxicity following treatment with SW5E6 or YLI-90 mAbs. This is indeed the case for ST61 (H60low), because the level of cytotoxicity increased with increasing concentrations of SW5E6 (Figure 2A) and, to a lesser extent, YLI-90 mAbs (Figure 2B), whereas the lysis of EL4 (H60neg) and ST63 (H60hi) targets was unchanged in the presence of either mAb. The high levels of ST63 killing were relatively unaltered by the SW5E6 or YLI-90 mAb treatment and may represent maximal activation through NKG2D at this E/T ratio. These observations were further confirmed by varying the E/T ratio and maintaining the mAb concentrations at 2 μg per well (Figure 2C-D). In neither case did blocking the Ly49C/I receptors achieve cytotoxicity levels against ST61 similar to those of ST63. Thus, while blocking inhibitory ligands increased the cytotoxicity, the expression level of the activating ligand was still important for the overall magnitude of NKG2D activation. To further test the influence of Ly49C/I receptors on NKG2D-mediated activation, we sorted Ly49C+/I+ and Ly49C-/I- NK subsets from B6 mice using SW5E6 mAb. Ly49C-/I- NK cells lysed both ST61 (H60low) and ST63 (H60hi) targets more efficiently than did Ly49C+/I+ NK cells (Figure 3).
Differential expression levels of H60 influence the Ly49C/I receptor-mediated inhibition. (A) The effect of inhibitory Ly49 receptors on cytotoxicity was tested using Ly49C/I-specific SW5E6 or (B) Ly49I-specific YLI-90 mAbs at titrated concentrations (ST63 •, ST61 ▴, and EL4 ▪; respective open symbols denote no antibody) and (C-D) with different E/T ratios using constant amounts of mAbs (2 μg per well) (histograms). Note different Y-axis scales for ST61 and ST63 data. Results presented as the mean percent cytotoxicity ± SD and are representative of 3 independent experiments.
Differential expression levels of H60 influence the Ly49C/I receptor-mediated inhibition. (A) The effect of inhibitory Ly49 receptors on cytotoxicity was tested using Ly49C/I-specific SW5E6 or (B) Ly49I-specific YLI-90 mAbs at titrated concentrations (ST63 •, ST61 ▴, and EL4 ▪; respective open symbols denote no antibody) and (C-D) with different E/T ratios using constant amounts of mAbs (2 μg per well) (histograms). Note different Y-axis scales for ST61 and ST63 data. Results presented as the mean percent cytotoxicity ± SD and are representative of 3 independent experiments.
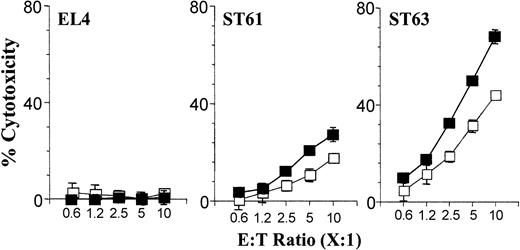
Expression levels of H60 differentially activate purified Ly49C+/I+ compared with Ly49C-/I- NK subsets. The effect of Ly49C/I receptors on NK cell-mediated cytotoxicity was tested as described in the legend to Figure 1B using sorted subsets of Ly49C+/I+ and Ly49C-/I- NK cells and are representative of 3 independent experiments. Data presented are the mean values of percent cytotoxicity against indicated target cells with Ly49C+/I+ (□) or Ly49C-/I- (▪).
Expression levels of H60 differentially activate purified Ly49C+/I+ compared with Ly49C-/I- NK subsets. The effect of Ly49C/I receptors on NK cell-mediated cytotoxicity was tested as described in the legend to Figure 1B using sorted subsets of Ly49C+/I+ and Ly49C-/I- NK cells and are representative of 3 independent experiments. Data presented are the mean values of percent cytotoxicity against indicated target cells with Ly49C+/I+ (□) or Ly49C-/I- (▪).
These results demonstrate that NKG2D-mediated lysis of H60-expressing targets can be inhibited by Ly49C and/or Ly49I inhibitory receptors. Specifically, cytotoxicity against ST61 (H60low) was strongly influenced by inhibitory Ly49C/I receptors. However, our observation that the ST63 (H60hi) targets were lysed to a greater extent than were ST61 (H60low) targets, even in the presence of Ly49C/I-blocking mAbs, demonstrates the critical role of the level of expression of H60 in determining the extent of NKG2D-mediated target cell lysis (Figure 2A). This suggests that expression of H60 at a sufficiently high level may be able to overcome Ly49C/I-mediated inhibition. These conclusions were further supported by our observation that even ST63 (H60hi) targets were more efficiently lysed by Ly49C-/I- than by Ly49C+/I+ NK subsets (Figure 3). Collectively, these results led us to hypothesize that the ability of an NK cell to lyse an H60-positive target may depend on the altered balance between activating and inhibiting signals.
Stronger interactions between Ly49A/G receptors and their ligand MHC class I, H2-Dd, significantly abrogate cytotoxicity via the NKG2D receptor
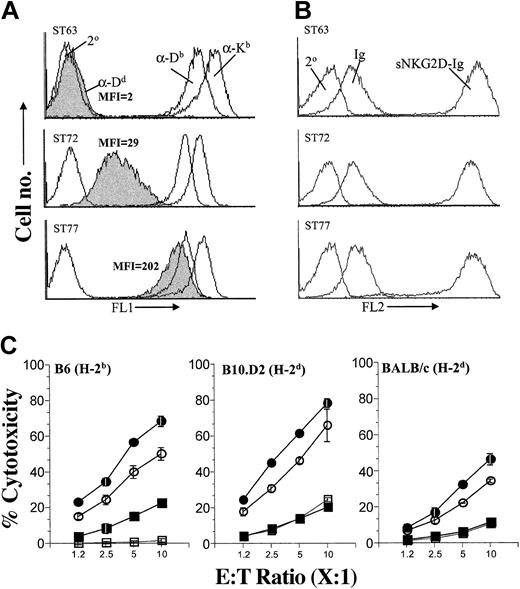
To test the hypothesis that the extent of H60-directed target cell lysis is influenced by the strength of the inhibitory signal, we expressed varying levels of ligands for Ly49 inhibitory receptors on H60-expressing targets. This was accomplished by generating variants of ST63 (H60hi) that expressed different levels of H2-Dd, which strongly interacts with Ly49A/G inhibitory receptors.23-25 As shown in Figure 4A, H2-Dd was absent on the parental ST63 line (H2-Dd-Neg) and was expressed at low levels on the ST72 variant (H2-Dd-Low) and at high levels on the ST77 variant (H2-Dd-High), whereas all 3 lines expressed H-2b-encoded class I molecules (Figure 4A) and H60 (Figure 4B) at equivalent levels. H2-Dd expression levels in ST77 (H2-Dd-High) were comparable to both H2-Kb and H2-Db molecules that are naturally expressed on EL4 cells (Figure 4A). Inhibitory Ly49A/G receptors are expressed on a major subset of NK cells from B6, B10.D2 (Figure S3), and BALB/c mice; therefore, as expected, the efficiency with which NK cells derived from either B6 (Figure 4C, left panel), B10.D2 (Figure 4C, middle panel), or BALB/c (Figure 4C, right panel) mice lysed H60-expressing targets was inversely correlated with the level of H2-Dd expression on the target cells. Thus, not only can blockade of the inhibitory signaling increase cytotoxicity (Figures 2 and 3) but the inverse, providing additional inhibitory signals, can decrease the cytotoxicity (Figure 4C).
Coexpression of H2-Dd on H60-positive target cells significantly inhibits NK cell cytotoxicity. (A) ST63 (H60hi) cells were transfected with H2-Dd-encoding plasmids to obtain sublines expressing low (ST72) and high (ST77) levels of H2-Dd. Expression levels of MHC class I were quantified by flow cytometric analysis following staining with anti-Dd (α-Dd), anti-Kb (α-Kb), and anti-Db (α-Db) mAbs. Secondary antibody alone (2°) was used to determine background staining levels. (B) Comparable levels of H60 on ST63, ST72, and ST77 were verified by flow cytometry following staining with sNKG2D-Ig. Soluble immunoglobulin Fc (Ig) or the secondary antibody alone (2°) was used to determine background staining levels. (C) Effect of H2-Dd expression on NKG2D-mediated cytotoxicity of NK cells derived from B6 (left), B10.D2 (middle), or BALB/c (right) mice was tested using targets that expressed high levels of H60 and were either negative for H2-Dd (ST63 •) or expressed H2-Dd at low (ST72 ○) or high (ST77 ▪) levels. H60-negative targets (EL4 □) were used as controls. Cytotoxicity assays were performed and data presented as described in the legend to Figure 1B and are representative of 5 independent experiments.
Coexpression of H2-Dd on H60-positive target cells significantly inhibits NK cell cytotoxicity. (A) ST63 (H60hi) cells were transfected with H2-Dd-encoding plasmids to obtain sublines expressing low (ST72) and high (ST77) levels of H2-Dd. Expression levels of MHC class I were quantified by flow cytometric analysis following staining with anti-Dd (α-Dd), anti-Kb (α-Kb), and anti-Db (α-Db) mAbs. Secondary antibody alone (2°) was used to determine background staining levels. (B) Comparable levels of H60 on ST63, ST72, and ST77 were verified by flow cytometry following staining with sNKG2D-Ig. Soluble immunoglobulin Fc (Ig) or the secondary antibody alone (2°) was used to determine background staining levels. (C) Effect of H2-Dd expression on NKG2D-mediated cytotoxicity of NK cells derived from B6 (left), B10.D2 (middle), or BALB/c (right) mice was tested using targets that expressed high levels of H60 and were either negative for H2-Dd (ST63 •) or expressed H2-Dd at low (ST72 ○) or high (ST77 ▪) levels. H60-negative targets (EL4 □) were used as controls. Cytotoxicity assays were performed and data presented as described in the legend to Figure 1B and are representative of 5 independent experiments.
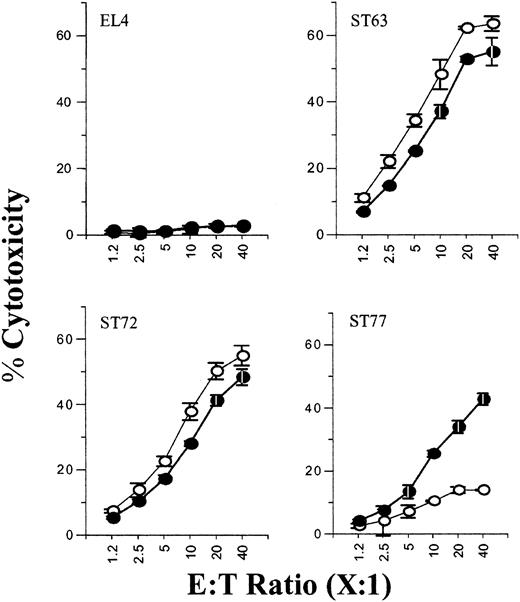
Because the B6-derived NK cells contained both Ly49A+/G+ and Ly49A-/G- subsets, we further hypothesized that only the cytotoxicity of the Ly49A+/G+ subsets that are able to interact with H2-Dd will be inhibited. To test this, we sorted the Ly49A+/G+ and Ly49A-/G- NK subsets. Figure 5 illustrates EL4 was not lysed while ST63 was equally lysed by both Ly49A+/G+ and Ly49A-/G- NK subsets. No major differences were seen in the lysis of ST72 (H2-Dd-Low) by both Ly49A/G-positive and -negative NK subsets (Figure 5). In contrast, there were significant differences in the cytotoxicity toward ST77 (H2-Dd-High) (Figure 5, bottom right). Ly49A+/G+ NK subsets were unable to mediate a strong cytotoxic response against ST77 (13.9%) as compared with ST63 (63.5%). Furthermore, the cytotoxicity by Ly49A-/G- subset to ST77 was significantly higher (42.8%). Interestingly, increasing the E/T ratio to 20:1 or 40:1 did not allow the Ly49A+/G+ subset to overcome the inhibition and mediate cytotoxicity against the H2-Dd-positive ST77 line (Figure 5).
Purified Ly49A+/G+ NK subsets are significantly inhibited by the expression of H2-Dd on the target cells. The effect of inhibitory Ly49A/G receptors on NK cell-mediated cytotoxicity of ST63, ST72, and ST77 targets was tested using sorted subsets of Ly49A+/G+ (○) and Ly49A-/G- (•) NK cells. Data are presented as described in the legend to Figure 1B and are representative of 3 independent experiments.
Purified Ly49A+/G+ NK subsets are significantly inhibited by the expression of H2-Dd on the target cells. The effect of inhibitory Ly49A/G receptors on NK cell-mediated cytotoxicity of ST63, ST72, and ST77 targets was tested using sorted subsets of Ly49A+/G+ (○) and Ly49A-/G- (•) NK cells. Data are presented as described in the legend to Figure 1B and are representative of 3 independent experiments.
These results demonstrate that target cell lysis under these conditions, which was H60-directed and therefore NKG2D-mediated, can be inhibited by Ly49A/G inhibitory receptors These studies extend the earlier observation4,26 that allogeneic targets must be negative for H2-Dd to be lysed by Ly49A+/G+ NK cells by showing that the interaction between H2-Dd and Ly49A/G can also inhibit NKG2D-mediated target cell lysis. Furthermore, these results establish that expression of a stronger inhibitory ligand such as H2-Dd can protect even targets that overexpress activating ligands. Our observations provide support for the hypothesis that the ability of an NK cell to lyse an H60-positive target depends on the relative strength of the activating, relative to the inhibitory, signal.
Cross-linking of Ly49A/G receptors with mAbs can down-regulate anti-NKG2D antibody-mediated cytokine production by NK cells
NK cell activation is a complex phenomenon in view of the fact that these effector cells are able to express multiple activating and inhibitory receptors simultaneously. To test whether signals transduced through selective Ly49 inhibitory receptors alone were capable of modulating NKG2D-mediated activation, we used anti-NKG2D mAb cross-linking in combination with immobilized anti-Ly49A or anti-Ly49G2 mAbs. Use of these specific mAbs allowed us to generate “pure signals,” thereby recreating the interplay between Ly49 and NKG2D receptors. Following earlier methodologies used for murine NK1.127 or human 2B428 activating receptors, we cultured the NK cells with immobilized activating anti-NKG2D and inhibiting Ly49 mAbs. Anti-Ly49A or anti-Ly49G2 or both together were titrated against a constant amount of anti-NKG2D mAb (A10; 5 μg/mL). Culture supernatants were collected from the activated NK cells and used to quantify the levels of both IFN-γ and GM-CSF. Results presented in Figure 6 demonstrate that significant quantities of IFN-γ (about 40 ng/mL) and GM-CSF (about 5000 pg/mL) were generated with the immobilized anti-NKG2D mAb, indicating a robust activation through NKG2D receptor using the platebound A10 mAb. To the contrary, a simultaneous cross-linking of coimmobilized anti-Ly49A or anti-Ly49G2 mAbs significantly reduced the production of both IFN-γ and GM-CSF in a concentration-dependent manner (Figure 6). Although combination of anti-Ly49A and anti-Ly49G2 mAbs also resulted in a significant reduction, the inhibition on cytokine production reached a plateau and an increase in the anti-Ly49 antibody concentration did not result in further increase in the inhibition. This indicates that Ly49A-/G- NK subsets were still able to produce these cytokines, because they were not affected by the 2 specific anti-Ly49 mAbs. Our observations are not due to nonspecific mAb interactions, because the isotype-matched control antibodies had no or negligible effect on the cytokine secretion by the NK cells. Results obtained in this section using “target cell-free” systems further validate our hypothesis that there is a strong interplay between the inhibitory Ly49 and the activating NKG2D receptors. This interplay is vital in redefining “self,” “missing self,” and “induced self” in the context of target cells that are expressing activating ligands and MHC class I molecules.
Immobilized anti-Ly49A or anti-Ly49G mAbs inhibit NKG2D receptor-mediated IFN-γ and GM-CSF production by NK cells. ELISA plates (Immunolon; Nunc, Rochester, NY) were coated with 5 μg/mL anti-NKG2D mAb in the presence or absence of titrated concentrations of either anti-Ly49A (A1), anti-Ly49G2 (4D11) alone, or both together. Independently, appropriate isotype antibody controls were also titrated in similar concentrations to define the specificity of the inhibition. NK cells (105 per well) derived from B6 were added to the mAb-coated plates and incubated for 18 to 20 hours before harvesting the culture supernatants. Culture supernatants were assayed for the effect of inhibitory Ly49 mAbs on NKG2D-mediated IFN-γ (A) or GM-CSF (B) productions. In the top panels of A and B,  indicates anti-NKG2D mAb A10 alone; □, isotype control, IgG1; ▪, anti-Ly49A; ○, isotype control, IgG2; and •, anti-Ly49G2. In the bottom bar graphs, □ indicates A10 alone, and
indicates anti-NKG2D mAb A10 alone; □, isotype control, IgG1; ▪, anti-Ly49A; ○, isotype control, IgG2; and •, anti-Ly49G2. In the bottom bar graphs, □ indicates A10 alone, and  , anti-Ly49A and anti-Ly49G2 combined with indicated mAb concentrations.
, anti-Ly49A and anti-Ly49G2 combined with indicated mAb concentrations.
Immobilized anti-Ly49A or anti-Ly49G mAbs inhibit NKG2D receptor-mediated IFN-γ and GM-CSF production by NK cells. ELISA plates (Immunolon; Nunc, Rochester, NY) were coated with 5 μg/mL anti-NKG2D mAb in the presence or absence of titrated concentrations of either anti-Ly49A (A1), anti-Ly49G2 (4D11) alone, or both together. Independently, appropriate isotype antibody controls were also titrated in similar concentrations to define the specificity of the inhibition. NK cells (105 per well) derived from B6 were added to the mAb-coated plates and incubated for 18 to 20 hours before harvesting the culture supernatants. Culture supernatants were assayed for the effect of inhibitory Ly49 mAbs on NKG2D-mediated IFN-γ (A) or GM-CSF (B) productions. In the top panels of A and B,  indicates anti-NKG2D mAb A10 alone; □, isotype control, IgG1; ▪, anti-Ly49A; ○, isotype control, IgG2; and •, anti-Ly49G2. In the bottom bar graphs, □ indicates A10 alone, and
indicates anti-NKG2D mAb A10 alone; □, isotype control, IgG1; ▪, anti-Ly49A; ○, isotype control, IgG2; and •, anti-Ly49G2. In the bottom bar graphs, □ indicates A10 alone, and  , anti-Ly49A and anti-Ly49G2 combined with indicated mAb concentrations.
, anti-Ly49A and anti-Ly49G2 combined with indicated mAb concentrations.
Ly49A/G receptor engagement to H2-Dd reduces NF-κB nuclear translocation in NK cells
Engagement of NKG2D activates multiple signal transduction pathways, some of which culminate in activation of NF-κB, that ultimately lead to target cell lysis by NK cells (Figure 7A). On the other hand, ligation of inhibitory Ly49 receptors has been shown to activate SH2 domain-containing phosphatases, which results in dephosphorylation of positive signaling molecules. Consequently, this results in the down-regulation of signal transduction pathways, possibly including nuclear translocation of NF-κB. We hypothesized, therefore, that NF-κB activation would be reduced in NK cells that recognized targets expressing both activating and inhibitory ligands relative to those that expressed only the activating ligands. To test this hypothesis, nuclear extracts were isolated from B6-derived NK cells following their incubation with EL4 (H60neg), ST63 (H60hi, H2-Dd-Neg), or ST77 (H60hi, H2-Dd-High) targets, and gel-shift assays were used to quantify the amount of NF-κB present in nuclear extracts. Gel supershift assays in the presence of an NF-κB-specific mAb were used to confirm the authenticity of the NF-κB protein bands. As shown in Figure 7B, the extent of nuclear translocation of NF-κB was reduced in NK cells incubated with ST77 (H60hi, H2-Dd-High) relative to ST63 (H60hi, H2-Dd-Neg) targets. These results indicate that interaction of H2-Dd with Ly49A/G inhibitory receptors has the downstream consequence of inhibiting NF-κB activation in response to NKG2D-mediated recognition of H60-expressing target cells.
Inhibition of the NKG2D activation pathway is mediated through the reduced translocation of NF-κB into the nuclei of NK cells. (A) Diagram depicting the hypothesized interactions between activating NKG2D and inhibitory Ly49 pathways. Binding of H60 with NKG2D receptor leads to phosphorylation of DAP10 or DAP12, resulting in recruitment of protein tyrosine kinases (PTKs) belonging to the SFK family. The subsequent activation of PTKs results in the phosphorylation of downstream signaling proteins, eventually leading to nuclear translocation of NF-κB. Phosphorylation of ITIM sequences at the cytoplasmic domain of Ly49 molecules recruits and activates phosphatases, which in turn dephosphorylate substrates that are part of the NKG2D activation pathway. This results in the impaired translocation of NF-κB into the nuclei. (B) Gel-shift analysis was performed using nuclear extracts from fresh NK cells incubated with indicated target cells at a 40:1 E/T ratio for 2 hours at 37°C. Supershift analysis was performed on nuclear extracts from NK cells incubated with ST63 in the presence of 32P-labelled oligonucleotide and a mAb specific for NF-κB subunit, p50. Data presented are representative of 2 independent experiments.
Inhibition of the NKG2D activation pathway is mediated through the reduced translocation of NF-κB into the nuclei of NK cells. (A) Diagram depicting the hypothesized interactions between activating NKG2D and inhibitory Ly49 pathways. Binding of H60 with NKG2D receptor leads to phosphorylation of DAP10 or DAP12, resulting in recruitment of protein tyrosine kinases (PTKs) belonging to the SFK family. The subsequent activation of PTKs results in the phosphorylation of downstream signaling proteins, eventually leading to nuclear translocation of NF-κB. Phosphorylation of ITIM sequences at the cytoplasmic domain of Ly49 molecules recruits and activates phosphatases, which in turn dephosphorylate substrates that are part of the NKG2D activation pathway. This results in the impaired translocation of NF-κB into the nuclei. (B) Gel-shift analysis was performed using nuclear extracts from fresh NK cells incubated with indicated target cells at a 40:1 E/T ratio for 2 hours at 37°C. Supershift analysis was performed on nuclear extracts from NK cells incubated with ST63 in the presence of 32P-labelled oligonucleotide and a mAb specific for NF-κB subunit, p50. Data presented are representative of 2 independent experiments.
Discussion
Recent studies have demonstrated that NKG2D is a major activation receptor that associates with novel activation motif containing DAP10 or ITAM containing KARAP/DAP12 adaptor molecules. A fundamental question is whether NK cell activation initiated via the H60-NKG2D interaction overrides the negative inhibition generated by the engagement of MHC class I to Ly49 receptors. Although an altered balance in the signaling strength of activating and inhibiting pathways of NK cells has been previously postulated,1,29 recent findings illustrate that NK cell activation via NKG2D receptor can occur despite the normal expression of MHC class I molecules on the target cells.10-12 Here, by varying the levels of H60 expression and introducing additional MHC class I molecules on the target cells, we provide the first set of evidence demonstrating that the inhibitory Ly49 receptors can indeed down-regulate NKG2D-mediated NK cell functions.
In evaluating the role of inhibitory receptors on the NKG2D-mediated cytotoxicity, we determined the effect of Ly49C/I receptor interaction to H2-Kb, and Ly49A/G interaction to H2-Dd, using H60-expressing target cells. Blocking Ly49C/I receptors with specific mAbs or using purified Ly49C+/I+ or Ly49C-/I- NK subsets, we demonstrate that the expression levels of H60 on target cells play a major role in balancing the effect of inhibitory Ly49C/I receptors. At a lower level of H60 expression, activation through NKG2D receptor is highly subject to Ly49C/I receptor-mediated inhibition because blocking Ly49C/I-Kb interactions resulted in a more than 50% increase in the cytotoxicity. In contrast, even without the mAb blocking, similar Ly49C/I-mediated inhibition could be significantly, if not completely, overridden by an increased expression of H60 on the target cells. Interestingly, in the present study, because mAb specific for Ly49I (YLI-90) alone could relieve significant levels of inhibition compared with mAb that reacted to both Ly49C and Ly49I (SW5E6), we speculate that Ly49I may be the major inhibitory receptor for H2-Kb ligand.
Compared with Ly49C/I-mediated inhibition, introduction of an additional MHC class I molecule such as H2-Dd that serves as a ligand for inhibitory Ly49A/G receptors significantly reduced NKG2D-mediated cytotoxicity. Remarkably, in these experiments, even an increased level of H60 expression could not override the Ly49A/G receptor-mediated inhibition. Therefore, we conclude a successful immune surveillance by NK cells is determined not only by the interaction of NKG2D to “induced” H60 molecules but is also dependent on the scanning for specific MHC class I molecules on the target cells by the inhibitory Ly49 receptors.
The only other well-defined murine NK cell-activating receptor is Ly49D, which recognizes H2-Dd as the activating ligand.20,30 Evidently, our present observations with NKG2D receptor are analogous with the effect of inhibitory Ly49C/I receptor on the activating Ly49D receptor. Although interaction of Ly49C/I receptors with H2-Dd has been shown to mediate inhibition, Ly49D+/C+/I+ NK subsets successfully lysed H2-Dd-positive target cells.31,32 In contrast, the same study also found that the Ly49D+/A+/G+ NK subsets were inhibited by H2-Dd-positive target cells.32 These earlier studies thereby concluded that the inhibitory effect generated by the interaction of Ly49C/I with its MHC ligands is weaker, thereby allowing the activation of NK cells.
The strong inhibitory abilities of Ly49A/G compared with Ly49C/I receptors need further analysis. One possible explanation is provided by recent x-ray crystal structure studies25 demonstrating that the intermolecular electrostatic interactions such as hydrogen bonds, van der Waals contacts, and salt bridges for Ly49A-Dd are far more extensive compared with Ly49C-Kb complexes.25 Based on these earlier observations and our current results, the functionality of the “missing-self” hypothesis in NKG2D-mediated activation is conclusively evident due to the following reasons. In the case of H-2d-positive strain-derived NK cells (BALB/c and B10.D2), the cytotoxicity through NKG2D receptor is significantly abolished when the target cells (ST77) express normal levels of self-MHC (H2-Dd), thereby establishing “self.” Even a higher expression level of H60 could not activate these NK cells to mediate cytotoxicity. Therefore, “down-regulation” of self-MHC (level of H2-Dd as in the case of ST72) is necessitated to establish “missing self” for these NK cells to mediate the efficient lysis. Similarly, the recognition of “self” is also evident in NK cells from an H-2b-positive strain (B6), but the extent of inhibition is strongly subjected to the level of activating ligand due to the insufficient inhibition mediated by Ly49C/I receptors. The evolutionary advantage of differential inhibitory abilities of Ly49 receptors has not been currently understood.
Apart from mediating cytotoxicity, NK cells also modulate both innate and adaptive immune responses through the secretion of proinflammatory cytokines.33 Activation of NK cells through the cross-linking of NKG2D receptor in the presence of immobilized anti-Ly49 mAbs further validated the “altered-balance” hypothesis. In our experiments, co-cross-linking Ly49A or Ly49G molecules along with anti-NKG2D mAb significantly abrogated the generation of IFN-γ and GM-CSF by the NK cells. These results strongly suggest that the cascade of inhibitory signals generated by the engagement of Ly49 receptors with respective MHC can regulate not only the NKG2D-mediated cytotoxicity but also the cytokine secretions. Although mAbs directed to both Ly49A and Ly49G receptors effectively mediated similar levels of inhibitions on the IFN-γ and GM-CSF production, the roles of other major inhibitory receptors such as Ly49C, Ly49I, and CD94/NKG2A remain to be determined. Furthermore, the role of “altered balance” on other cytokine or chemokine productions and noncytotoxic functions of NK cells will be imperative to delineate “global” versus “specific” impacts of the inhibitory signals. It is also significant that our results presented here on NKG2D-mediated cytokine secretion parallels earlier observations with other activating receptors such as NK1.127 and 2B4 (human).28 Collectively, these results confirm that the successful production of cytokines by NK cells needs both the induction of activating ligands and concomitant down-regulation of MHC class I on target cells.
Engagement of inhibitory Ly49 receptors by MHC class I molecules results in the recruitment of SH2 domain-containing phosphatases to immunotyrosine-based inhibitory motifs (ITIMs).34,35 This results in the activation of phosphatases, which dephosphorylate signaling molecules that are part of activation pathways.36 Although the mechanism by which the phosphatases are activated has been well defined, the substrates, which are targeted by these phosphatases, are yet to be identified. Therefore, we analyzed the role of Ly49A/G-mediated inhibitory signals on the nuclear translocation of NF-κB and established that the inhibition resulted in a significant reduction of NF-κB translocation. The diminished nuclear translocation of NF-κB, which results in decreased NKG2D-mediated cytotoxicity, can be explained by the fact that the transcription of perforin is dependent on the binding of NF-κB to an upstream enhancer region in the perforin gene.37 In addition, that the NKG2D-mediated activation pathway is defective in perforin-deficient animals further supports this hypothesis.38 Interestingly, earlier studies showed that NK cells derived from fetal liver and thymus lack the expression of inhibitory Ly49 receptors but were able to distinguish between MHC class I-positive and -negative target cells.39 It is of further interest that expression of NKG2D occurs very early in the development of NK cells, when these cells are still lacking the expression of many inhibitory Ly49 receptors.40,41 Although it is assumed that NK cells at this particular stage during their development express either a low level of NKG2D receptor or other non-Ly49 inhibitory receptors to maintain a balance, the exact mechanism by which these fetal NK cells obtain a proper balance in distinguishing between “self” and “nonself” is not very well understood.41,42
Another explanation for the observed regulation is that the inhibitory LY49 receptors indeed target other ITAM-bearing activation receptors that associate with NKG2D. A detailed study involving the intracellular signaling molecules is needed to delineate the specific activation pathway(s) that is targeted by the Ly49-recruited phosphatases. Nonetheless, our observations highlight the significance of both the levels of activating ligands for NKG2D receptor and the nature of MHC class I expressed on target cells. As for the murine system, parallel analysis is required for human NK cells in the formulation of cellular immunotherapy for leukemia ablation that is based on NKG2D receptor-mediated activation. Because NKG2D receptor can associate with 2 distinct adaptor molecules (ie, DAP10 or KARAP/DAP12) to mediate intracellular signal transduction, it would be of interest to analyze the effect of inhibitory Ly49 receptors on each of these activation pathways. Our present findings also set the stage to understand the role of inhibitory receptors on NKG2D-mediated activation in γδ-positive T cells,43 because NKG2D is one of the major activation receptors involved in their effector functions. Furthermore, inhibitory Ly49 receptors are also expressed on T44 and B cells,45 with previous studies demonstrating a causal relationship between the Ly49A expression and down-regulation of T- and B-cell activations; however, the role of an “altered balance” between activating and inhibiting signals is yet to be established.
Prepublished online as Blood First Edition Paper, August 24, 2004; DOI 10.1182/blood-2004-03-1075.
Supported by the Roche Organ Transplantation Research Foundation (ROTRF 111662730) (S.M.). S.M. is a Scholar of the American Cancer Society (RSG-02-172-01-LIB) and Young Investigator of the American Society for Blood and Marrow Transplantation (ASBMT 25045). J.R. is a recipient of a Northwestern Mutual Postdoctoral Fellowship.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs L. Gorski and D. Newman for the critical review of the manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal